Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair
Abstract
:1. Introduction
2. Materials and Methods
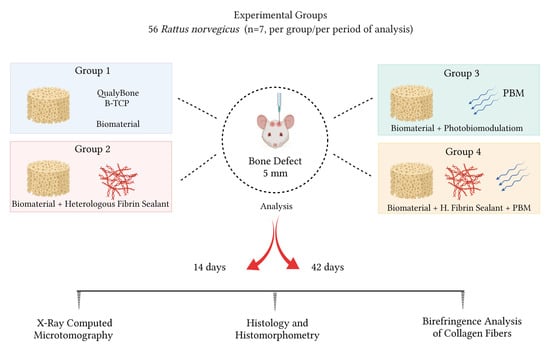
2.1. Experimental Design
2.2. Sample Characterization–Biomaterial (BCP)
2.3. Heterologous Fibrin Biopolymer (FB)
2.4. Experimental Surgery
2.5. Photobiomodulation Protocol (PBM)
2.6. Euthanasia and X-ray Computed Microtomography (µ-CT)
2.7. Sample Collection and Histological Procedure
2.8. Birefringence Analysis of Collagen Fibers (Picrosirius-Red Staining)
2.9. Histomorphometric Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Sample Characterization
3.2. Qualitative Analysis of Two-Dimensional Microtomographic Images
3.3. Histomorphological Analysis
3.4. Description Birefringence Analysis of Collagen Fibers
3.5. Histomorphometric Analysis
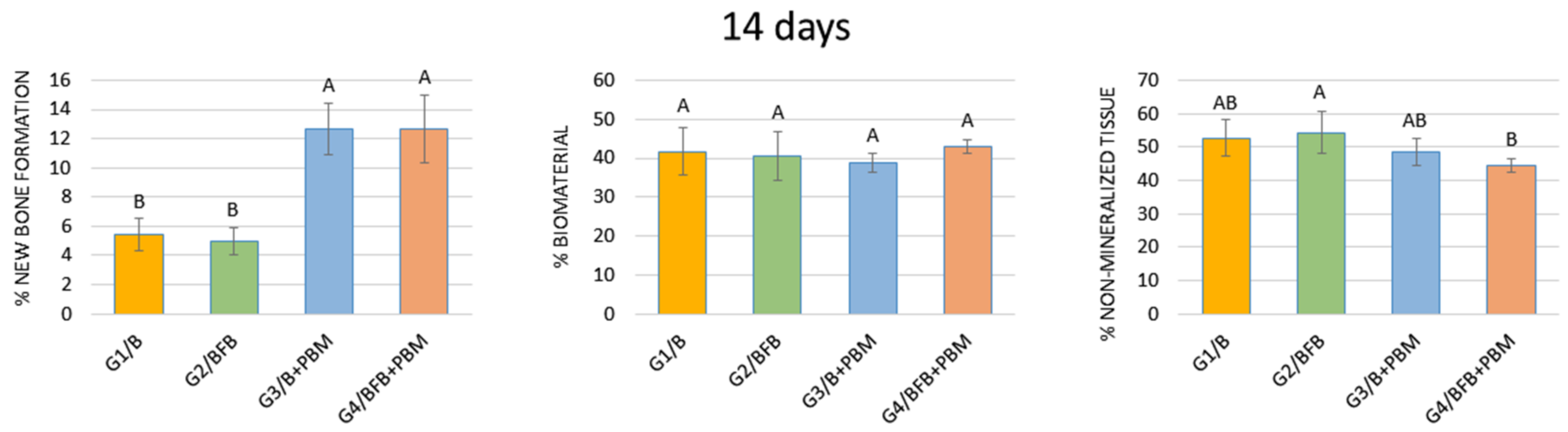
3.5.1. 14 Days
3.5.2. 42 Days
3.5.3. Comparison of Groups in the Two Trial Periods (14 vs. 42 Days)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Moraes, R.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; Duarte, M.A.H.; Alcalde, M.P.; Buchaim, R.L.; Pomini, K.T.; Machado, E.G.; Munhoz, M.A.E.S.; Cunha, F.B.; et al. Suitability of the use of an elastin matrix combined with bone morphogenetic protein for the repair of cranial defects. Am. J. Transl. Res. 2019, 11, 5261–5271. [Google Scholar] [PubMed]
- Munhoz, M.; Pomini, K.T.; Plepis, A.M.G.; Martins, V.; Machado, E.G.; de Moraes, R.; Cunha, F.B.; Santos, A.R., Jr.; Cardoso, G.B.C.; Duarte, M.A.R.; et al. Elastin-derived scaffolding associated or not with bone morphogenetic protein (BMP) or hydroxyapatite (HA) in the repair process of metaphyseal bone defects. PLoS ONE 2020, 15, e0231112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Neel, E.A.; Chrzanowski, W.; Salih, V.M.; Kim, H.W.; Knowles, J.C. Tissue engineering in dentistry. J. Dent. 2014, 42, 915–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.S.B.M.; Roque, D.D.; Rosso, M.P.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Jones, E.; Einhorn, T.A. Fracture healing and bone repair. Injury 2011, 42, 549–550. [Google Scholar] [CrossRef]
- Rai, R.; Raval, R.; Khandeparker, R.V.; Chidrawar, S.K.; Khan, A.A.; Ganpat, M.S. Tissue Engineering: Step Ahead in Maxillofacial Reconstruction. J. Int. Oral Health 2015, 7, 138–142. [Google Scholar]
- Trevisiol, C.H.; Turner, R.T.; Pfaff, J.E.; Hunter, J.C.; Menagh, P.J.; Hardin, K.; Ho, E.; Iwaniec, U.T. Impaired osteoinduction in a rat model for chronic alcohol abuse. Bone 2007, 41, 175–180. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Rodrigues, A.C.; Buchaim, D.V.; Dias, D.V.; Daré, L.R.; Roque, D.D.; Roque, J.S. The action of demineralized bovine bone matrix on bone neoformation in rats submitted to experimental alcoholism. Arq. Bras. Med. Vet. Zootec. 2013, 63, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Cunha, F.B.; Pomini, K.T.; Plepis, A.M.G.; Martins, V.; Machado, E.G.; de Moraes, R.; Munhoz, M.A.E.S.; Machado, M.V.R.; Duarte, M.A.H.; Alcalde, M.P.; et al. In Vivo Biological Behavior of Polymer Scaffolds of Natural Origin in the Bone Repair Process. Molecules 2021, 26, 1598. [Google Scholar] [CrossRef]
