Overview: Polycarbonates via Ring-Opening Polymerization, Differences between Six- and Five-Membered Cyclic Carbonates: Inspiration for Green Alternatives
Abstract
:1. Introduction
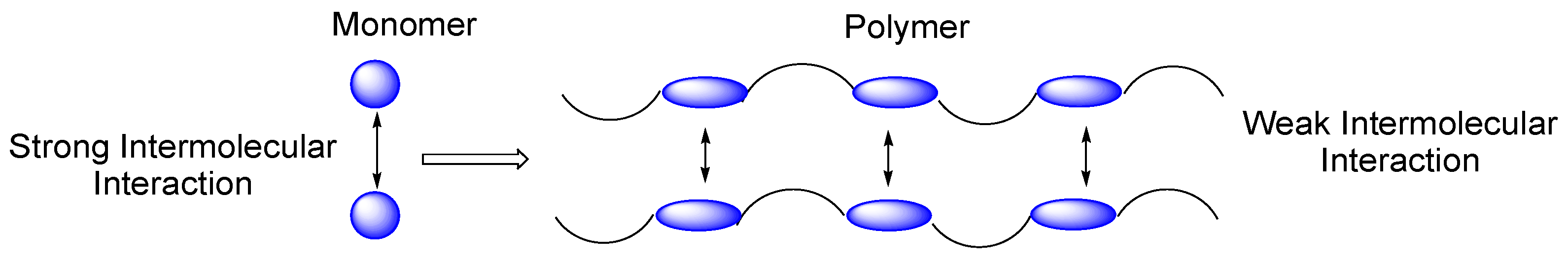
- Packaging is potentially the largest use of biodegradable polymers [10]. Biodegradable polymeric materials and blends that have passed toxicological and cytocompatibility testing for in vivo use have obvious benefits as biodegradable materials for pharmaceutical products, drugs, dressings for wounds, and surgical fixation (sutures, clips, bone pins, and plates). Biodegradable implants can be used as alternatives to metal implants for the fixation of fractured bones and joints [11].
- Controlled drug delivery may be the most important and versatile application of these polymers [12]. It has applications in the medical, veterinary, and agrochemical fields. Active ingredients, ranging from pesticides to contraceptives, can be delivered by sustained release followed by the ultimate biodegradation of the carrier medium.
2. Synthesis of Polycarbonates and ROP
2.1. Kinetic and Thermodynamic Aspects of Cyclic Carbonates Ring-Opening Polymerization
2.1.1. Kinetic Control
2.1.2. Thermodynamic Control
- Kp: rate constant of propagation
- Kb: rate constant of back-biting
- Kte: rate constant of transesterification
2.2. Mechanistic Aspects
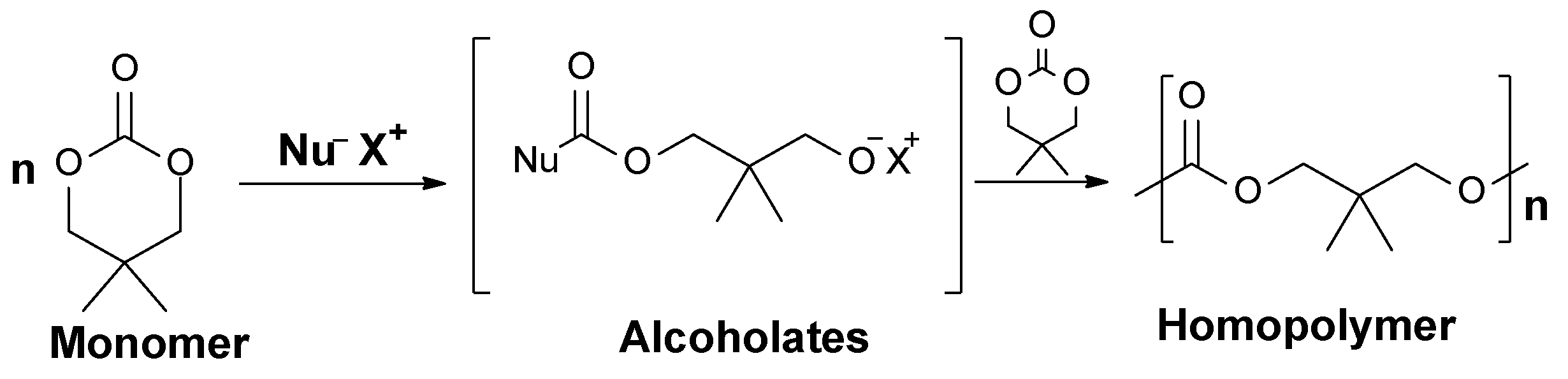
2.2.1. Anionic Polymerization
2.2.2. Coordination-Insertion Mechanism
2.2.3. Cationic Polymerization
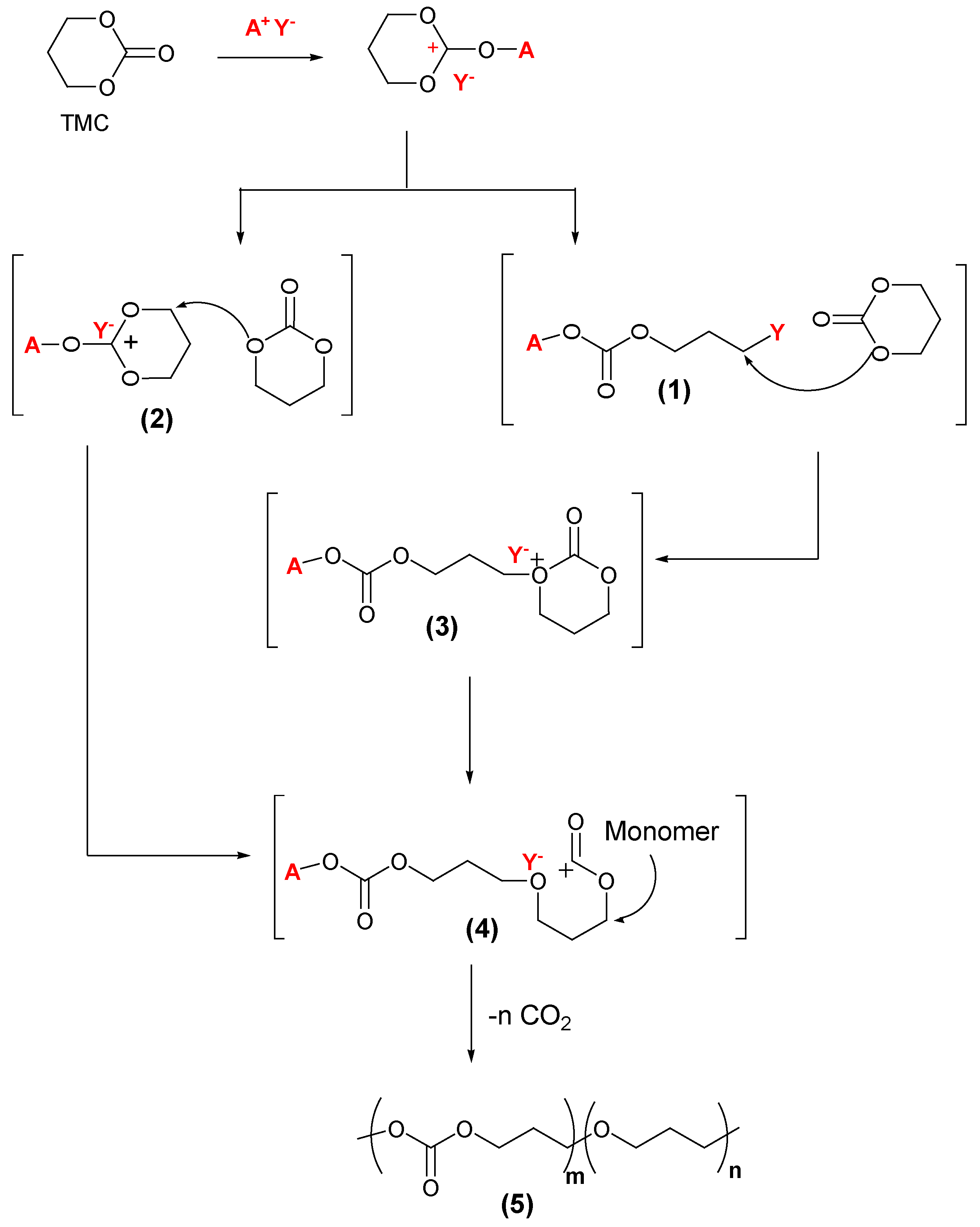
- Use of excess methyl triflate leads to formation of (1) where the counter-ion is covalently bonded to the initiated monomer, a nucleophilic attack on the covalent counter-ion adduct results in (3), and this attack gives an intermediate oxycarbonyl cation (4).
