Space Charge Behavior of Thermally Aged Polyethylene Insulation of Track Cables
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
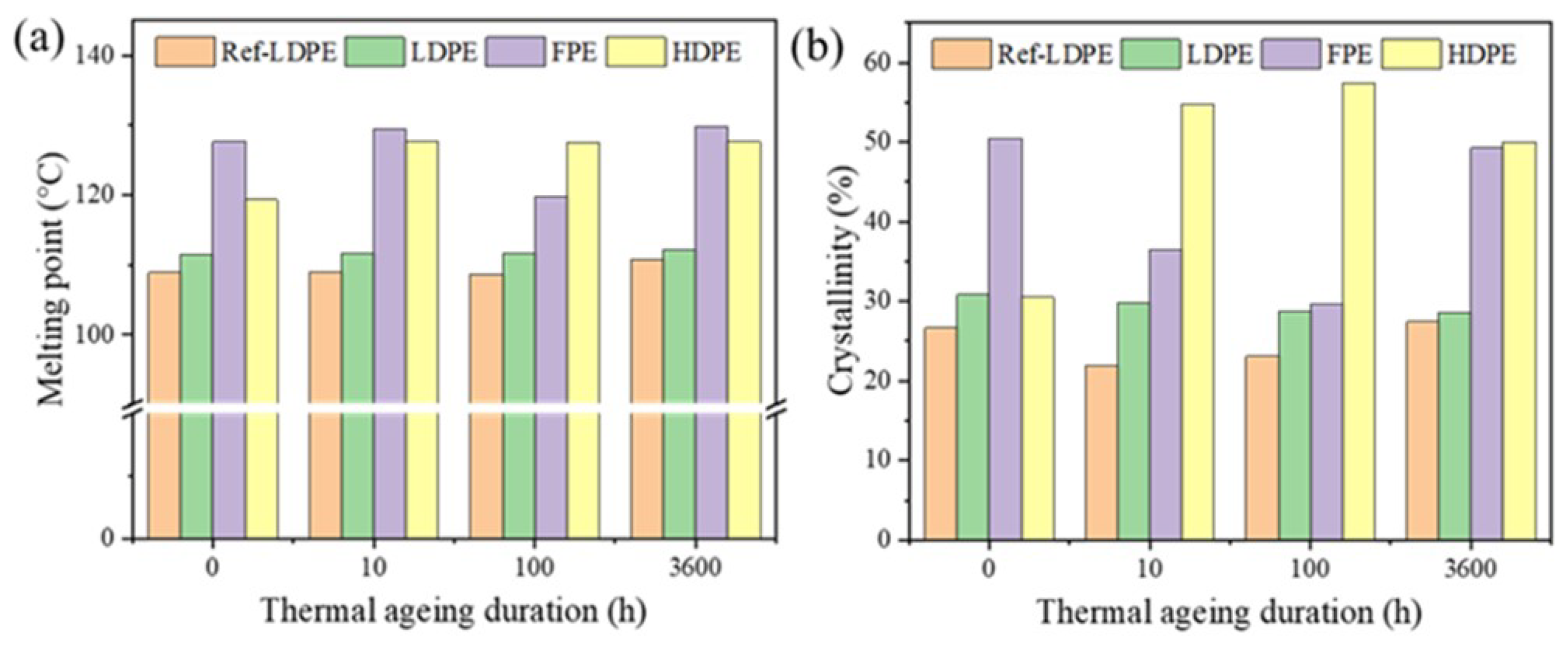
