Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review
Abstract
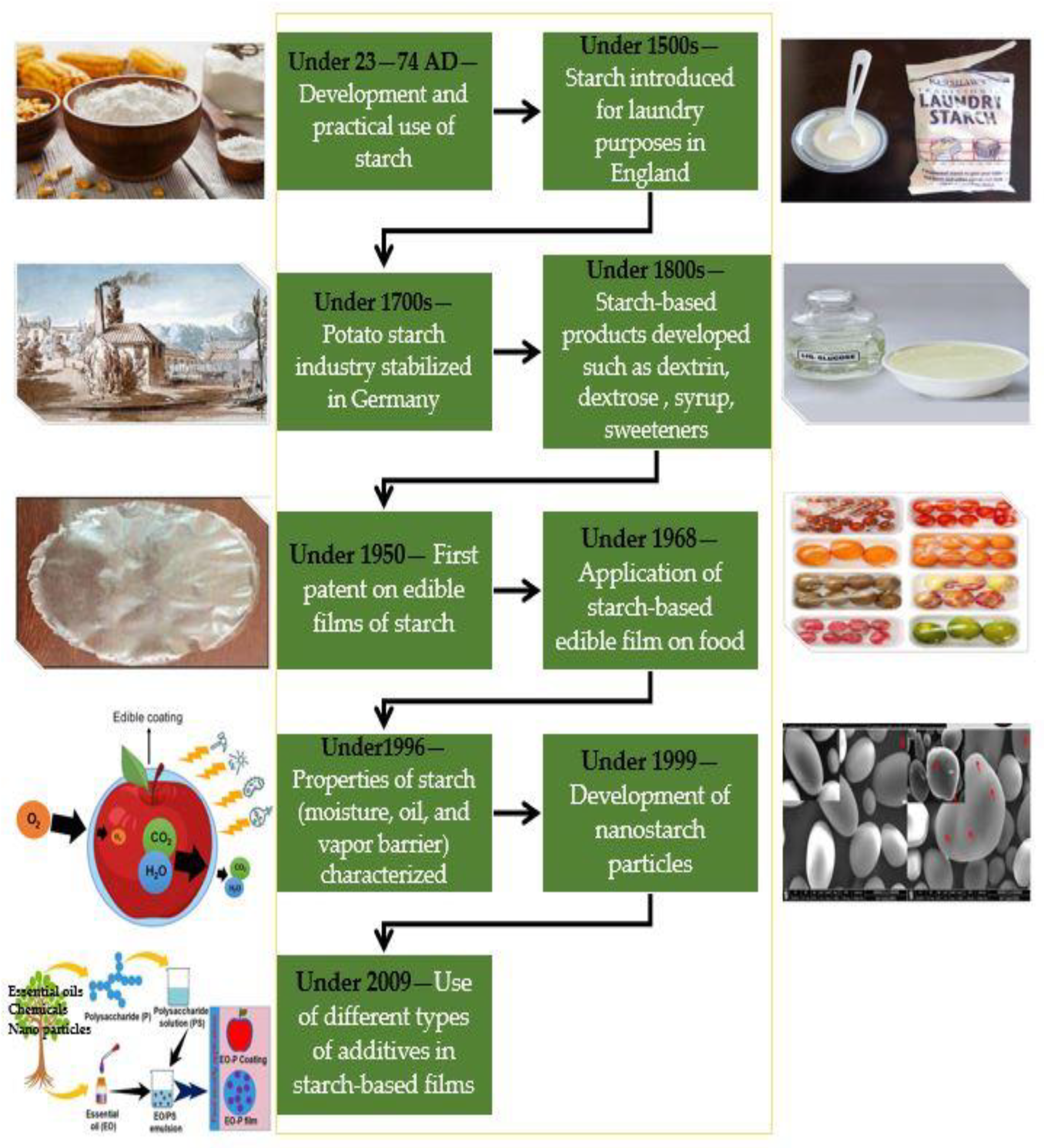
:1. Introduction
2. Starch-Based Edible Coatings/Films
3. Food Additives
4. Functions of Essential Oils and Extracts as Additives
5. Function of Chemicals as Additives
6. Function of Pigments and Others as Additives
7. Effect of Incorporation of Additives on Various Properties of Starch-Based Films
7.1. Thickness
7.2. Morphology
7.3. Optical Properties
7.4. Water Barrier Properties
7.5. Biodegradability
7.6. Mechanical Properties
7.7. Antioxidant and Antimicrobial Properties
7.8. Oxygen Barrier Properties
8. Food Applications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Punia, S. Barley starch: Structure, properties and in vitro digestibility—A review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Farajpour, R.; Djomeh, Z.E.; Moeini, S.; Tavakolipour, H.; Safayan, S. Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Major factors affecting the characteristics of starch-based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Sinhmar, A.; Nehra, M.; Thory, R.; Pathera, A.K.; Sundarraj, A.A.; Nain, V. Impact on various properties of native starch after synthesis of starch nanoparticles: A review. Food Chem. 2021, 364, 130416. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Dhull, S.B.; Sandhu, K.S.; Kaur, M.; Purewal, S.S. Kidney bean (Phaseolus vulgaris) starch: A review. Legum. Sci. 2020, 2, e52. [Google Scholar] [CrossRef]
- Fan, X.-J.; Zhang, B.; Yan, H.; Feng, J.-T.; Ma, Z.-Q.; Zhang, X. Effect of lotus leaf extract incorporated composite coating on the postharvest quality of fresh goji (Lycium barbarum L.) fruit. Postharvest Biol. Technol. 2019, 148, 132–140. [Google Scholar] [CrossRef]
- Perazzo, K.K.N.C.L.; Carlos De Vasconcelos Conceição, A.; Pires Dos Santos, J.C.; De Jesus Assis, D.; Souza, C.O.; Druzian, J.I. Properties and antioxidant action of actives cassava starch films incorporated with green tea and palm oil extracts. PLoS ONE 2014, 9, e105199. [Google Scholar] [CrossRef] [Green Version]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Lin, X. Effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. Carbohydr. Polym. 2012, 90, 1595–1600. [Google Scholar] [CrossRef]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef]
- Saha, T.; Hoque, M.E.; Mahbub, T. Biopolymers for Sustainable Packaging in Food, Cosmetics, and Pharmaceuticals. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–214. [Google Scholar]
- Molavi, H.; Behfar, S.; Shariati, M.A.; Kaviani, M.; Atarod, S. A review on biodegradable starch based film. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 456. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, C.; Perez-Rea, D.; Bergenståhl, B.; Carballo, S.; Sjöö, M.; Nilsson, L. Physicochemical and structural properties of starch from five Andean crops grown in Bolivia. Int. J. Biol. Macromol. 2019, 125, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Hananinur, Z.A. Surface properties of biodegradable polymers for food packaging. In Polymers for Food Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 131–147. [Google Scholar]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch–methylcellulose based edible films: Rheological properties of film-forming dispersions. J. Food Eng. 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Saxena, A.; Sharma, L.; Maity, T. Enrichment of Edible Coatings and Films with Plant Extracts or Essential Oils for the Preservation of Fruits and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128168981. [Google Scholar]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.M.; Lim, L.-T.; Dias, Á.R.G.; da Rosa Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef]