- Formation of (2) via alkylation of the exocyclic oxygen atom of the carbonate linkage, generating a trioxocarbenium ion, which undergoes a nucleophilic attack from another monomer to give adduct (4).
2.2.4. Challenge of Decarboxylation (Back-Biting)
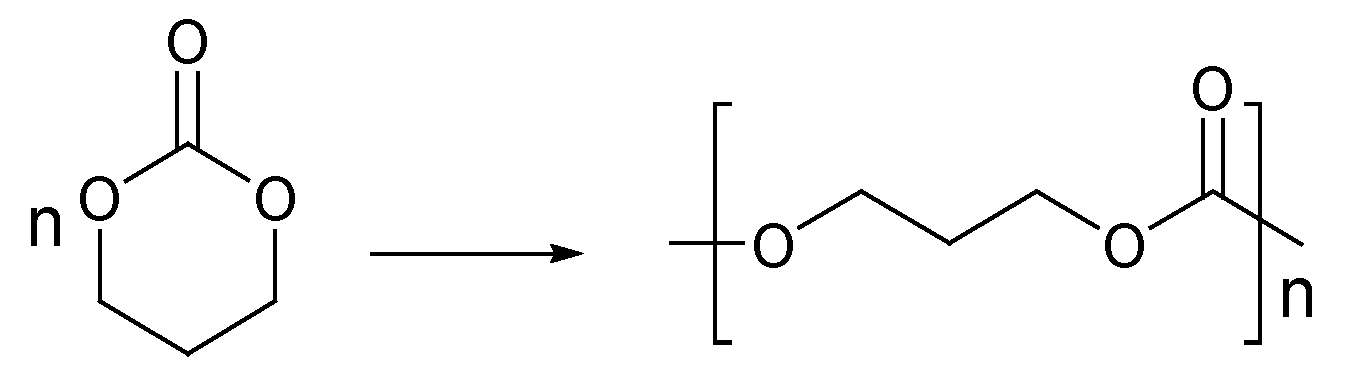
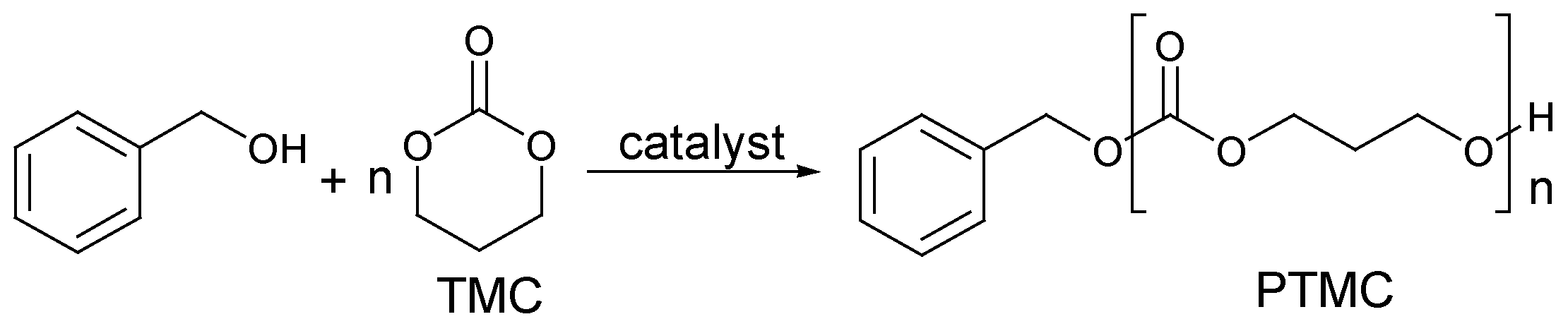
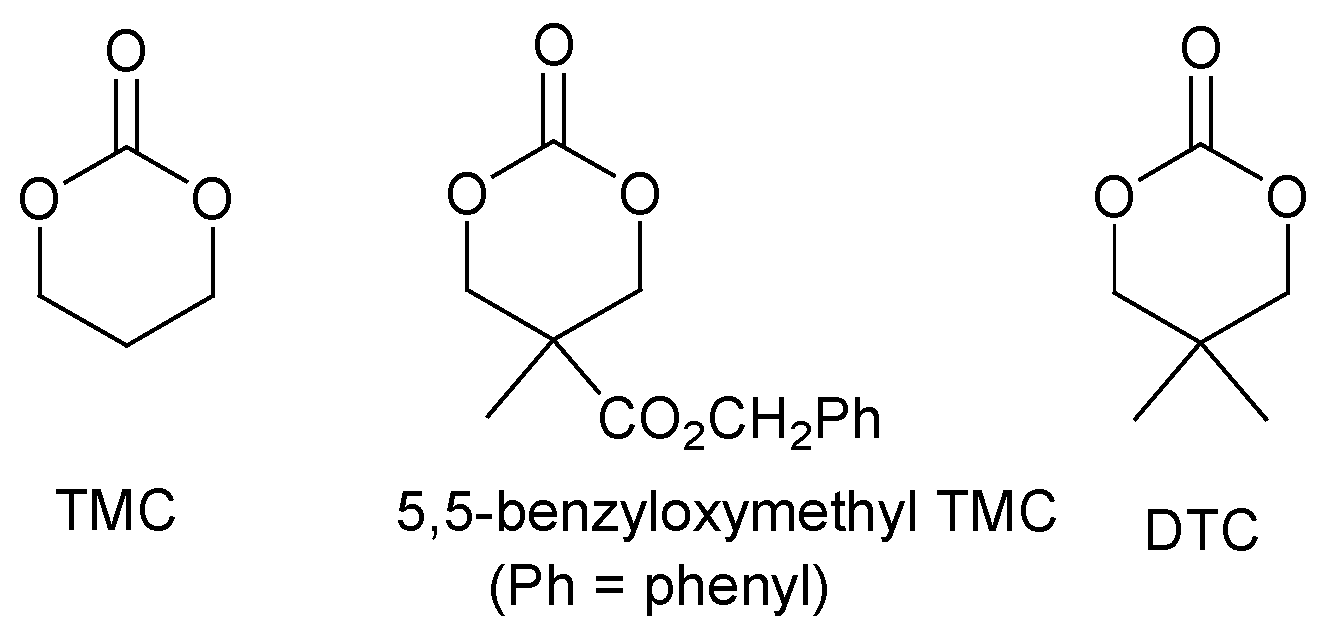
2.3. Cyclic Carbonate Monomers
3. Catalyst-Initiator Systems in ROP of Six-Membered Cyclic Carbonates
3.1. Organometallic Initiating Systems
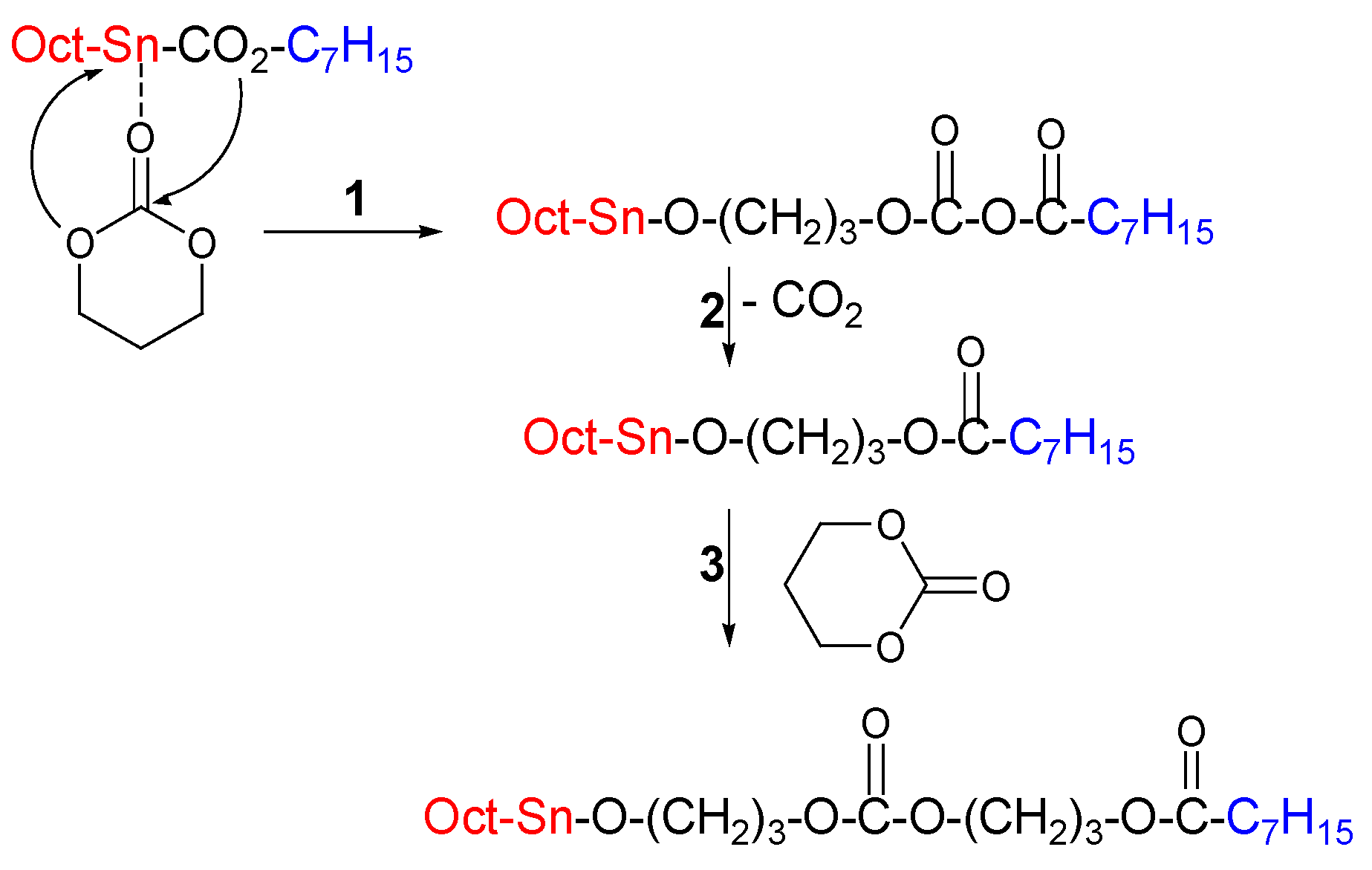
3.1.1. Main Group Metals and Transition Metals
3.1.2. Lanthanides
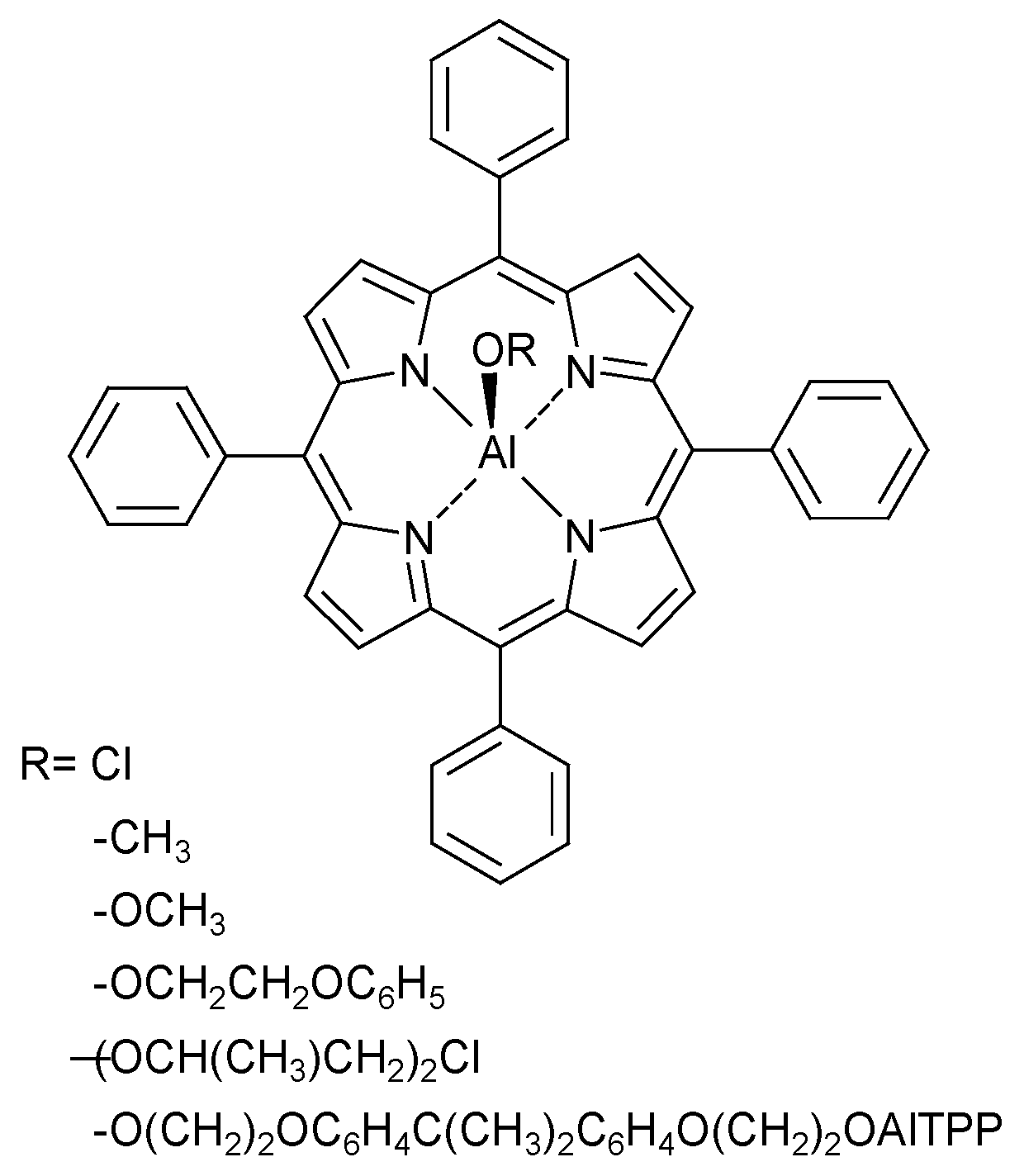
3.1.3. Lewis Acids
3.2. Organic Initiating Systems
3.2.1. Brönsted Bases
3.2.2. Brönsted Acids
3.3. Enzymes
4. Catalyst-Initiator Systems in ROP of Five-Membered Cyclic Carbonates
4.1. Main Group Metals and Transition Metals Initiating System
4.2. Brönsted Bases Initiating Systems
4.3. Dual Initiating Systems-Lewis Base-Lewis Acid
4.4. Glycerol Carbonate RO-Polymerization/Oligomerization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artham, T.; Doble, M. Biodegradation of Aliphatic and Aromatic Polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef]
- Scharfenberg, M.; Hilf, J.; Frey, H. Functional Polycarbonates from Carbon Dioxide and Tailored Epoxide Monomers: Degradable Materials and Their Application Potential. Adv. Funct. Mater. 2018, 28, 1704302. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A Review of Biodegradable Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Dubois, P.; Coulembier, O.; Raquez, J.-M. Handbook of Ring-Opening Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 3-527-31953-0. [Google Scholar]
- Taraghi, I.; Paszkiewicz, S.; Grebowicz, J.; Fereidoon, A.; Roslaniec, Z. Nanocomposites of Polymeric Biomaterials Containing Carbonate Groups: An Overview. Macromol. Mater. Eng. 2017, 302, 1700042. [Google Scholar] [CrossRef]
- Fukushima, K. Poly(Trimethylene Carbonate)-Based Polymers Engineered for Biodegradable Functional Biomaterials. Biomater. Sci. 2016, 4, 9–24. [Google Scholar] [CrossRef]
- Han, Y.; Jin, X.; Yang, J.; Fan, Z.; Lu, Z.; Zhang, Y.; Li, S. Totally Bioresorbable Composites Prepared from Poly(l-Lactide)-Co-(Trimethylene Carbonate) Copolymers and Poly(l-Lactide)-Co-(Glycolide) Fibers as Cardiovascular Stent Material. Polym. Eng. Sci. 2012, 52, 741–750. [Google Scholar] [CrossRef]
- Mohajeri, S.; Amsden, B.G. In Vivo Degradation Mechanism and Biocompatibility of a Biodegradable Aliphatic Polycarbonate: Poly(Trimethylene Carbonate- Co -5-Hydroxy Trimethylene Carbonate). ACS Appl. Bio Mater. 2021, 4, 3686–3696. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Dove, A.P. Synthesis, Properties and Biomedical Applications of Hydrolytically Degradable Materials Based on Aliphatic Polyesters and Polycarbonates. Biomater. Sci. 2017, 5, 9–21. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, F.; Zhou, D.; Mo, J.; Hu, H.; Lin, L.; Wu, J.; Yu, M.; Zhang, M.; Chen, H. Degradation Behaviors of Biodegradable Aliphatic Polyesters and Polycarbonates. J. Biobased Mater. Bioenergy 2020, 14, 155–168. [Google Scholar] [CrossRef]
- Ploypetchara, N.; Suppakul, P.; Atong, D.; Pechyen, C. Blend of Polypropylene/Poly (Lactic Acid) for Medical Packaging Application: Physicochemical, Thermal, Mechanical, and Barrier Properties. Energy Procedia 2014, 56, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. Advanced Drug and Gene Delivery Systems Based on Functional Biodegradable Polycarbonates and Copolymers. J. Controlled Release 2014, 190, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Abts, G.; Eckel, T.; Wehrmann, R. Polycarbonates. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–18. ISBN 978-3-527-30673-2. [Google Scholar]