- Da Silva Brum, I.; de Carvalho, J.J.; da Silva Pires, J.L.; de Carvalho, M.A.A.; Dos Santos, L.B.F.; Elias, C.N. Nanosized hydroxyapatite and β-tricalcium phosphate composite: Physico-chemical, cytotoxicity, morphological properties and in vivo trial. Sci. Rep. 2019, 9, 19602. [Google Scholar] [CrossRef] [PubMed]
- Zima, A.; Czechowska, J.; Szponder, T.; Ślósarczyk, A. In vivo behavior of biomicroconcretes based on α-tricalcium phosphate and hybrid hydroxyapatite/chitosan granules and sodium alginate. J. Biomed. Mater. Res. A 2020, 108, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Brum, I.; Frigo, L.; Lana Devita, R.; da Silva Pires, J.L.; Hugo Vieira de Oliveira, V.; Rosa Nascimento, A.L.; de Carvalho, J.J. Histomorphometric, Immunohistochemical, Ultrastructural Characterization of a Nano-Hydroxyapatite/Beta-Tricalcium Phosphate Composite and a Bone Xenograft in Sub-Critical Size Bone Defect in Rat Calvaria. Materials 2020, 13, 4598. [Google Scholar] [CrossRef]
- Trindade, H.F.; Batista, C.A.; Farsoun, A.; Farsoun, J.; Trindade, M.L.C. Injerto de materiales biológicos: Injertos óseos sintéticos frente a xenoinjertos I en la elevación del seno maxilar. Quintessence 2018, 6, 54–58. [Google Scholar]
- Shi, W.; Que, Y.; Zhang, X.; Bian, L.; Yu, X.; Tang, X.; Yang, G.; Dai, Y.; Bi, S.; Lv, D.; et al. Functional tissue-engineered bone-like graft made of a fibrin scaffold and TG2 gene-modified EMSCs for bone defect repair. NPG Asia Mater. 2021, 13, 28. [Google Scholar] [CrossRef]
- Abbade, L.P.F.; Barraviera, S.; Silvares, M.R.C.; Lima, A.; Haddad, G.R.; Gatti, M.A.N.; Medolago, N.B.; Carneiro, M.T.R.; dos Santos, L.D.; Ferreira, R.S., Jr.; et al. Treatment of Chronic Venous Ulcers with Heterologous Fibrin Sealant: A Phase I/II Clinical Trial. Front. Immunol. 2021, 12, 627541. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.; Coletta, B.B.D.; Pomini, K.T.; Rosso, M.P.O.; Campos, L.M.G.; Ferreira, R.S., Jr.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190038. [Google Scholar] [CrossRef]
- Machado, E.G.; Issa, J.P.; Figueiredo, F.A.; Santos, G.R.; Galdeano, E.A.; Alves, M.C.; Chacon, E.L.; Ferreira, R.S., Jr.; Barraviera, B.; da Cunha, M.R. A new heterologous fibrin sealant as scaffold to recombinant human bone morphogenetic protein-2 (rhBMP-2) and natural latex proteins for the repair of tibial bone defects. Acta Histochem. 2015, 117, 288–296. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.O.; della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Rosa, G.M.; Shindo, J.V.T.C.; et al. Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Boo, J.S.; Ozawa, R.; Nagasaka, T.; Okazaki, Y.; Hata, K.; Ueda, M. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J. Craniomaxillofac. Surg. 2003, 31, 27–33. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Fideles, S.O.M.; Pomini, K.T.; Reis, C.H.B.; Bueno, C.R.S.; Pereira, E.; Rossi, J.O.; Novais, P.C.; Pilon, J.P.G.; Rosa, G.M., Jr.; et al. Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells 2021, 10, 2323. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005, 70, 247–299. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.C.; Ferreira, R.S., Jr.; Barraviera, S.R.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.; Toscano, E.; Barraviera, B. A new fibrin sealant from Crotalus durissus terrificus venom: Applications in medicine. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 553–571. [Google Scholar] [CrossRef]