- Johnson, K.A.; Mauer, L.J. Effects of controlled relative humidity storage on moisture sorption and amylopectin retrogradation in gelatinized starch lyophiles. J. Food Sci. 2019, 84, 507–523. [Google Scholar] [CrossRef]
- Nizam, N.H.M.; Rawi, N.F.M.; Ramle, S.F.M.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Kassim, M.H.M. Physical, thermal, mechanical, antimicrobial and physicochemical properties of starch based film containing aloe vera: A review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- World Health Organization & Joint FAO/WHO Expert Committee on Food Additive. Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2017; ISBN 9241210028. [Google Scholar]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.-D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Sui, J.; Yu, B.; Yuan, C.; Guo, L.; Abd El-Aty, A.M.; Cui, B. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohydr. Polym. 2021, 254, 117314. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Cirmi, S.; Navarra, M. Effectiveness of citrus fruits on Helicobacter pylori. Evid. Based Complement. Altern. Med. 2017, 2017, 8379262. [Google Scholar] [CrossRef] [Green Version]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and Characterization of Corn Starch/PVA Active Films Incorporated with Carvacrol Nanoemulsions; Elsevier B.V.: Cham, Switzerland, 2020; Volume 164, ISBN 8618353321707. [Google Scholar]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; do Amaral Sobral, P.J.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef]
- De Souza, A.G.; dos Santon, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Bodini, R.B.; Pugine, S.M.P.; de Melo, M.P.; de Carvalho, R.A. Antioxidant and anti-inflammatory properties of orally disintegrating films based on starch and hydroxypropyl methylcellulose incorporated with Cordia verbenacea (erva baleeira) extract. Int. J. Biol. Macromol. 2020, 159, 714–724. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Gao, W.; Wu, W.; Liu, P.; Hou, H.; Li, X.; Cui, B. Preparation and evaluation of hydrophobic biodegradable films made from corn/octenylsuccinated starch incorporated with different concentrations of soybean oil. Int. J. Biol. Macromol. 2020, 142, 376–383. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of natural antioxidants from rice straw into renewable starch films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Lukin, N.D. Structure and properties of biodegradable maize starch/chitosan composite films as affected by PVA additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef]
- Leal, I.L.; da Silva Rosa, Y.C.; da Silva Penha, J.; Cruz Correia, P.R.; da Silva Melo, P.; Guimarães, D.H.; Barbosa, J.D.V.; Druzian, J.I.; Machado, B.A.S. Development and application starch films: PBAT with additives for evaluating the shelf life of Tommy Atkins mango in the fresh-cut state. J. Appl. Polym. Sci. 2019, 136, 1–19. [Google Scholar] [CrossRef]