- Sugiyama, J.; Nagahata, R.; Goyal, M.; Asai, M.; Ueda, M.; Takeuchi, K. Polymerization of Uniform Macrocyclic Carbonate Initiated by Neutral or Weak Basic Salt; American Chemical Society: Washington, DC, USA, 1999; Volume 218, pp. U456–U457. [Google Scholar]
- Nagahata, R.; Sugiyama, J.; Goyal, M.; Asai, M.; Ueda, M.; Takeuchi, K. Synthesis of Ultra High-molecular-weight Polycarbonate. Polym. Adv. Technol. 2000, 11, 727–732. [Google Scholar] [CrossRef]
- Serini, V. Polycarbonates. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Shelnutt, S.; Kind, J.; Allaben, W. Bisphenol A: Update on Newly Developed Data and How They Address NTP’s 2008 Finding of “Some Concern”. Food Chem. Toxicol. 2013, 57, 284–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriano, F.; Coulembier, O.; Hedrick, J.L.; Dubois, P. Functionalized Cyclic Carbonates: From Synthesis and Metal-Free Catalyzed Ring-Opening Polymerization to Applications. Polym. Chem. 2011, 2, 528–533. [Google Scholar] [CrossRef]
- Yokoe, M.; Aoi, K.; Okada, M. Biodegradable Polymers Based on Renewable Resources. VII. Novel Random and Alternating Copolycarbonates from 1, 4: 3, 6-dianhydrohexitols and Aliphatic Diols. J. Polym. Sci. Part Polym. Chem. 2003, 41, 2312–2321. [Google Scholar] [CrossRef]
- Keul, H. Polycarbonates. In Handbook of Ring-Opening Polymerization; Dubois, P., Coulembier, O., Raquez, J.-M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 307–327. ISBN 978-3-527-62840-7. [Google Scholar]
- Hovestadt, W.; Keul, H.; Höcker, H. Tetraphenylporphyrin-Aluminium Compounds as Initiators for the Ring-Opening Polymerization of 2, 2-Dimethyltrimethylene Carbonate: Synthesis of Homopolymers and Copolymers with ε-Caprolactone, Ethylene Oxide and Propylene Oxide. Polymer 1992, 33, 1941–1948. [Google Scholar] [CrossRef]
- Olsén, P.; Odelius, K.; Keul, H.; Albertsson, A.-C. Macromolecular Design via an Organocatalytic, Monomer-Specific and Temperature-Dependent “On/Off Switch”. High Precision Synthesis of Polyester/Polycarbonate Multiblock Copolymers. Macromolecules 2015, 48, 1703–1710. [Google Scholar] [CrossRef]
- Clements, J.H. Reactive Applications of Cyclic Alkylene Carbonates. Ind. Eng. Chem. Res. 2003, 42, 663–674. [Google Scholar] [CrossRef]
- Takata, T.; Sanda, F.; Ariga, T.; Nemoto, H.; Endo, T. Cyclic Carbonates, Novel Expandable Monomers on Polymerization. Macromol. Rapid Commun. 1997, 18, 461–469. [Google Scholar] [CrossRef]
- Keul, H.; Höcker, H. Expected and Unexpected Reactions in Ring-opening (Co) Polymerization. Macromol. Rapid Commun. 2000, 21, 869–883. [Google Scholar] [CrossRef]
- Haba, O.; Tomizuka, H.; Endo, T. Anionic Ring-Opening Polymerization of Methyl 4, 6-O-Benzylidene-2, 3-O-Carbonyl-α-d-Glucopyranoside: A First Example of Anionic Ring-Opening Polymerization of Five-Membered Cyclic Carbonate without Elimination of CO2. Macromolecules 2005, 38, 3562–3563. [Google Scholar] [CrossRef]
- Duda, A.; Kowalski, A.; Libiszowski, J.; Penczek, S. Thermodynamic and Kinetic Polymerizability of Cyclic Esters. Macromol. Symp. 2005, 224, 71–84. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and Mechanism of Cyclic Esters Polymerization Initiated with Tin(II) Octoate. 3. Polymerization of l, l -Dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. On the Difference of Reactivities of Various Aggregated Forms of Aluminium Triisopropoxide in Initiating Ring-Opening Polymerizations. Macromol. Rapid Commun. 1995, 16, 67–76. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Stricker, A. Polymers of Carbonic Acid, 28. SnOct2-Initiated Polymerizations of Trimethylene Carbonate (TMC, 1,3-Dioxanone-2). Macromol. Chem. Phys. 2000, 201, 2557–2565. [Google Scholar] [CrossRef]
