Enhancement of Epoxy Thermosets with Hyperbranched and Multiarm Star Polymers: A Review
Abstract
:1. Introduction
2. Epoxy Thermosets
3. Effect of the Addition of HBPs or MASPs on the Curing Process
4. Thermal Properties
5. Thermomechanical Characteristics
6. Rheological Properties
7. Fire Retardancy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.H. Hyperbranched Polyarylene. U.S. Patent 4857630, 15 August 1989. [Google Scholar]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.; Moeller, M. Uniform highly branched polymers by anionic grafting: Arborescent graft polymers. Macromolecules 1991, 24, 4548–4553. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures-synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Wooley, K.L.; Fréchet, J.M.J.; Hawker, C.J. Influence of shape on the reactivity and properties of dendritic, hyperbranched and linear aromatic polyesters. Polymer 1994, 35, 4489–4495. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Monodispersed dendritic polyesters with removable chain ends: A versatile approach to globular macromolecules with chemically reversible polarities. J. Chem. Soc. Perkin Trans. 1992, 1, 2459–2469. [Google Scholar] [CrossRef]
- Voit, B. New developments in hyperbranched polymers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2505–2525. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M.A. Dendritic Aromatic Polyamides and Polyimides. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1293–1309. [Google Scholar] [CrossRef]
- Carlmark, A.; Hawker, C.; Hult, A.; Malkoch, M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 2009, 38, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.I.; Ahmadi, Y.; Ahmad, S. Recent Advances in Structural Modifications of Hyperbranched Polymers and Their Applications. Ind. Eng. Chem. Res. 2018, 57, 10754–10785. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Hyperbranched Polymers for Coating Applications: A Review. Polym.-Plast. Technol. Eng. 2016, 55, 92–117. [Google Scholar] [CrossRef]
- Dhevi, D.M.; Anand Prabu, A.; Kim, K.J. Hyperbranched polyester as a crosslinker in polyurethane formation: Real-time monitoring using in situ FTIR. Polym. Bull. 2016, 73, 2867–2888. [Google Scholar] [CrossRef]
- Schulze, A.; Went, M.; Prager, A. Membrane Functionalization with Hyperbranched Polymers. Materials 2016, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Aloorkar, N.H.; Kulkarni, A.S.; Patil, R.A.; Ingale, D.J. Star Polymers: An Overview. Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1675–1684. [Google Scholar] [CrossRef]
- Däbritz, F.; Voit, B.; Naguib, M.; Sangermano, M. Hyperstar poly(ester-methacrylate)s as additives in thermally and photocured epoxy resins. Polymer 2011, 52, 5723–5731. [Google Scholar] [CrossRef]
- Lagunas, C.; Fernández-Francos, X.; Ferrando, F.; Flores, M.; Serra, À.; Morancho, J.M.; Salla, J.M.; Ramis, X. New epoxy thermosets modified with amphiphilic multiarm star polymers as toughness enhancer. React. Funct. Polym. 2014, 83, 132–143. [Google Scholar] [CrossRef]
- Alfurhood, J.A.; Bachler, P.R.; Sumerlin, B.S. Hyperbranched polymers via RAFT self-condensing vinyl polymerization. Polym. Chem. 2016, 7, 3361–3369. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H. Recent Progress on Hyperbranched Polymers Synthesized via Radical-Based Self-Condensing Vinyl Polymerization. Polymers 2017, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Guo, W.; Xu, Z.; Chen, S.; Cheng, J.; Miao, M.; Zhang, D. Synthesis of degradable hyperbranched epoxy resins with high tensile, elongation, modulus and low-temperature resistance. Compos. Part B 2020, 192, 108005. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Zhu, X.; Yan, D.; Chen, R. Synthesis and therapeutic applications of biocompatible or biodegradable hyperbranched polymers. Polym. Chem. 2015, 6, 2794–2812. [Google Scholar] [CrossRef]
- Sun, F.; Luo, X.; Kang, L.; Peng, X.; Lu, C. Synthesis of hyperbranched polymers and their applications in analytical chemistry. Polym. Chem. 2015, 6, 1214–1225. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Zhu, X.; Yan, D. Synthesis and applications of stimuli-responsive hyperbranched polymers. Prog. Polym. Sci. 2017, 64, 114–153. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J. Hyperbranched polymers from A2 + B3 strategy: Recent advances in description and control of fine topology. Polym. Chem. 2016, 7, 3643–3663. [Google Scholar] [CrossRef]
- Mou, Q.; Ma, Y.; Jin, X.; Zhu, X. Designing hyperbranched polymers for gene delivery. Mol. Syst. Des. Eng. 2016, 1, 25–39. [Google Scholar] [CrossRef]
- Boogh, L.; Petterson, B.; Manson, J.-A.E. Dendritic hyperbranched polymers as tougheners for epoxy resins. Polymer 1999, 40, 2249–2261. [Google Scholar] [CrossRef]
- Mezzenga, R.; Boogh, L.; Månson, J.A.E. A review of dendritic hyperbranched polymer as modifiers in epoxy composites. Compos. Sci. Technol. 2001, 61, 787–795. [Google Scholar] [CrossRef]