- López, O.V.; Giannuzzi, L.; Zaritzky, N.E.; García, M.A. Potassium sorbate controlled release from corn starch films. Mater. Sci. Eng. C 2013, 33, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Olivato, J.B.; Grossmann, M.V.E.; Bilck, A.P.; Yamashita, F. Effect of organic acids as additives on the performance of thermoplastic starch/polyester blown films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Kohli, D.; Maiti, M.; Jana, A.K.; Bajpai, S. Effect of cross linking of PVA/starch and reinforcement of modified barley husk on the properties of composite films. Carbohydr. Polym. 2016, 151, 926–938. [Google Scholar] [CrossRef]
- Leroy, E.; Decaen, P.; Jacquet, P.; Coativy, G.; Pontoire, B.; Reguerre, A.L.; Lourdin, D. Deep eutectic solvents as functional additives for starch based plastics. Green Chem. 2012, 14, 3063–3066. [Google Scholar] [CrossRef]
- Sartori, T.; Menegalli, F.C. Development and characterization of unripe banana starch films incorporated with solid lipid microparticles containing ascorbic acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Souza, C.O.; Silva, L.T.; Silva, J.R.; López, J.A.; Veiga-Santos, P.; Druzian, J.I. Mango and acerola pulps as antioxidant additives in cassava starch bio-based film. J. Agric. Food Chem. 2011, 59, 2248–2254. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.Q.; Lopes, S.M.; Costa, T.M.H.; Flôres, S.H.; Rios, A.; de Oliveira Rios, A. Active biodegradable cassava starch films incorporated lycopene nanocapsules. Ind. Crop. Prod. 2017, 109, 818–827. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Li, S.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef] [PubMed]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2020, 118, 108758. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, F.; Yuan, L.; Hu, H.; Yao, X.; Liu, J. Comparison of the physical and functional properties of starch/polyvinyl alcohol films containing anthocyanins and/or betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef]
- Bagde, P.; Nadanathangam, V. Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose. Carbohydr. Polym. 2019, 222, 115021. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Cao, L.; Ge, T.; Meng, F.; Xu, S.; Li, J.; Wang, L. An edible oil packaging film with improved barrier properties and heat sealability from cassia gum incorporating carboxylated cellulose nano crystal whisker. Food Hydrocoll. 2020, 98, 105251. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Najwa, N.A.I.S.; Guerrero, P.; de la Caba, K.; Nur Hanani, Z.A. Physical and antioxidant properties of starch/gelatin films incorporated with Garcinia atroviridis leaves. Food Packag. Shelf Life 2020, 26, 100583. [Google Scholar] [CrossRef]
- Silva, V.D.M.; Macedo, M.C.C.; Rodrigues, C.G.; dos Santos, A.N.; e Loyola, A.C.D.F.; Fante, C.A. Biodegradable edible films of ripe banana peel and starch enriched with extract of Eriobotrya japonica leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Mościcki, L.; Mitrus, M.; Wójtowicz, A.; Oniszczuk, T.; Rejak, A.; Janssen, L. Application of extrusion-cooking for processing of thermoplastic starch (TPS). Food Res. Int. 2012, 47, 291–299. [Google Scholar] [CrossRef]