- Nemoto, N.; Sanda, F.; Endo, T. Cationic Ring-Opening Polymerization of Six-Membered Cyclic Carbonates with Ester Groups. J. Polym. Sci. Part Polym. Chem. 2001, 39, 1305–1317. [Google Scholar] [CrossRef]
- Azuma, N.; Takata, T.; Sanda, F.; Endo, T. Cationic Ring-Opening Polymerization Behavior of Six-Membered Cyclic Sulfite. Macromolecules 1995, 28, 7331–7334. [Google Scholar] [CrossRef]
- Kameshima, H.; Nemoto, N.; Sanda, F.; Endo, T. Cationic Ring-Opening Polymerization of Five-Membered Cyclic Thiocarbonate Bearing an Adamantane Moiety via Selective Ring-Opening Direction. Macromolecules 2002, 35, 5769–5773. [Google Scholar] [CrossRef]
- Rokicki, G. Aliphatic Cyclic Carbonates and Spiroorthocarbonates as Monomers. Prog. Polym. Sci. 2000, 25, 259–342. [Google Scholar] [CrossRef]
- Ariga, T.; Takata, T.; Endo, T. Cationic Ring-Opening Polymerization of Cyclic Carbonates with Alkyl Halides to Yield Polycarbonate without the Ether Unit by Suppression of Elimination of Carbon Dioxide. Macromolecules 1997, 30, 737–744. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weegen-Schulz, B.; Jenssen, J. Cationic Polymerization of Aliphatic Cyclocarbonates. Macromol. Symp. 1998, 132, 421–430. [Google Scholar] [CrossRef]
- Lee, J.-C.; Litt, M.H. Ring-Opening Polymerization of Ethylene Carbonate and Depolymerization of Poly(Ethylene Oxide-Co-Ethylene Carbonate). Macromolecules 2000, 33, 1618–1627. [Google Scholar] [CrossRef]
- Endo, T.; Sanda, F. Novel Ring-Opening Polymerization and Its Application to Polymeric Materials. Macromol. Symp. 2000, 159, 1–8. [Google Scholar] [CrossRef]
- Carothers, W.H.; Natta, F.J.V. Studies on Polymerization and Ring Formation. III Glycol Esters of Carbonic Acid. J. Am. Chem. Soc. 1930, 52, 314–326. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Choi, W.; Ganguly, P.; Richers, C.P. Biometal Derivatives as Catalysts for the Ring-Opening Polymerization of Trimethylene Carbonate. Optimization of the Ca(II) Salen Catalyst System. Macromolecules 2006, 39, 4374–4379. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhuo, R.-X.; Huang, S.-W.; Liu, L.-J.; He, F. Synthesis, Characterization and In Vitro Cytotoxicity of Poly[(5-Benzyloxy-Trimethylene Carbonate)-Co-(Trimethylene Carbonate)]. Macromol. Chem. Phys. 2002, 203, 985–990. [Google Scholar] [CrossRef]
- Mei, H.; Zhong, Z.; Long, F.; Zhuo, R. Synthesis and Characterization of Novel Glycerol-Derived Polycarbonates with Pendant Hydroxyl Groups. Macromol. Rapid Commun. 2006, 27, 1894–1899. [Google Scholar] [CrossRef]
- Stridsberg, K.M.; Ryner, M.; Albertsson, A.-C. Controlled Ring-Opening Polymerization: Polymers with Designed Macromolecular Architecture. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany; pp. 41–65.
- Chen, W.; Yang, H.; Wang, R.; Cheng, R.; Meng, F.; Wei, W.; Zhong, Z. Versatile Synthesis of Functional Biodegradable Polymers by Combining Ring-Opening Polymerization and Postpolymerization Modification via Michael-Type Addition Reaction. Macromolecules 2010, 43, 201–207. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Ganguly, P.; Billodeaux, D. Ring-Opening Polymerization of Trimethylene Carbonate Using Aluminum(III) and Tin(IV) Salen Chloride Catalysts. Macromolecules 2005, 38, 5406–5410. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Ganguly, P.; Choi, W. Metal Salen Derivatives as Catalysts for the Alternating Copolymerization of Oxetanes and Carbon Dioxide to Afford Polycarbonates. Inorg. Chem. 2006, 45, 3831–3833. [Google Scholar] [CrossRef]