- Gatti, M.A.N.; Vieira, L.M.; Barraviera, B.; Barraviera, S.R.C.S. Treatment of venous ulcers with fibrin sealant derived from snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 226–229. [Google Scholar] [CrossRef]
- Gasparotto, V.P.; Landim-Alvarenga, F.C.; Oliveira, A.L.; Simões, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A., Jr.; Kaneno, R.; de Assis Golim, M.; Dos Santos, D.C.; Creste, C.F.Z.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Venante, H.S.; Chappuis-Chocano, A.P.; Marcillo-Toala, O.O.; da Silva, R.A.; da Costa, R.M.B.; Pordeus, M.D.; Barraviera, B.; Ferreira, R.S., Jr.; Lara, V.S.; Neppelenbroek, K.H.; et al. Fibrin Biopolymer Incorporated with Antimicrobial Agents: A Proposal for Coating Denture Bases. Materials 2021, 14, 1618. [Google Scholar] [CrossRef]
- De Oliveira, C.T.B.; Leonel, B.C.; de Oliveira, A.C.; de Brito Paiva, M.; Ramos, J.; Barraviera, B.; Ferreira, R.S., Jr.; Shimano, A.C. Effects of fibrin sealant and bone fragments on defect regeneration performed on rat tibiae: An experimental study. J. Mech. Behav. Biomed. Mater. 2020, 104, 103662. [Google Scholar] [CrossRef]
- Iatecola, A.; Barraviera, B.; Ferreira, R.S., Jr.; dos Santos, G.R.; Neves, J.I.; da Cunha, M.R. Use of a new fibrin sealant and laser irradiation in the repair of skull defects in rats. Braz. Dent. J. 2013, 24, 456–461. [Google Scholar] [CrossRef]
- Rahal, S.C.; Amaral, M.S.P.; Pai, V.D.; Barraviera, S.R.C.S.; Caporali, E.H.G.; Crocci, A.J. Effect of fibrin glue derived from snake venom on the viability of autogenous split-thickness skin graft. J. Venom. Anim. Toxins Incl. Trop. Dis. 2004, 10, 161–172. [Google Scholar] [CrossRef]
- Bayat, M.; Virdi, A.; Jalalifirouzkouhi, R.; Rezaei, F. Comparison of effects of LLLT and LIPUS on fracture healing in animal models and patients: A systematic review. Progr. Biophys. Mol. Biol. 2018, 132, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Dalvi, S.; Amaroli, A.; de Angelis, N.; Benedicenti, S. Effects of photobiomodulation on bone defects grafted with bone substitutes: A systematic review of in vivo animal studies. J. Biophotonics 2021, 14, e202000267. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Magri, A.M.P.; Parisi, J.R.; de Andrade, A.L.M.; Rennó, A.C.M. Bone substitutes and photobiomodulation in bone regeneration: A systematic review in animal experimental studies. J. Biomed. Mater. Res. A 2021, 109, 1765–1775. [Google Scholar] [CrossRef]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 nm Diode Laser Therapy at Higher Fluence on the in Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Escudero, J.S.B.; Perez, M.G.B.; de Oliveira Rosso, M.P.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef]
- Gerbi, M.; Miranda, J.M.; Arruda, J.A.A.; Moreno, L.M.M.; Carneiro, V.S.M.; Brasilino, N.C.; Menezes, R.F.; Brugnera, A., Jr.; Pinheiro, A.L.B. Photobiomodulation Therapy in Bone Repair Associated with Bone Morphogenetic Proteins and Guided Bone Regeneration: A Histomorphometric Study. Photomed. Laser Surg. 2018, 36, 581–588. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Ferreira, R.S., Jr.