- Vanier, N.L.; Paraginski, R.T.; Berrios, J.D.J.; da Conceição Oliveira, L.; Elias, M.C. Thiamine content and technological quality properties of parboiled rice treated with sodium bisulfite: Benefits and food safety risk. J. Food Compos. Anal. 2015, 41, 98–103. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Frígols, B.; Martí, M.; Salesa, B.; Hernández-Oliver, C.; Aarstad, O.; Teialeret Ulset, A.-S.; Inger Sætrom, G.; Aachmann, F.L.; Serrano-Aroca, Á. Graphene oxide in zinc alginate films: Antibacterial activity, cytotoxicity, zinc release, water sorption/diffusion, wettability and opacity. PLoS ONE 2019, 14, e0212819. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Liu, Z. Edible films and coatings from starches. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005; pp. 318–337. [Google Scholar]
- Maniglia, B.C.; Laroque, D.A.; de Andrade, L.M.; Carciofi, B.A.M.; Tenório, J.A.S.; de Andrade, C.J. Production of active cassava starch films; effect of adding a biosurfactant or synthetic surfactant. React. Funct. Polym. 2019, 144, 104368. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Estevez-Areco, S.; Goyanes, S. Potato starch-based biocomposites with enhanced thermal, mechanical and barrier properties comprising water-resistant electrospun poly (vinyl alcohol) fibers and yerba mate extract. Carbohydr. Polym. 2019, 215, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Olsson, E.; Menzel, C.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef]
- Stoll, L.; Silva, A.M.D.; Iahnke, A.O.E.S.; Costa, T.M.H.; Flores, S.H.; Rios, A.D.O. Active biodegradable film with encapsulated anthocyanins: Effect on the quality attributes of extra-virgin olive oil during storage. J. Food Process. Preserv. 2017, 41, e13218. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile properties, barrier properties, and biodegradation in soil of compression—Molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Degradation behavior of biocomposites based on cassava starch buried under indoor soil conditions. Carbohydr. Polym. 2014, 101, 20–28. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as cross-linking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.D.A.; Carvalho, R.D. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Yoon, S.D.; Chough, S.H.; Park, H.R. Properties of starch-based blend films using citric acid as additive. II. J. Appl. Polym. Sci. 2006, 100, 2554–2560. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Sun, J.; Fang, Y.; Liu, J.; Jin, C. Comparison of the structural characterization and physicochemical properties of starches from seven purple sweet potato varieties cultivated in China. Int. J. Biol. Macromol. 2018, 120, 1632–1638. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef] [Green Version]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, optical and mechanical properties of chitosan–starch films with natural extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [Green Version]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Darbasi, M.; Askari, G.; Kiani, H.; Khodaiyan, F. Development of chitosan based extended-release antioxidant films by control of fabrication variables. Int. J. Biol. Macromol. 2017, 104, 303–310. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.C.; Luchini, A.C.; Seito, L.N.; Gomes, J.C.; Crespo-López, M.E.; Di Stasi, L.C. Cordia verbenacea and secretion of mast cells in different animal species. J. Ethnopharmacol. 