- Helou, M.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S.M. Highly Effective and Green Catalytic Approach Toward α, ω-Dihydroxy-Telechelic Poly(Trimethylenecarbonate). Macromol. Rapid Commun. 2009, 30, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, P.; Pastusiak, M.; Bero, M. Less Toxic Acetylacetonates as Initiators of Trimethylene Carbonate and 2,2-Dimethyltrimethylene Carbonate Ring Opening Polymerization. J. Polym. Sci. Part Polym. Chem. 2005, 43, 1913–1922. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Kasperczyk, J. Synthesis of Biodegradable Copolymers with Low-Toxicity Zirconium Compounds. V. Multiblock and Random Copolymers OfL-Lactide with Trimethylene Carbonate Obtained in Copolymerizations Initiated with Zirconium(IV) Acetylacetonate. J. Polym. Sci. Part Polym. Chem. 2006, 44, 3184–3201. [Google Scholar] [CrossRef]
- Brignou, P.; Guillaume, S.M.; Roisnel, T.; Bourissou, D.; Carpentier, J.-F. Discrete Cationic Zinc and Magnesium Complexes for Dual Organic/Organometallic-Catalyzed Ring-Opening Polymerization of Trimethylene Carbonate. Chem.-Eur. J. 2012, 18, 9360–9370. [Google Scholar] [CrossRef]
- Sheng, H.; Xu, F.; Yao, Y.; Zhang, Y.; Shen, Q. Novel Mixed-Metal Alkoxide Clusters of Lanthanide and Sodium: Synthesis and Extremely Active Catalysts for the Polymerization of ε-Caprolactone and Trimethylene Carbonate. Inorg. Chem. 2007, 46, 7722–7724. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zhou, L.; Zhang, Y.; Yao, Y.; Shen, Q. Anionic Lanthanide Phenoxide Complexes as Novel Single-Component Initiators for the Polymerization of ε-Caprolactone and Trimethylene Carbonate. J. Polym. Sci. Part Polym. Chem. 2007, 45, 1210–1218. [Google Scholar] [CrossRef]
- Yasuda, H.; Aludin, M.-S.; Kitamura, N.; Tanabe, M.; Sirahama, H. Syntheses and Physical Properties of Novel Optically Active Poly(Ester−carbonate)s by Copolymerization of Substituted Trimethylene Carbonate with ε-Caprolactone and Their Biodegradation Behavior. Macromolecules 1999, 32, 6047–6057. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, H.; Chen, J.; Yao, Y.; Shen, Q. Homoleptic Lanthanide Guanidinate Complexes: The Effective Initiators for the Polymerization of Trimethylene Carbonate and Its Copolymerization with?—Caprolactone. J. Polym. Sci. Part Polym. Chem. 2005, 43, 1778–1786. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yasuda, H.; Yamamoto, K.; Tsutsumi, C.; Jerome, R.; Lecomte, P. Comparison of Sm Complexes with Sn Compounds for Syntheses of Copolymers Composed of Lactide and Cyclic Carbonates and Their Biodegradabilities. React. Funct. Polym. 2005, 63, 95–105. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Zhou, L.; Sun, H.; Shen, Q. Homoleptic Lanthanide Amidinate Complexes: A Single-Component Initiator for Ring-Opening Polymerization of Trimethylene Carbonate and Copolymerization with ε-Caprolactone. J. Appl. Polym. Sci. 2006, 102, 22–28. [Google Scholar] [CrossRef]
- Zhao, B.; Lu, C.R.; Shen, Q. Ring-Opening Polymerization of Trimethylenecarbonate and Its Copolymerization with ε-Caprolactone by Lanthanide (II) Aryloxide Complexes. J. Appl. Polym. Sci. 2007, 106, 1383–1389. [Google Scholar] [CrossRef]
- Palard, I.; Schappacher, M.; Belloncle, B.; Soum, A.; Guillaume, S.M. Unprecedented Polymerization of Trimethylene Carbonate Initiated by a Samarium Borohydride Complex: Mechanistic Insights and Copolymerization with ɛ-Caprolactone. Chem.-Eur. J. 2007, 13, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cui, D. Polymerization of 2,2′-Dimethyltrimethylene Carbonate by Lutetium Complexes Bearing Amino-Phosphine Ligands. J. Appl. Polym. Sci. 2009, 112, 3110–3118. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Shen, L.; Zhang, T. Ring-Opening Copolymerization of 2,2-Dimethyltrimethylene Carbonate and ε-Caprolactone Using Rare Earth Aryloxides Substituted by Various Alkyl Groups. Chin. J. Chem. 2010, 28, 1019–1026. [Google Scholar] [CrossRef]