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.; Silvares, M.R.C.; de Pontes, L.G.; dos Santos, L.D.; Barraviera, B. Heterologous fibrin sealant derived from snake venom: From bench to bedside—An overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ressler, A.; Antunović, M.; Teruel-Biosca, L.; Ferrer, G.G.; Babić, S.; Urlić, I.; Ivanković, M.; Ivanković, H. Osteogenic differentiation of human mesenchymal stem cells on substituted calcium phosphate/chitosan composite scaffold. Carbohydr. Polym. 2022, 277, 118883. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Andreo, J.C.; Pomini, K.T.; Barraviera, B.; Ferreira, R.S.; Duarte, M.A.H.; Alcalde, M.P.; Reis, C.H.B.; Teixeira, D.B.; Bueno, C.R.S.; et al. A biocomplex to repair experimental critical size defects associated with photobiomodulation therapy. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210056. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Ferreira, R.S., Jr.; Andreo, J.C.; Rodrigues, A.C.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B 2016, 162, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, A.C.; Torquato, L.C.; Gonçalves, P.R.; Ribeiro, T.C.; Nunes, C.M.; Bernardo, D.V.; Gomes, M.S.; Jardini, M.A.N.; Santamaria, M.P. The Effect of Photobiomodulation Therapy in Different Doses on Bone Repair of Critical Size Defects in Rats: A Histomorphometric Study. J. Lasers Med. Sci. 2021, 12, e53. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.A.P.; Santos, T.D.; Cunha, J.L.S.; Matos, F.S.; Albuquerque, R.L.C., Jr.; Ribeiro, M.A.G. Effect of laser photobiomodulation associated with a bioceramic cement on the repair of bone tissue in the femur of rats. J. Photochem. Photobiol. B 2020, 205, 111813. [Google Scholar] [CrossRef]
- De Oliveira, D.; de Oliveira Puttini, I.; Silva Gomes-Ferreira, P.H.; Palin, L.P.; Matsumoto, M.A.; Okamoto, R. Effect of intermittent teriparatide (PTH 1-34) on the alveolar healing process in orchiectomized rats. Clin. Oral Investig. 2019, 23, 2313–2322. [Google Scholar] [CrossRef]
- Oliveira, D.; Hassumi, J.S.; Gomes-Ferreira, P.H.; Polo, T.O.; Ferreira, G.R.; Faverani, L.P.; Okamoto, R. Short term sodium alendronate administration improves the peri-implant bone quality in osteoporotic animals. J. Appl. Oral Sci. 2017, 25, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Ramalho-Ferreira, G.; Faverani, L.P.; Momesso, G.A.C.; Luvizuto, E.R.; de Oliveira Puttini, I.; Okamoto, R. Effect of antiresorptive drugs in the alveolar bone healing. A histometric and immunohistochemical study in ovariectomized rats. Clin. Oral Investig. 2017, 21, 1485–1494. [Google Scholar] [CrossRef]
- Garcia, V.J.; Arnabat, J.; Comesaña, R.; Kasem, K.; Ustrell, J.M.; Pasetto, S.; Segura, O.P.; ManzanaresCéspedes, M.C.; Carvalho-Lobato, P. Effect of low-level laser therapy after rapid maxillary expansion: A clinical investigation. Lasers Med. Sci. 2016, 31, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Puttini, I.; Gomes-Ferreira, P.H.D.S.; de Oliveira, D.; Hassumi, J.S.; Gonçalves, P.Z.; Okamoto, R. Teriparatide improves alveolar bone modelling after tooth extraction in orchiectomized rats. Arch. Oral Biol. 2019, 102, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, K.B.; do Egito Vasconcelos, B.C.; de Assis Limeira, F., Jr.; de Sousa, F.B.; de Souza Andrade, E.S.; de Holanda Vasconcellos, R.J. Histomorphometric evaluation of calcium phosphate bone grafts on bone repair. Braz. J. Otorhinolaryngol. 