2011, 135, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Jonhed, A.; Andersson, C.; Järnström, L. Effects of film forming and hydrophobic properties of starches on surface sized packaging paper. Packag. Technol. Sci. Int. J. 2008, 21, 123–135. [Google Scholar] [CrossRef]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect of glycerol and amylose enrichment on cassava starch film properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Chavan, P.; Sinhmar, A.; Sharma, S.; Dufresne, A.; Thory, R.; Kaur, M.; Sandhu, K.S.; Nehra, M.; Nain, V. Nanocomposite Starch Films: A New Approach for Biodegradable Packaging Materials. Starch-Stärke 2022, 74, 2100302. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Júnior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- de Aquino, A.B.; Blank, A.F.; de Aquino Santana, L.C.L. Impact of edible chitosan–cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015, 171, 108–116. [Google Scholar] [CrossRef]
- Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; El Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of sweet potato starch-based nanocomposite films activated with thyme essential oil on the shelf-life of baby spinach leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radha Krishnan, K.; Babuskin, S.; Rakhavan, K.R.; Tharavin, R.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Potential application of corn starch edible films with spice essential oils for the shelf life extension of red meat. J. Appl. Microbiol. 2015, 119, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- dos Caetano, S.K.; Lopes, N.A.; Costa, T.M.H.; Brandelli, A.; Rodrigues, E.; Flôres, S.H.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Supardan, M.D.; Annisa, Y.; Arpi, N.; Satriana, S.; Wan Mustapha, W.A. Cassava starch edible film incorporated with lemongrass oil: Characteristics and application. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and Applicability of Starch-Based Films: An Eco-Friendly Approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
| Sources of Starch | Additives | Amounts Incorporated | Plasticizers Used | Key Features of Developed Films | Reference |
|---|---|---|---|---|---|
| Cassava starch | Green tea extract (2.5, 5.0 and 7.5%) and palm oil colorant (0.01, 0.05 and 1.00%) | 2.5–7.5% 0.01–1.0% | Glycerol |
| [16] |
| Corn starch and banana peel flour | Eriobotrya japonica leaf extract | 4% | Glycerol |
| [17] |
| Corn starch | Zanthoxylum bungeanum essential oil | 0.5%, 1.0%, and 2.0% v/v | Glycerol |
| [40] |
| Chitosan and sugar palm starch | Extra virgin olive oil | 1%, 2%, and 5% | Glycerol |
| [43] |
| Corn starch | Cinnamomum camphora, cardamom, and cinnamon oil | 2% and 5% | Glycerol |
| [44] |
| Corn starch | Carvacrol nanoemulsions (CNE) | 5 mL, 10 mL, 15 mL, and 20 mL | Glycerol |
| [45] |
| Chitosan and cassava starch | Pitanga (Eugenia uniflora L.) leaf extract and natamycin | 1 g and 2.25 g | Glycerol |
| [46] |
| Cassava starch | Mannosylerythritol lipid-B | 20 g | Glycerol |
| [47] |
| Corn starch | Grapefruit seed extract (GFSE) | 0.5 g and 1.5 g | Glycerol and sorbitol |
| [48] |
| Corn starch | Carvacrol essential oil (EO) and montmorillonite (MMT) | 4.5 wt%, 9 wt%, and 15 wt% | Glycerol |
| [49] |