- Xu, N.; Liu, X.; Liu, Y.; Zhu, W.; Chen, F.; Shen, Z. Synthesis and Properties of Random Copolymers of 2,2-Dimethyltrimethylene Carbonate and Ethylene Carbonate Catalyzed by Lanthanide Tris(2,6-Di- Tert -Butyl-4-Methylphenolate). J. Appl. Polym. Sci. 2010, 115, 46–51. [Google Scholar] [CrossRef]
- Nomura, N.; Taira, A.; Tomioka, T.; Okada, M. A Catalytic Approach for Cationic Living Polymerization: Sc(OTf) 3 -Catalyzed Ring-Opening Polymerization of Lactones. Macromolecules 2000, 33, 1497–1499. [Google Scholar] [CrossRef]
- Kunioka, M.; Wang, Y.; Onozawa, S. Poly(Lactic Acid) Polymerized by Aluminum Triflate. Macromol. Symp. 2005, 224, 167–180. [Google Scholar] [CrossRef]
- Gorczynski, J.L.; Chen, J.; Fraser, C.L. Iron Tris(Dibenzoylmethane)-Centered Polylactide Stars: Multiple Roles for the Metal Complex in Lactide Ring-Opening Polymerization. J. Am. Chem. Soc. 2005, 127, 14956–14957. [Google Scholar] [CrossRef]
- Nomura, N.; Taira, A.; Nakase, A.; Tomioka, T.; Okada, M. Ring-Opening Polymerization of Lactones by Rare-Earth Metal Triflates and by Their Reusable System in Ionic Liquids. Tetrahedron 2007, 63, 8478–8484. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Yao, C.; Sheng, H.; Shen, Q. Homo- and Copolymerization of 2,2-Dimethyltrimethylene Carbonate Promoted by Samarium Thiolate Derivatives: Novel and Versatile Initiators. J. Polym. Sci. Part Polym. Chem. 2005, 43, 1312–1316. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, L.; Shen, Z. Random Copolymerization of 2,2-Dimethyltri Methylene Carbonate Andɛ-Caprolactone Using a Rare-Earth Tris(4-Tert-Butylphenolate)s as a Single-Component Initiator. Polym. Int. 2004, 53, 1485–1490. [Google Scholar] [CrossRef]
- Guillaume, S.M.; Carpentier, J.-F. Recent Advances in Metallo/Organo-Catalyzed Immortal Ring-Opening Polymerization of Cyclic Carbonates. Catal. Sci. Technol. 2012, 2, 898. [Google Scholar] [CrossRef]
- Möller, M.; Kånge, R.; Hedrick, J.L. Sn (OTf) 2 and Sc (OTf) 3: Efficient and Versatile Catalysts for the Controlled Polymerization of Lactones. J. Polym. Sci. Part Polym. Chem. 2000, 38, 2067–2074. [Google Scholar] [CrossRef]
- Cervellera, R.; Ramis, X.; Salla, J.M.; Mantecón, A.; Serra, A. N,N-Dimethylaminopyridine as Initiator in the Copolymerization of Diglycidylether of Bisphenol A with Six-Membered Cyclic Carbonates. J. Polym. Sci. Part Polym. Chem. 2006, 44, 2873–2882. [Google Scholar] [CrossRef]
- Helou, M.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S.M. Organocatalysts for the Controlled “Immortal” Ring-Opening Polymerization of Six-Membered-Ring Cyclic Carbonates: A Metal-Free, Green Process. Chem.-Eur. J. 2010, 16, 13805–13813. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, H.; Ochiai, B.; Endo, T. Synthesis and Properties of Star-Shaped Polymers by the Ring-Opening Polymerization of Cyclic Carbonate Initiated with a Trifunctional, Poly(Ethylene Glycol)-Based Surfactant. J. Polym. Sci. Part Polym. Chem. 2006, 44, 6633–6639. [Google Scholar] [CrossRef]
- Morinaga, H.; Ochiai, B.; Mori, H.; Endo, T. Synthesis and Characterization of Block Copolymers by Metal- and Solvent-Free Ring-Opening Polymerization of Cyclic Carbonates Initiated from PEG-Based Surfactants. J. Polym. Sci. Part Polym. Chem. 2006, 44, 1985–1996. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Liu, L. Ring Opening Polymerization of Aliphatic Cyclic Carbonates in the Presence of Natural Amino Acids. J. Appl. Polym. Sci. 2008, 107, 3275–3279. [Google Scholar] [CrossRef]