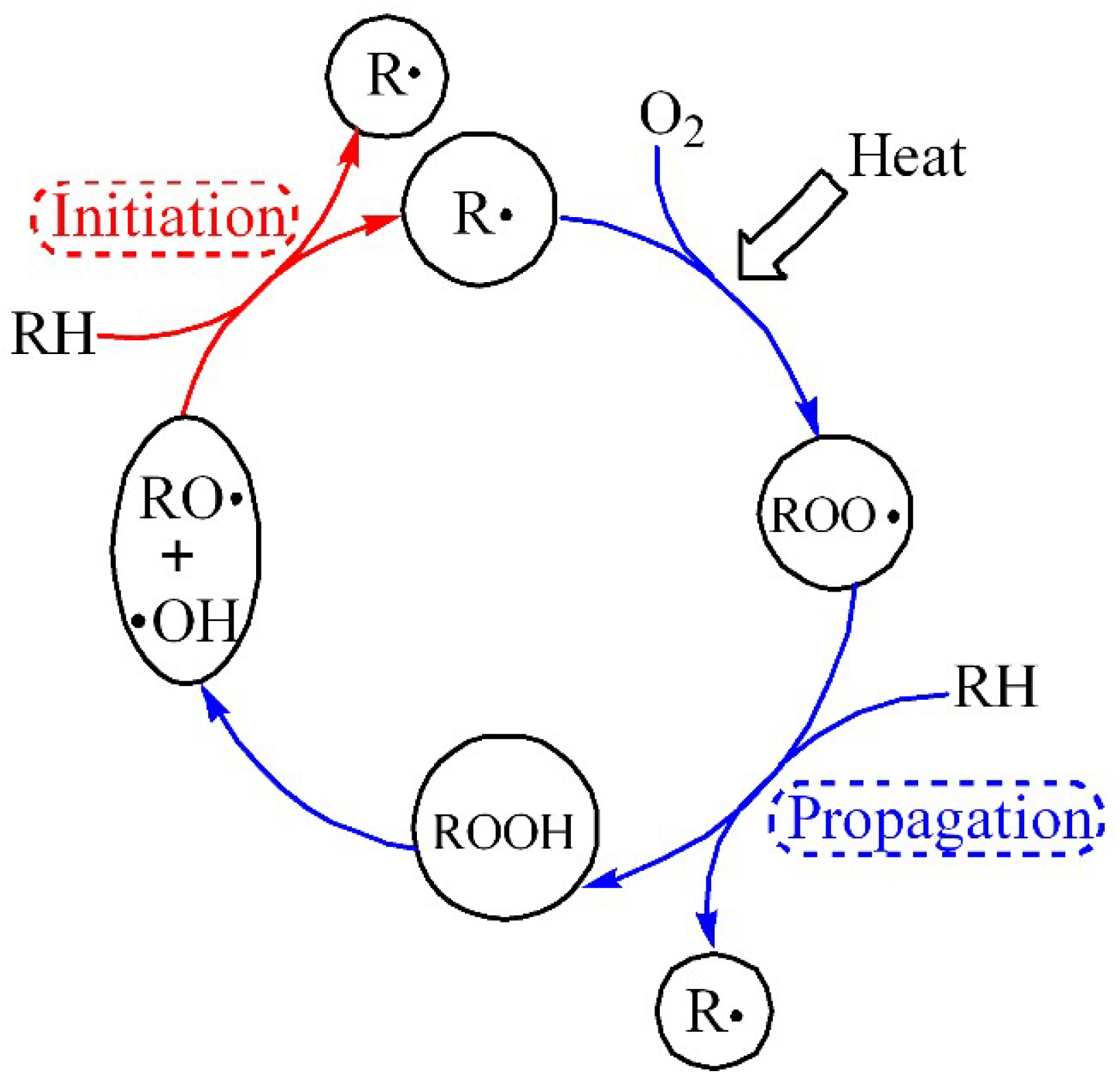
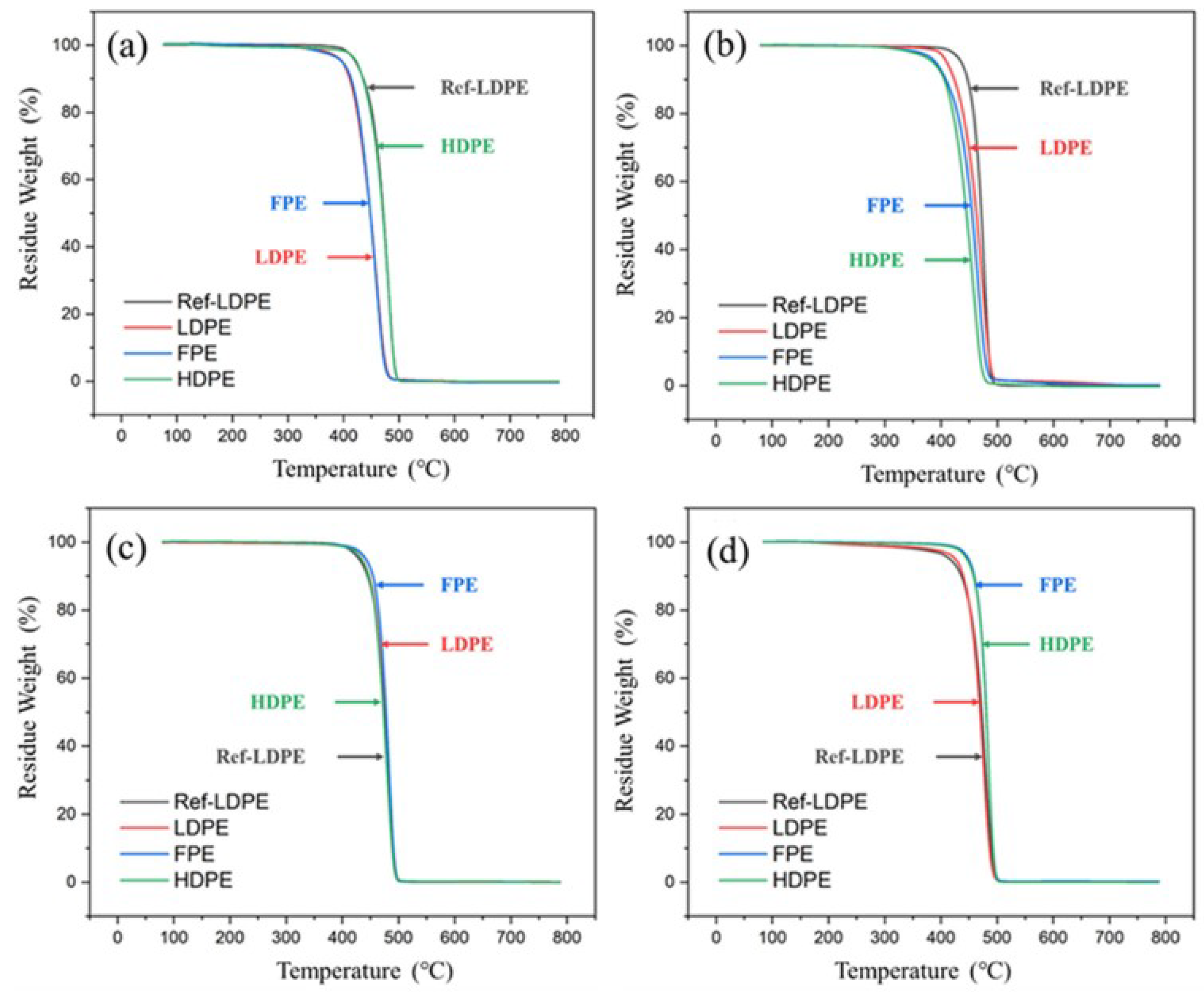
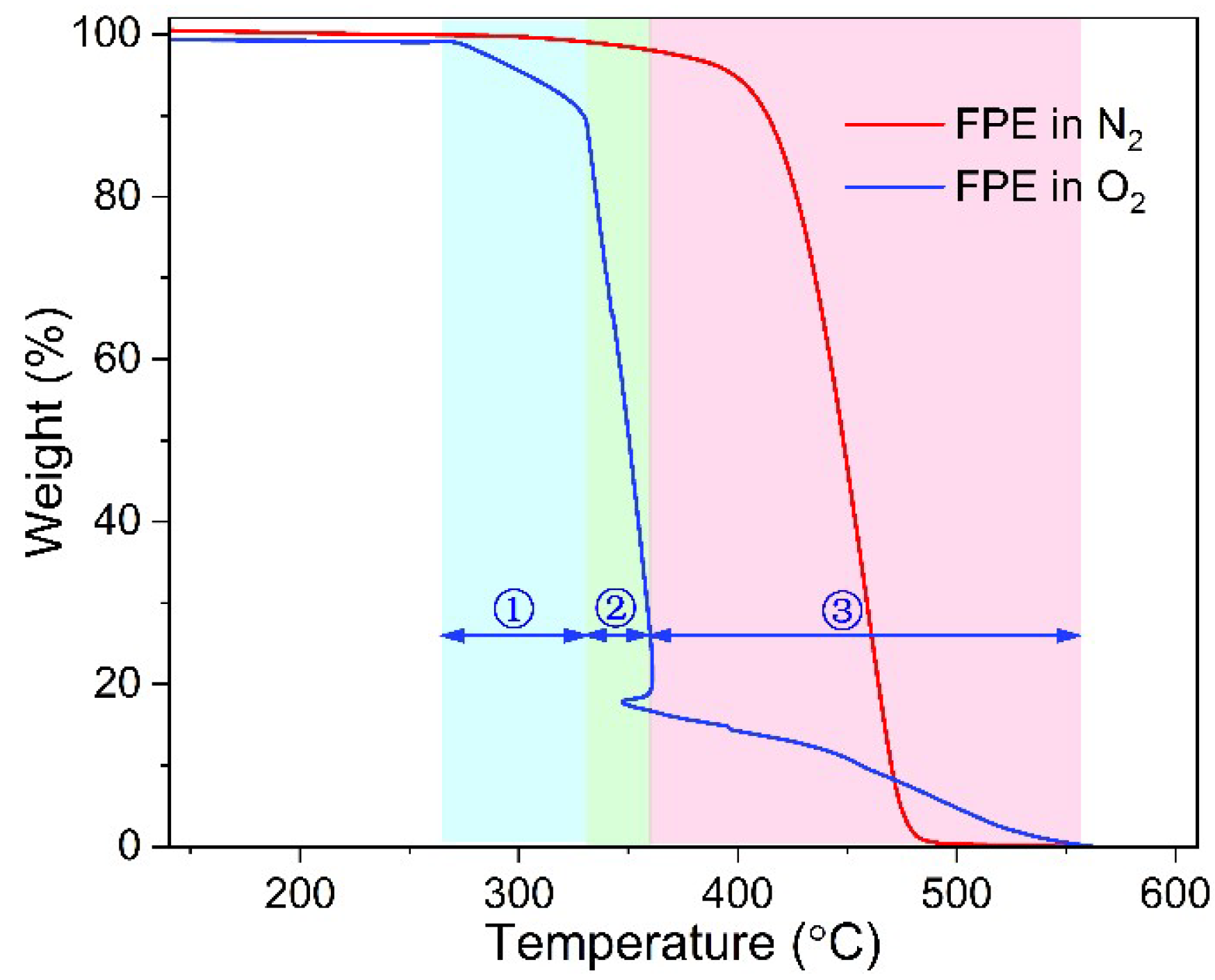
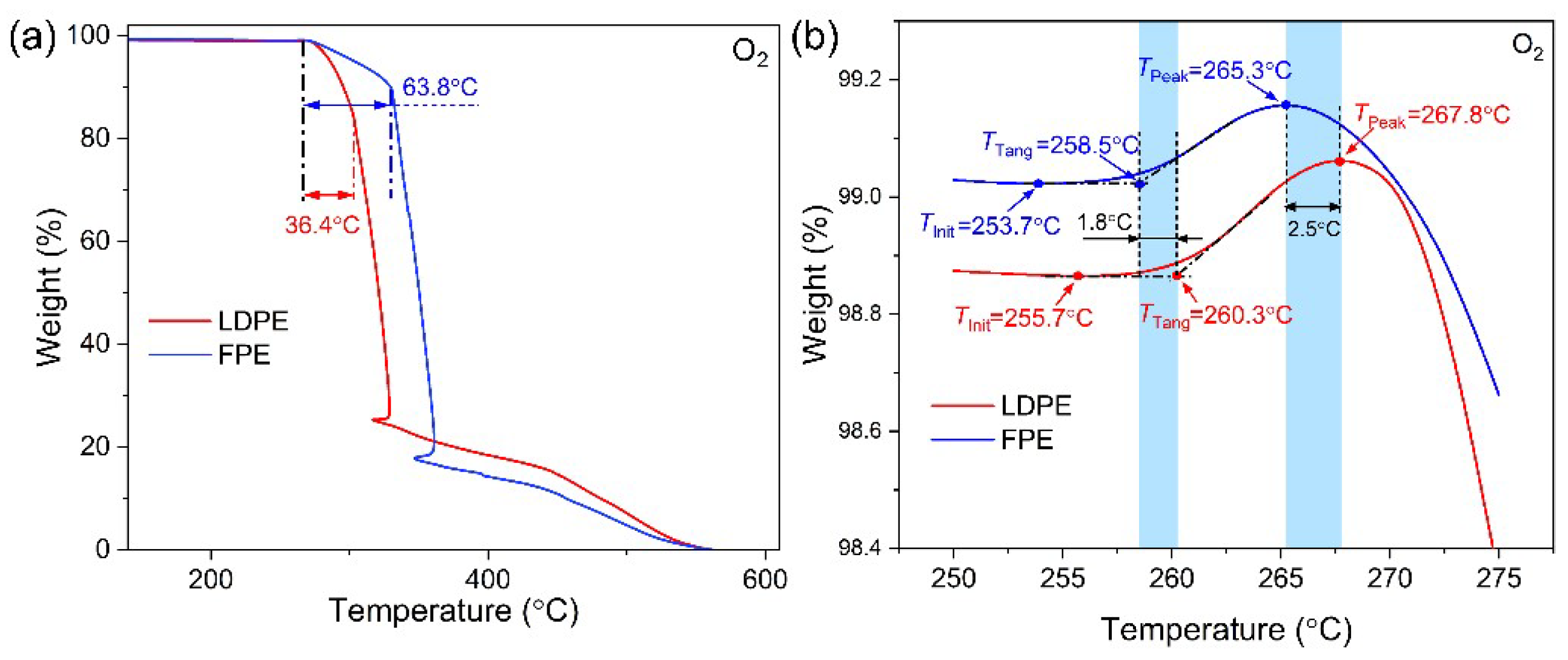
3.1. Thermal Property