| Pregelatinized starch | Cordia verbenacea (erva baleeira) | 0.25 mg, 0.50 mg, and 0.75 mg | Sorbitol |
| [50] |
| Cassava starch | Yerba mate (Ilex paraguariensis) leaf extract | 10 wt% and 20 wt% | Glycerol |
| [51] |
| Corn starch and chitosan | Thymus kotschyanus essential oil and pomegranate peel extract | 0.5%, 1%, and 2% (w/w) | Glycerol |
| [52] |
| Corn/octenylsuccinated starch (C/OS) | Soybean oil (SO) | 0.5%, 1.0%, 1.5%, and 2.0% w/w | Glycerol |
| [53] |
| Potato starch | Rice straw waste | 2 g, 3 g, and 4 g | Glycerol |
| [54] |
| Tapioca starch | Garcinia atroviridis leaves | 1%, 3%, and 5% | Glycerol |
| [55] |
| Sources of Starch | Additives | Amounts Incorporated | Plasticizers Used | Key Features of Developed Films | Reference |
|---|---|---|---|---|---|
| Cassava starch | Sodium nitrite | 1%, 2%, and 5% | Glycerol |
| [31] |
| Cassava starch | Sodium-dodecyl-sulphate | 20 g | Glycerol |
| [46] |
| Corn starch | Potassium sorbate (KS) | 0.2 g and 1.5 g | Glycerol and sorbitol |
| [48] |
| Maize starch chitosan | Polyvinyl alcohol (PVA) | 0–40 wt% | Glycerol |
| [55] |
| Cassava starch | Poly (butylene adipate-co-terephthalate) (40, 60 g), coconut nanocellulose (0.55 g), annatto (0.5, 1.0 g), and citric acid (1.0 g) | 40–60 g 0.55 g 0.5 g 1.0 g | Glycerol |
| [56] |
| Corn starch | Potassium sorbate | 0.1–0.5% | Glycerol |
| [57] |
| Thermoplastic starch with polybutylene adipate terephthalate | Sodium nitrite | 1–5% | Glycerol |
| [61] |
| Corn starch | Urea | 16.8 wt% | Glycerol |
| [62] |
| Corn starch | Deep eutectic solvents (DES) (urea–choline chloride and glycerol–choline chloride) | 2:1 | Glycerol |
| [63] |
| Banana starch | Lauric acid, oleic acid, and ascorbic acid | 0.9% | Glycerol |
| [64] |
| Sources of Starch | Additives | Amounts Incorporated | Plasticizers Used | Key Features of Developed Films | Reference |
|---|---|---|---|---|---|
| Cassava Starch | Acerola and mango pulps | 0–20% | — |
| [65] |
| Rice starch/ Chitosan | Anthocyanins, betalains, resveratrol, thymol, and carvacrol | 5% | Glycerol |
| [66] |
| Cassava Starch | Lycopene or lycopene nanocapsules | 2%, 5%, and 8% (w/w) | Glycerol |
| [68] |
| Cassava starch | Lactic acid bacteria (Lactobacillus plantarum and Pedocococcus pentosaceus) | 0.5 g, 1 g, 1.5 g, and 2 g | Glycerol |
| [69] |
| Cassava starch | Anthocyanins /betacyanins | 3:1, 1:1, and 1:3 | Polyvinyl alcohol (PVA) |
| [71] |
| Corn starch | Immobilized bacteriocin | 1% | Glycerol |
| [72] |
| Cassava Starch | Papain | 5–15% | — |
| [73] |
| Thermoplastic starch with polybutylene adipate terephthalate | Zinc oxide nanoparticles | 1–5% | — |
| [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. https://doi.org/10.3390/polym14101987
Singh GP, Bangar SP, Yang T, Trif M, Kumar V, Kumar D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers. 2022; 14(10):1987. https://doi.org/10.3390/polym14101987
Chicago/Turabian StyleSingh, Gurvendra Pal, Sneh Punia Bangar, Tianxi Yang, Monica Trif, Vinod Kumar, and Dinesh Kumar. 2022. "Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review" Polymers 14, no. 10: 1987. https://doi.org/10.3390/polym14101987
APA StyleSingh, G. P., Bangar, S. P., Yang, T., Trif, M., Kumar, V., & Kumar, D. (2022). Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers, 14(10), 1987. https://doi.org/10.3390/polym14101987