- Nederberg, F.; Lohmeijer, B.G.G.; Leibfarth, F.; Pratt, R.C.; Choi, J.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic Ring Opening Polymerization of Trimethylene Carbonate. Biomacromolecules 2007, 8, 153–160. [Google Scholar] [CrossRef]
- Mindemark, J.; Hilborn, J.; Bowden, T. End-Group-Catalyzed Ring-Opening Polymerization of Trimethylene Carbonate. Macromolecules 2007, 40, 3515–3517. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Bagrov, V.; Lozhkin, B.; Zakirova, G.; Ivchenko, P.; Legon’kova, O. Theoretical and Experimental Studies of 1,5,7-Triazabicyclo[4.4.0]Dec-5-Ene-Catalyzed Ring Opening/Ring Closure Reaction Mechanism for 5-, 6- and 7-Membered Cyclic Esters and Carbonates. React. Kinet. Mech. Catal. 2016, 117, 447–476. [Google Scholar] [CrossRef]
- Naumann, S.; Thomas, A.W.; Dove, A.P. Highly Polarized Alkenes as Organocatalysts for the Polymerization of Lactones and Trimethylene Carbonate. ACS Macro Lett. 2016, 5, 134–138. [Google Scholar] [CrossRef]
- Matsuo, J.; Nakano, S.; Sanda, F.; Endo, T. Ring-Opening Polymerization of Cyclic Carbonates by Alcohol-Acid Catalyst. J. Polym. Sci. Part Polym. Chem. 1998, 36, 2463–2471. [Google Scholar] [CrossRef]
- Makiguchi, K.; Ogasawara, Y.; Kikuchi, S.; Satoh, T.; Kakuchi, T. Diphenyl Phosphate as an Efficient Acidic Organocatalyst for Controlled/Living Ring-Opening Polymerization of Trimethylene Carbonates Leading to Block, End-Functionalized, and Macrocyclic Polycarbonates. Macromolecules 2013, 46, 1772–1782. [Google Scholar] [CrossRef]
- He, X.; Ji, Y.; Jin, Y.; Kan, S.; Xia, H.; Chen, J.; Liang, B.; Wu, H.; Guo, K.; Li, Z. Bifunctional Imidodiphosphoric Acid-Catalyzed Controlled/Living Ring-Opening Polymerization of Trimethylene Carbonate Resulting Block, α,ω-Dihydroxy Telechelic, and Star-Shaped Polycarbonates. J. Polym. Sci. Part Polym. Chem. 2014, 52, 1009–1019. [Google Scholar] [CrossRef]
- Delcroix, D.; Martín-Vaca, B.; Bourissou, D.; Navarro, C. Ring-Opening Polymerization of Trimethylene Carbonate Catalyzed by Methanesulfonic Acid: Activated Monomer versus Active Chain End Mechanisms. Macromolecules 2010, 43, 8828–8835. [Google Scholar] [CrossRef]
- Kobayashi, S.; Makino, A. Enzymatic Polymer Synthesis: An Opportunity for Green Polymer Chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kaihara, S.; Toshima, K.; Matsumura, S. High-Molecular-Weight Polycarbonates Synthesized by Enzymatic ROP of a Cyclic Carbonate as a Green Process. Macromol. Biosci. 2009, 9, 968–978. [Google Scholar] [CrossRef]
- Tasaki, H.; Toshima, K.; Matsumura, S. Enzymatic Synthesis and Polymerization of Cyclic Trimethylene Carbonate Monomer with/without Methyl Substituent. Macromol. Biosci. 2003, 3, 436–441. [Google Scholar] [CrossRef]
- Namekawa, S.; Uyama, H.; Kobayashi, S.; Kricheldorf, H.R. Lipase-Catalyzed Ring-Opening Polymerization and Copolymerization of Cyclic Dicarbonates. Macromol. Chem. Phys. 2000, 201, 261–264. [Google Scholar] [CrossRef]
- Yu, X.-H.; Zhuo, R.-X.; Feng, J.; Liao, J. Enzymatic Ring-Opening Polymerization of 2,2-Dimethyltrimethylene Carbonate Catalyzed by PPL Immobilized on Silica Nanoparticles. Eur. Polym. J. 2004, 40, 2445–2450. [Google Scholar] [CrossRef]
- Vogdanis, L.; Martens, B.; Uchtmann, H.; Hensel, F.; Heitz, W. Synthetic and Thermodynamic Investigations in the Polymerization of Ethylene Carbonate. Makromol. Chem. 1990, 191, 465–472. [Google Scholar] [CrossRef]
- Vogdanis, L.; Heitz, W. Carbon Dioxide as a Monomer, 3. The Polymerization of Ethylene Carbonate. Makromol. Chem. Rapid Commun. 1986, 7, 543–547. [Google Scholar] [CrossRef]