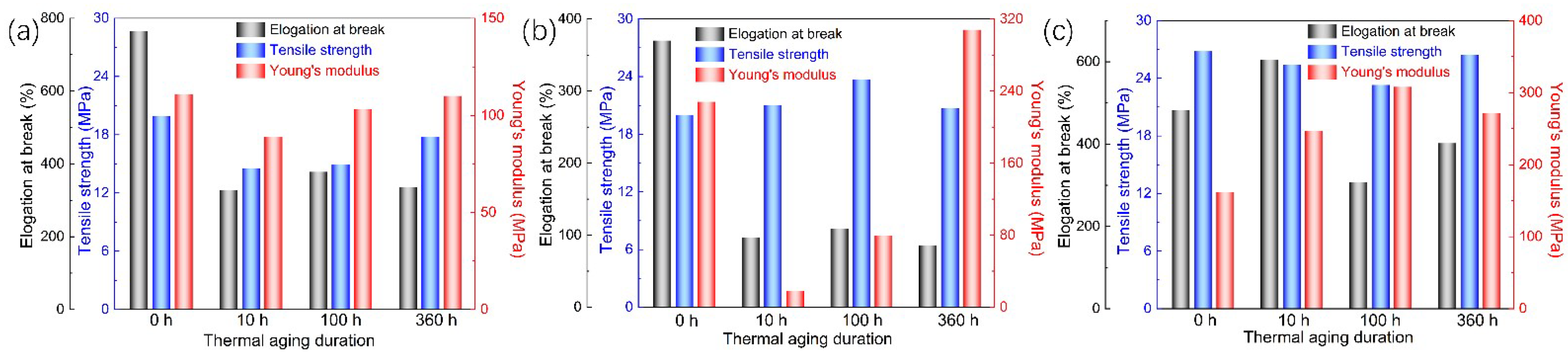
3.2. Mechanical Property
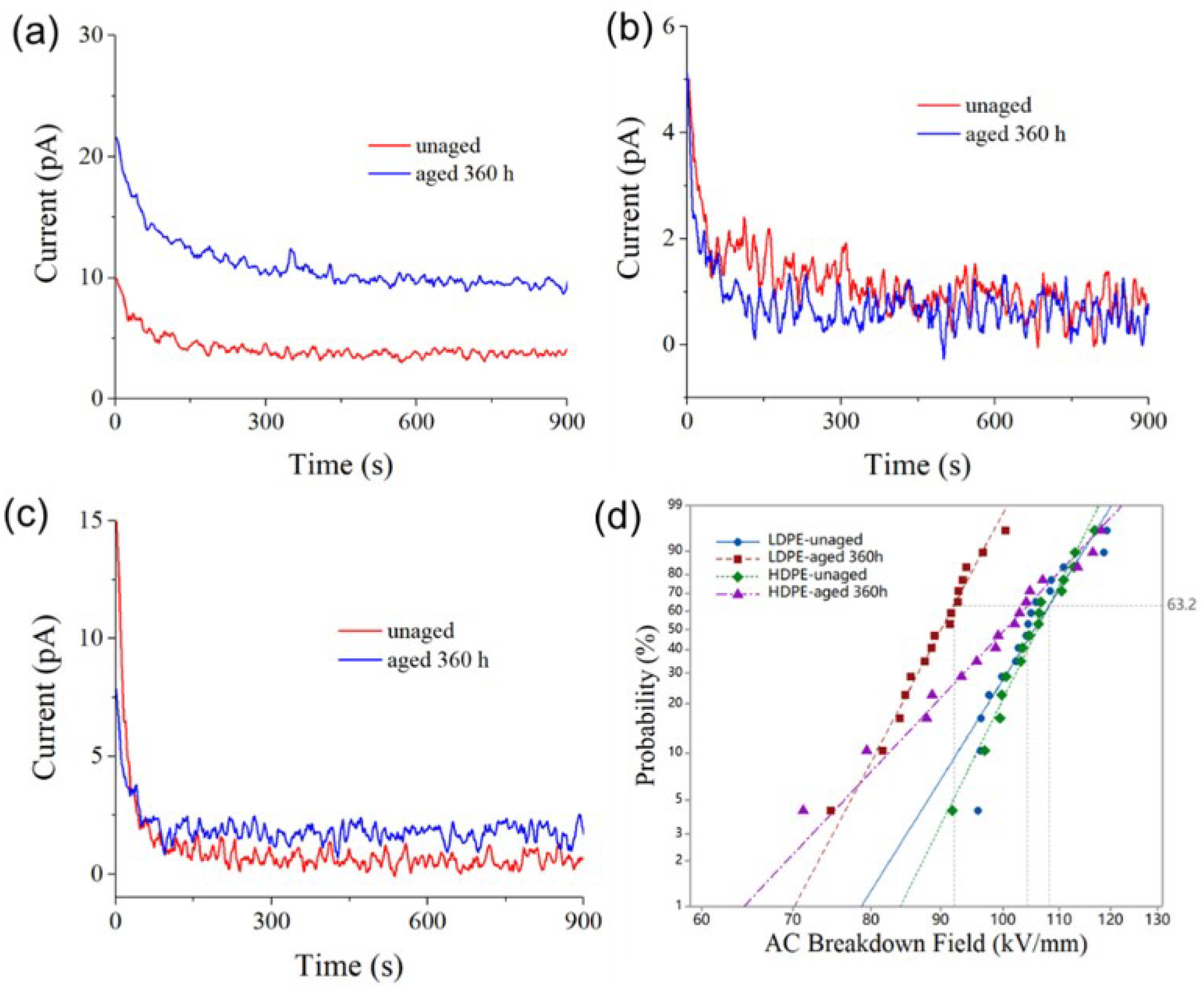
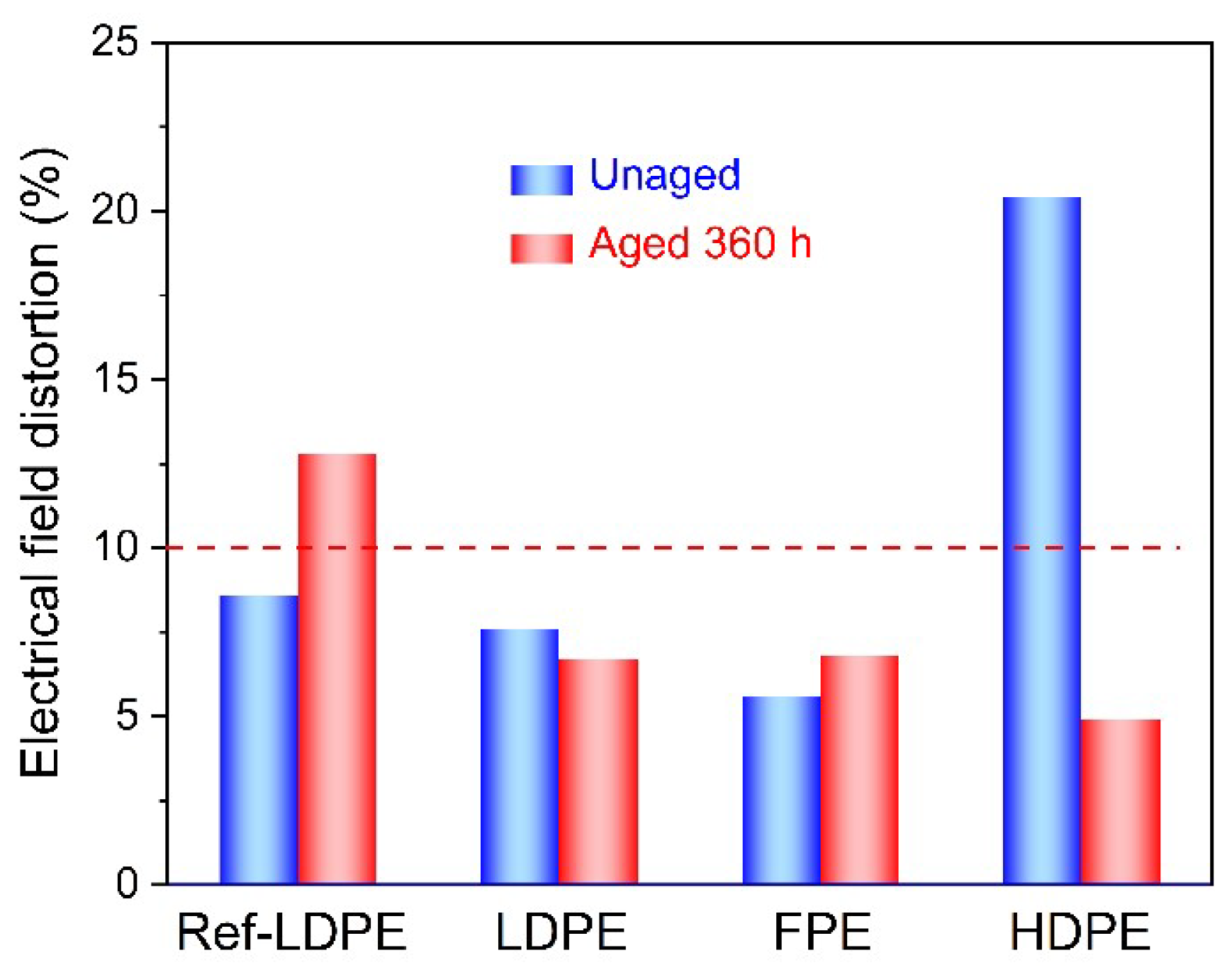
3.3. Electrical Property