2011, 77, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petronis, S.; Jakusonoka, R.; Linovs, V.; Jumtins, A. Filling Bone Defects after Hip Arthroplasty Revision Using Hydroxyapatite/β-tricalcium Phosphate: A Case Report with Long-term Result. J. Orthop. Case Rep. 2021, 11, 5–9. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M. Biphasic calcium phosphates (BCP) of hydroxyapatite (HA) and tricalcium phosphate (TCP) as bone substitutes: Importance of physicochemical characterizations in biomaterials studies. Data Brief 2016, 10, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Seifert, A.; Tylek, T.; Blum, C.; Hemmelmann, N.; Böttcher, B.; Gbureck, U.; Groll, J. Calcium phosphate-based biomaterials trigger human macrophages to release extracellular traps. Biomaterials 2022, 285, 121521. [Google Scholar] [CrossRef]
- MacMillan, A.K.; Lamberti, F.V.; Moulton, J.N.; Geilich, B.M.; Webster, T.J. Similar healthy osteoclast and osteoblast activity on nanocrystalline hydroxyapatite and nanoparticles of tri-calcium phosphate compared to natural bone. Int. J. Nanomed. 2014, 9, 5627–5637. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, D.M.B.; Figadoli, A.L.F.; Alcantara, P.L.; Pomini, K.T.; Santos German, I.J.; Reis, C.H.B.; Rosa, G.M., Jr.; Rosso, M.P.O.; da Silva Santos, P.S.; Zangrando, M.S.R.; et al. Biological Behavior of Xenogenic Scaffolds in Alcohol-Induced Rats: Histomorphometric and Picrosirius Red Staining Analysis. Polymers 2022, 14, 584. [Google Scholar] [CrossRef]
- Pinheiro, A.L.; Martinez Gerbi, M.E.; de Assis Limeira, F., Jr.; Carneiro Ponzi, E.A.; Marques, A.M.; Carvalho, C.M.; Santos, R.C.; Oliveira, P.C.; Nóia, M.; Ramalho, L.M.P. Bone repair following bone grafting hydroxyapatite guided bone regeneration and infra-red laser photobiomodulation: A histological study in a rodent model. Lasers Med. Sci. 2009, 24, 234–240. [Google Scholar] [CrossRef]
- Torquato, L.C.; Suárez, E.A.C.; Bernardo, D.V.; Pinto, I.L.R.; Mantovani, L.O.; Silva, T.I.L.; Jardini, M.A.N.; Santamaria, M.P.; de Marco, A.C. Bone repair assessment of critical size defects in rats treated with mineralized bovine bone (Bio-Oss®) and photobiomodulation therapy: A histomorphometric and immunohistochemical study. Lasers Med. Sci. 2021, 36, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Amid, R.; Kadkhodazadeh, M.; Ahsaie, M.G.; Hakakzadeh, A. Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J. Lasers Med. Sci. 2014, 5, 163–170. [Google Scholar] [PubMed]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonasson, T.H.; Zancan, R.; de Oliveira Azevedo, L.; Fonseca, A.C.; Silva, M.C.D.; Giovanini, A.F.; Zielac, J.C.; de Araújo, M.R. Effects of low-level laser therapy and platelet concentrate on bone repair: Histological, histomorphometric, immunohistochemical, and radiographic study. J. Craniomaxillofac. Surg. 2017, 45, 1846–1853. [Google Scholar] [CrossRef]
- Cunha, M.R.; Menezes, F.A.; dos Santos, G.R.; Pinto, C.L.A.; Barraviera, B.; Martins, V.C.A.; Plepis, A.M.G.; Ferreira, R.S., Jr. Hydroxyapatite and a New Fibrin Sealant Derived from Snake Venom as Scaffold to Treatment of Cranial Defects in Rats. Mater. Res. 2015, 18, 196–203. [Google Scholar] [CrossRef] [Green Version]