- Kéki, S.; Török, J.; Deák, G.; Zsuga, M. Ring-Opening Oligomerization of Propylene Carbonate Initiated by the Bisphenol A/KHCO 3 System: A Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Study of the Oligomers Formed. Macromolecules 2001, 34, 6850–6857. [Google Scholar] [CrossRef]
- Soós, L.; Deák, G.; Kéki, S.; Zsuga, M. Anionic Bulk Oligomerization of Ethylene and Propylene Carbonate Initiated by Bisphenol-A/Base Systems. J. Polym. Sci. Part Polym. Chem. 1999, 37, 545–550. [Google Scholar] [CrossRef]
- Wu, M.; Guo, J.; Jing, H. Organic Base Catalyzed Oligomerization of Propylene Carbonate and Bisphenol A: Unexpected Polyether Diol Formation. Catal. Commun. 2008, 9, 120–125. [Google Scholar] [CrossRef]
- Kéki, S.; Török, J.; Deák, G.; Zsuga, M. Mechanism of Ring-Opening and Elimination Cooligomerization of Cyclic Carbonates and ε-Caprolactone: Formation of Cyclic Cooligomers. Eur. Polym. J. 2005, 41, 1478–1483. [Google Scholar] [CrossRef]
- Von Seggern, N.; Schindler, T.; Naumann, S. Dual Catalytic Ring-Opening Polymerization of Ethylene Carbonate for the Preparation of Degradable PEG. Biomacromolecules 2020, 21, 2661–2669. [Google Scholar] [CrossRef]
- Rokicki, G.; Rakoczy, P.; Parzuchowski, P.; Sobiecki, M. Hyperbranched Aliphatic Polyethers Obtained from Environmentally Benign Monomer: Glycerol Carbonate. Green Chem. 2005, 7, 529–539. [Google Scholar] [CrossRef]
- Mouloungui, Z.; Marechal, P.; Troung Dinh, N. Glycerol Polycarbonate, Organic Compositions Containing Same and Method for Obtaining Said Compositions. U.S. Patent US7928182B2, 26 February 2009. [Google Scholar]
- Claude, S.; Mouloungui, Z.; Yoo, J.-W.; Gaset, A. Method for Preparing Glycerol Carbonate. U.S. Patent US6025504A, 15 February 2000. [Google Scholar]
- Hino, T.; Inoue, N.; Endo, T. Detailed Study of the Ring-Opening Metathesis Polymerization of Norbornene Bearing a Five- or Six-Membered Ring Cyclic Carbonate along with Volume Expansion. J. Polym. Sci. Part Polym. Chem. 2006, 44, 395–405. [Google Scholar] [CrossRef]
- Sugimoto, H.; Ohshima, H.; Inoue, S. Alternating Copolymerization of Carbon Dioxide and Epoxide by Manganese Porphyrin: The First Example of Polycarbonate Synthesis from 1-Atm Carbon Dioxide. J. Polym. Sci. Part Polym. Chem. 2003, 41, 3549–3555. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. One-Pot Non-Isocyanate Synthesis of Polyurethanes from Bisepoxide, Carbon Dioxide, and Diamine. J. Polym. Sci. Part Polym. Chem. 2005, 43, 6613–6618. [Google Scholar] [CrossRef]







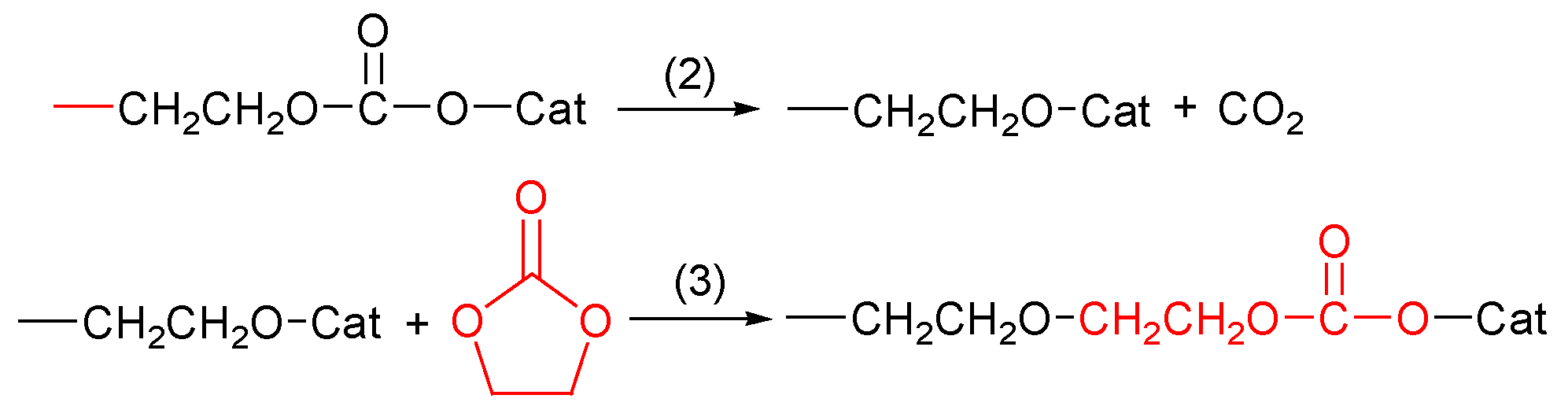

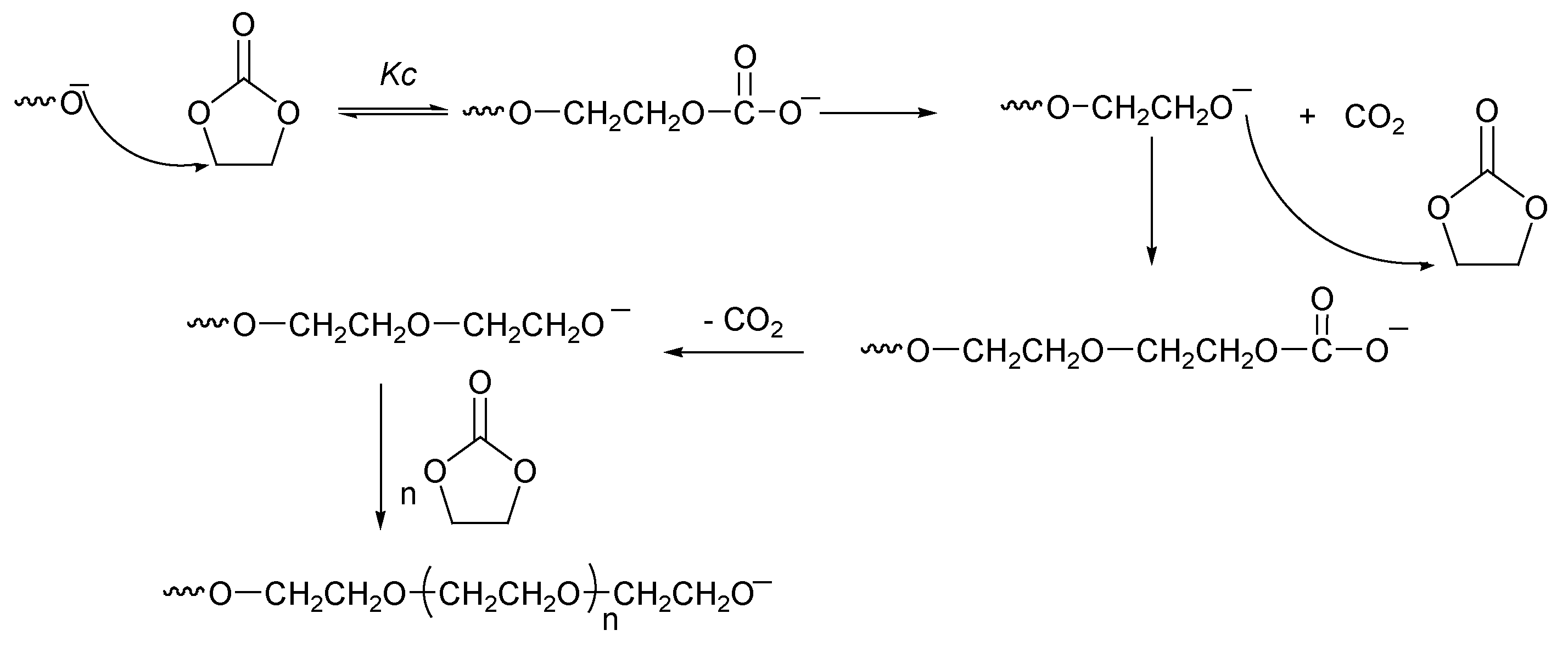
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Baki, Z.; Dib, H.; Sahin, T. Overview: Polycarbonates via Ring-Opening Polymerization, Differences between Six- and Five-Membered Cyclic Carbonates: Inspiration for Green Alternatives. Polymers 2022, 14, 2031. https://doi.org/10.3390/polym14102031
Abdel Baki Z, Dib H, Sahin T. Overview: Polycarbonates via Ring-Opening Polymerization, Differences between Six- and Five-Membered Cyclic Carbonates: Inspiration for Green Alternatives. Polymers. 2022; 14(10):2031. https://doi.org/10.3390/polym14102031
Chicago/Turabian StyleAbdel Baki, Zaher, Hanna Dib, and Tuba Sahin. 2022. "Overview: Polycarbonates via Ring-Opening Polymerization, Differences between Six- and Five-Membered Cyclic Carbonates: Inspiration for Green Alternatives" Polymers 14, no. 10: 2031. https://doi.org/10.3390/polym14102031
APA StyleAbdel Baki, Z., Dib, H., & Sahin, T. (2022). Overview: Polycarbonates via Ring-Opening Polymerization, Differences between Six- and Five-Membered Cyclic Carbonates: Inspiration for Green Alternatives. Polymers, 14(10), 2031. https://doi.org/10.3390/polym14102031