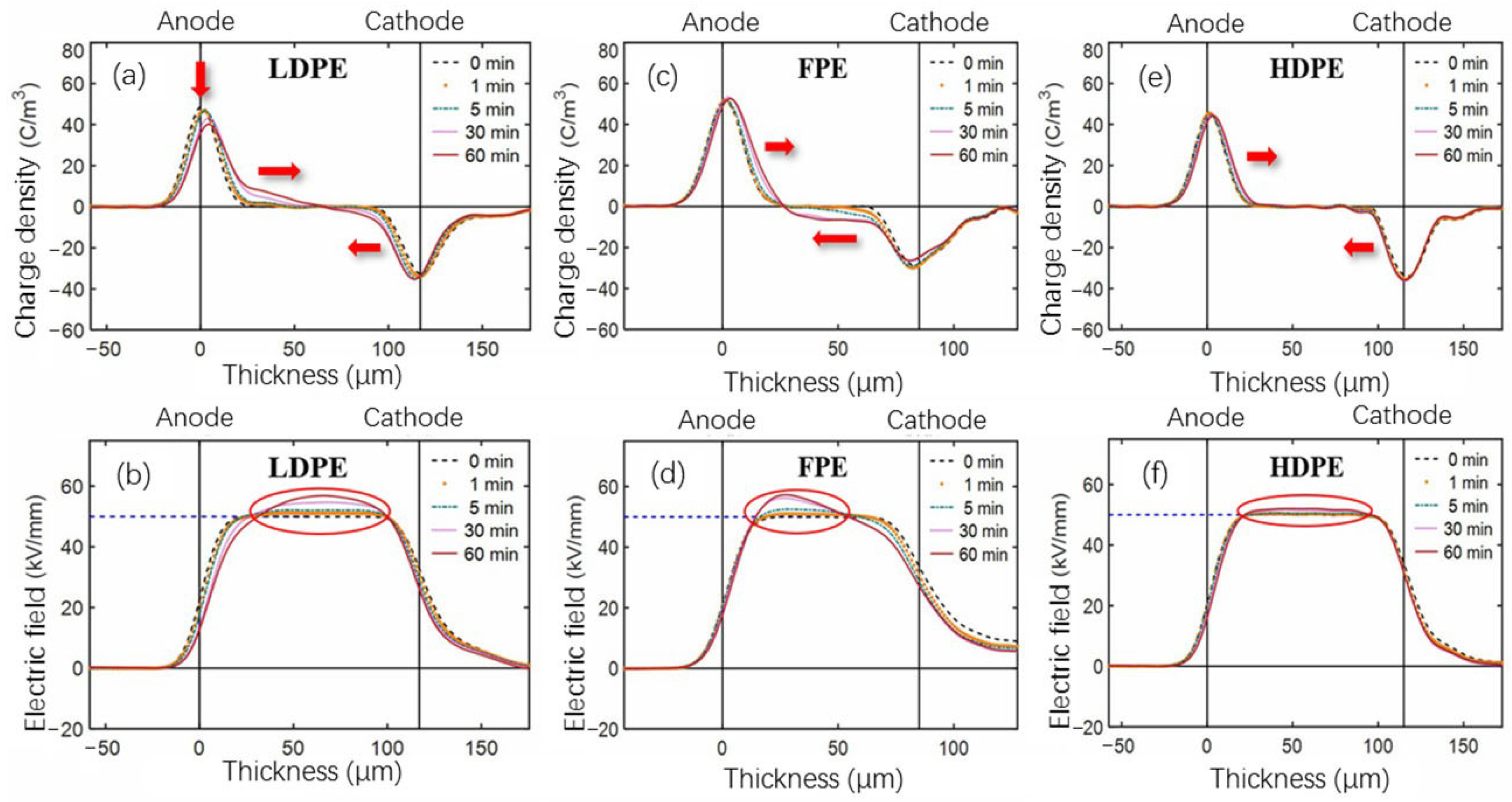
3.4. Space Charge Behavior of Single-Layer Samples
3.5. Space Charge Behavior of Double-Layer Samples
4. Conclusions
- (1)
- Both TGA and DSC are high-precision thermal analysis methods, and detailed thermal aging parameters were obtained in N2 and O2 atmospheres. Compared to additive-free LDPE, all three PE materials contain a certain amount of antioxidants. LDPE had the lowest melting point and thermal oxidation temperature region, while FPE had the highest melting point and crystallinity.