- De Miranda, J.R.; Choi, I.G.G.; Moreira, M.S.; Martins, M.D.; Cortes, A.R.G.; Yoshimoto, M. Histologic Evaluation of Early Bone Regeneration Treated with Simvastatin Associated with Low-Level Laser Therapy. J. Int. Oral Maxillofac. Implant. 2019, 34, 658–664. [Google Scholar] [CrossRef]
- AlGhamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Guarnieri, R.; Belleggia, F.; DeVillier, P.; Testarelli, L. Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after 24 Months of Healing. A Case Report. J. Funct. Biomater. 2018, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.d.C. Biocompatibility of anionic collagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- Spejo, A.B.; Chiarotto, G.B.; Ferreira, A.D.F.; Gomes, D.A.; Ferreira, R.S., Jr.; Barraviera, B.; Oliveira, A.L.R. Neuroprotection and immunomodulation following intraspinal axotomy of motoneurons by treatment with adult mesenchymal stem cells. J. Neuroinflamm. 2018, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Creste, C.F.Z.; Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A.; Golim, M.d.A.; Barraviera, B.; Ferreira, R.S., Jr. Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration. Materials 2020, 13, 2747. [Google Scholar] [CrossRef] [PubMed]











| Parameter | Unit/Description |
|---|---|
| Type of laser | GaAlAs |
| Output power | 30 mW |
| Wavelength | 830 nm |
| Power density | 258.6 mW/cm² |
| Energy density | 6.2 J/cm² |
| Beam area | 0.116 cm² |
| Total power | 2.9 J |
| Beam type | Positioned perpendicular to the skull |
| Emission mode | Continuous |
| Form of application | Four points around the surgical area |
| Irradiation duration | 24 s per point |
| Total time of each application | 96 s |
| Treatment time | Immediately after surgery and three times a week until euthanasia. |
| Groups | G1/B | G2/BFB | G3/B + PBM | G4/BFB + PBM |
|---|---|---|---|---|
| 14 days | 5.42 ± 1.12 Bb | 5.00 ± 0.94 Bb | 12.65 ± 1.78 Ba | 12.65 ± 2.32 Ba |
| 42 days | 21.49 ± 4.74 Ab | 21.77 ± 2.83 Ab | 29.29 ± 2.93 Aa | 31.38 ± 2.89 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, C.H.B.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.L.; et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers 2022, 14, 2075. https://doi.org/10.3390/polym14102075
Reis CHB, Buchaim RL, Pomini KT, Hamzé AL, Zattiti IV, Duarte MAH, Alcalde MP, Barraviera B, Ferreira Júnior RS, Pontes FML, et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers. 2022; 14(10):2075. https://doi.org/10.3390/polym14102075
Chicago/Turabian StyleReis, Carlos Henrique Bertoni, Rogerio Leone Buchaim, Karina Torres Pomini, Abdul Latif Hamzé, Isabella Vasconcelos Zattiti, Marco Antonio Hungaro Duarte, Murilo Priori Alcalde, Benedito Barraviera, Rui Seabra Ferreira Júnior, Fenelon Martinho Lima Pontes, and et al. 2022. "Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair" Polymers 14, no. 10: 2075. https://doi.org/10.3390/polym14102075
APA StyleReis, C. H. B., Buchaim, R. L., Pomini, K. T., Hamzé, A. L., Zattiti, I. V., Duarte, M. A. H., Alcalde, M. P., Barraviera, B., Ferreira Júnior, R. S., Pontes, F. M. L., Grandini, C. R., Ortiz, A. d. C., Fideles, S. O. M., Eugênio, R. M. d. C., Rosa Junior, G. M., Teixeira, D. d. B., Pereira, E. d. S. B. M., Pilon, J. P. G., Miglino, M. A., & Buchaim, D. V. (2022). Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers, 14(10), 2075. https://doi.org/10.3390/polym14102075