- (2)
- The electric field distortion and space charge accumulation of HDPE film samples are the most obvious among the four unaged groups. However, the electric field distortion ratio of 360 h aged HDPE samples is much smaller than that of unaged HDPE, which may be related to the newly generated deep traps due to the thermal aging.
- (3)
- A large amount of space charge accumulates at the unaged LDPE/FPE interface mainly due to the mismatching in DC conductivity between the two layers. After thermal aging, the space charge accumulation of the double-layer sample increases at first and decreases, which should be mainly attributed to the corresponding change of trap property and electrical conductivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.X.; Sun, Q.H.; Wang, N.H.; Wang, Y.S. Pre-stressing effect on DC breakdown in low-density polyethylene. J. Electrost. 2009, 67, 426–429. [Google Scholar] [CrossRef]
- An, Y.G.; Kim, C.H.; Nam, T.W.; Lee, J.H. A Study on the Behavior of Interfacial Space Charge at Polymer Dielectric Double-Layer Simulating the HVDC Cable Joints. In Proceedings of the 2020 International Symposium on Electrical Insulating Materials (ISEIM), Tokyo, Japan, 13–17 September 2020; pp. 158–161. [Google Scholar]
- Iguchi, K.; Tohmine, T.; Tohmine, H.; Tanaka, Y.; Takada, T.; Ida, Y.; Murata, Y.; Inoue, Y. Space charge accumulation characteristics in double-layered samples simulating DC cable joints. In Proceedings of the 2018 12th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Xi’an, China, 2 July 2018; pp. 119–123. [Google Scholar]
- Zhang, Y.W.; Lewine, J.; Alquie, C.; Hampton, N. Evidence of strong correlation between space-charge buildup and breakdown in cable insulation. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 778–783. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.Y.; Xiao, K.; Zhang, Z.X. Research on the Thermal Aging Behaviors of LDPE/TiO2 Nanocomposites. J. Nanomater. 2017, 2017, 5048382. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Tanaka, T.; Takada, T.; Zhong, L.S. Effect of Polyethylene Interface on Space Charge Formation. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T. Space charge injected via interfaces and tree initiation in polymers. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 733–743. [Google Scholar] [CrossRef]
- Liu, N. Space Charge Characteristics in Polymer Materials and Their Relation with Ageing; University of Southampton: Southampton, UK, 2017. [Google Scholar]
- Kerimli, G.M. On the mechanism of electrical aging of polymer insulation materials. Surf. Eng. Appl. Electrochem. 2014, 50, 485–490. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.X.; Teng, C.Y.; Zhang, Y.X.; Chen, M.; Cheng, Z.X. Transient dynamics of packet-like space charge in low-density polyethylene at high temperatures. J. Electrost. 2017, 88, 100–105. [Google Scholar] [CrossRef]
- Ren, C.Y.; Zhang, C.S.; Hu, D.; Zhang, C.; Kong, F.; Shao, T.; Yan, P. Trap distribution of polymeric materials and its effect on surface flashover in vacuum. Plasma Sci. Technol. 2020, 22, 044002. [Google Scholar] [CrossRef]
- Dissado, L.A. The origin and nature of ‘charge packets’: A short review. In Proceedings of the 2010 10th IEEE International Conference on Solid Dielectrics, Potsdam, Germany, 4–9 July 2010; p. 5568209. [Google Scholar]
- Carstensen, P.; Jonsson, J.; Farkas, A.A.; Campus, A.; Nilsson, U.H. A study on the origin of space charge accumulation in polymeric HVDC cables. In Proceedings of the 2004 IEEE International Conference on Solid Dielectrics, Toulouse, France, 5–9 July 2004; pp. 260–263. [Google Scholar]
- Aida, F.; Wang, S.; Fujita, M. Study of the mechanism of space charge formation in polyethylene. J. Electrost. 1997, 42, 3–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Christen, T.; Meng, X.; Chen, J.; Rocks, J. Research progress on space charge characteristics in polymeric insulation. J. Adv. Dielectr. 2016, 6, 1692001. [Google Scholar] [CrossRef] [Green Version]
- Sima, W.; Jiang, X.W.; Peng, Q.J.; Sun, P. Investigation of dielectric properties of polyethylene terephthalate under different aging temperatures. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3015–3023. [Google Scholar] [CrossRef]
- Fu, M.; Dissado, L.A.; Chen, G.; Fothergill, J.C. Space charge formation and its modified electric field under applied voltage reversal and temperature gradient in XLPE cable. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Rogti, F.; Ferhat, M. Temperature effect on space charge dynamics in multi-layer insulation Fluorinated Ethylene Propylene (FEP) Low Density Polyethylene (LDPE). In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Chenzhen, China, 20–23 October 2013; pp. 283–286. [Google Scholar]
- Xu, Z.; Chen, G. Interfacial characteristics of space charge in multi-layer LDPE. In Proceedings of the 2008 International Conference on Condition Monitoring and Diagnosis, Beijing, China, 21–24 April 2008; pp. 332–335. [Google Scholar]
- Etsu, K.; Ohki, T.; Tanaka, T.; Sekiguchi, Y.; Murata, Y.; Reddy, C.C. Space charge behavior in multi-layered dielectrics with LDPE and LDPE/MgO nanocomposites. In Proceedings of the 2010 10th IEEE International Conference on Solid Dielectrics, Potsdam, Germany, 4–9 July 2010; pp. 1–4. [Google Scholar]
- Dakka, M.A.; Bulinski, A.; Bamji, S. Space charge development and breakdown in cross-linked polyethylene under DC fields. In Proceedings of the Conference Record of the 2000 IEEE International Symposium on Electrical Insulation (Cat. No.00CH37075), Anaheim, CA, USA, 5 April 2000; pp. 489–492. [Google Scholar]
- Suzuoki, Y.; Matsukawa, Y.; Han, S.O.; Fujii, A.; Kim, J.S.; Mizutani, T.; Ieda, M.; Yoshifuji, N. Study of space-charge effects on dielectric breakdown of polymers by direct probing. IEEE Trans. Dielectr. Electr. Insul. 1992, 27, 758–762. [Google Scholar] [CrossRef]
- Hosier, I.L.; Vaughan, A.S.; Swingler, S.G. Structure—Property relationships in polyethylene blends: The effect of morphology on electrical breakdown strength. J. Mater. Sci. 1997, 32, 4523–4531. [Google Scholar] [CrossRef]
- Li, X.; Du, Q.G.; Kang, J.; Tu, D.M. Influence of microstructure on space charges of polypropylene. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 365–374. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.M.; Hu, J.; He, J.L. Polymeric insulation materials for HVDC cables: Development, challenges and future perspective. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1308–1318. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.X.; Zhou, Y.X.; Teng, C.Y.; Peng, Z.W.; Spinella, S. Crystalline modification and its effects on dielectric breakdown strength and space charge behavior in isotactic polypropylene. Polymers 2018, 10, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weingril, H.M.; Fauster, K.R.; Lucyshyn, T.; Zauner, C. High density polyethylene as phase-change material: Long-term stability and aging. Polym. Test. 2019, 76, 433–442. [Google Scholar] [CrossRef]
- Hu, S.X.; Wang, W.; Dang, B.; Zhou, Y.; Yuan, C.; Hu, J.; Li, Q.; He, J.L. Thermal Properties and Space Charge Behavior of Thermally Aged Polypropylene/Elastomer Blends Nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 521–527. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C.; Palmieri, F. Quantities extracted from space-charge measurements as markers for insulation aging. IEEE Trans. Dielectr. Electr. Insul. 2003, 2, 198–203. [Google Scholar] [CrossRef]
- IEC/TS 62758; Calibration of Space Charge Measuring Equipment Based on the Pulsed Electroacoustic (PEA) Measurement Principle. International Electrotechnical Commission: Paris, France, 2012.








| DSC OIT in O2 | TGA in O2 | |||||
|---|---|---|---|---|---|---|
| T2 (°C) | T3 (°C) | T4 (°C) | TInit (°C) | TTang (°C) | TPeak (°C) | |
| Ref-LDPE | − | − | − | 199.8 | 209.8 | 238.6 |
| LDPE | 249.7 | 255.9 | 267.6 | 255.7 | 260.3 | 267.8 |
| FPE | 245.5 | 252.8 | 260.5 | 253.7 | 258.5 | 265.3 |
| HDPE | 254.2 | 262.3 | 270.2 | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Z.; Wu, W.; Wang, Z.; Zhang, L.; Zhou, Y. Space Charge Behavior of Thermally Aged Polyethylene Insulation of Track Cables. Polymers 2022, 14, 2162. https://doi.org/10.3390/polym14112162
Qiao Z, Wu W, Wang Z, Zhang L, Zhou Y. Space Charge Behavior of Thermally Aged Polyethylene Insulation of Track Cables. Polymers. 2022; 14(11):2162. https://doi.org/10.3390/polym14112162
Chicago/Turabian StyleQiao, Zhichao, Wangsong Wu, Zhaowei Wang, Ling Zhang, and Yuanxiang Zhou. 2022. "Space Charge Behavior of Thermally Aged Polyethylene Insulation of Track Cables" Polymers 14, no. 11: 2162. https://doi.org/10.3390/polym14112162
APA StyleQiao, Z., Wu, W., Wang, Z., Zhang, L., & Zhou, Y. (2022). Space Charge Behavior of Thermally Aged Polyethylene Insulation of Track Cables. Polymers, 14(11), 2162. https://doi.org/10.3390/polym14112162