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Acebo, C.; Ramis, X.; Serra, À. Improved epoxy thermosets by the use of poly(ethyleneimine) derivatives. Phys. Sci. Rev. 2017, 2, 20160128. [Google Scholar] [CrossRef]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Serrano, E.; Tercjak, A.; Ocando, C.; Larrañaga, M.; Parellada, M.D.; Corona-Galván, S.; Mecerreyes, D.; Zafeiropoulos, N.E.; Stamm, M.; Mondragón, I. Curing Behavior and Final Properties of Nanostructured Thermosetting Systems Modified with Epoxidized Styrene-Butadiene Linear Diblock Copolymers. Macromol. Chem. Phys. 2007, 208, 2281–2292. [Google Scholar] [CrossRef]
- Kumar, K.D.; Kothandaraman, B. Modification of (DGEBA) epoxy resin with maleated depolymerised natural rubber. Express Polym. Lett. 2008, 2, 302–311. [Google Scholar] [CrossRef]
- Guo, Q.; Thomann, R.; Gronski, W.; Thurn-Albrecht, T. Phase Behavior, Crystallization, and Hierarchical Nanostructures in Self-Organized Thermoset Blends of Epoxy Resin and Amphiphilic Poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) Triblock Copolymers. Macromolecules 2002, 35, 3133–3144. [Google Scholar] [CrossRef]
- Ratna, D.; Banthia, A.K. Rubber toughened epoxy. Macromol. Res. 2004, 12, 11–21. [Google Scholar] [CrossRef]
- Lipic, P.M.; Bates, F.S.; Hillmyer, M.A. Nanostructured Thermosets from Self-Assembled Amphiphilic Block Copolymer/Epoxy Resin Mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Kinloch, I.A.; Othman, R.N. Low viscosity processing using hybrid CNT-coated silica particles to form electrically conductive epoxy resin composites. Polymer 2016, 98, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.L.; Fan, Y. Effect of the Compatibilizer on the Morphology and Properties of Dynamically Cured PP/POE/Epoxy Blends. J. Appl. Polym. Sci. 2012, 124, 2423–2429. [Google Scholar] [CrossRef]
- Liang, Y.L.; Pearson, R.A. The toughening mechanism in hybrid epoxy-silica-rubber nanocomposites (HESRNs). Polymer 2010, 51, 4880–4890. [Google Scholar] [CrossRef]
- Grishchuk, S.; Sorochynska, L.; Vorster, O.C.; Karger-Kocsis, J. Structure, thermal, and mechanical properties of DDM-hardened epoxy/benzoxazine hybrids: Effects of epoxy resin functionality and ETBN toughening. J. Appl. Polym. Sci. 2013, 127, 5082–5093. [Google Scholar] [CrossRef]
- Kelnar, I.; Rotrekl, J.; Kaprálková, L.; Hromádková, J.; Strachota, A. Effect of Amine-Terminated Butadiene-Acrylonitrile/Clay Combinations on the Structure and Properties of Epoxy Nanocomposites. J. Appl. Polym. Sci. 2012, 125, 3477–3483. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosur, M.; Zainuddin, S.; Jajam, K.C.; Tippur, H.V.; Jeelani, S. Mechanical characterization of epoxy composites modified with reactive polyol diluent and randomly-oriented amino-functionalized MWCNTs. Polym. Test. 2012, 31, 1083–1093. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Seidel, C.; Schulte, K. Preparation and characterization of graphite nano-platelet (GNP)/epoxy nano-composite: Mechanical, electrical and thermal properties. Eur. Polym. J. 2013, 49, 3878–3888. [Google Scholar] [CrossRef]
- Santiago, D.; Morell, M.; Fernández-Francos, X.; Serra, À.; Salla, J.M.; Ramis, X. Influence of the end groups of hyperbranched poly(glycidol) on the cationic curing and morphology of diglycidylether of bisfenol A thermosets. React. Funct. Polym. 2011, 71, 380–389. [Google Scholar] [CrossRef]
- Ratna, D.; Varley, R.; Singh Raman, R.K.; Simon, G.P. Studies on blends of epoxy-functionalized hyperbranched polymer and epoxy resin. J. Mater. Sci. 2003, 38, 147–154. [Google Scholar] [CrossRef]
- Chen, K.-L.; Zhang, T.; Cui, Y.; Wang, Z.-Y. Progress of hyperbranched polymers (HBPs) as modifiers in epoxy resins. Cailiao Gongcheng/J. Mater. Eng. 2019, 47, 11–18. [Google Scholar] [CrossRef]
- Guo, Q.; Habrard, A.; Park, Y.; Halley, P.J.; Simon, G.P. Phase separation, porous structure, and cure kinetics in aliphatic epoxy resin containing hyperbranched polyester. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 889–899. [Google Scholar] [CrossRef]
- Frigione, M.; Calò, E. Influence of an Hyperbranched Aliphatic Polyester on the Cure Kinetic of a Trifunctional Epoxy Resin. J. Appl. Polym. Sci. 2008, 107, 1744–1758. [Google Scholar] [CrossRef]
- Ratna, D.; Simon, G.P. Thermomechanical properties and morphology of blends of a hydroxy-functionalized hyperbranched polymer and epoxy resin. Polymer 2001, 42, 8833–8839. [Google Scholar] [CrossRef]
- Rozenberg, B.A. Kinetics, Thermodynamics and Mechanism of Reactions of Epoxy Oligomers with Amines. Adv. Polym. Sci. 1986, 75, 113–165. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Salla, J.M.; Cadenato, A.; Morancho, J.M.; Serra, À.; Mantecón, A.; Ramis, X. A New Strategy for Controlling Shrinkage of DGEBA Resins Cured by Cationic Copolymerization with Hydroxyl-Terminated Hyperbranched Polymers and Ytterbium Triflate as an Initiator. J. Appl. Polym. Sci. 2009, 111, 2822–2829. [Google Scholar] [CrossRef]
- Sangermano, M.; Amerio, E.; Di Gianni, A.; Priola, A.; Pospiech, D.; Voit, B. Hyperbranched polymers in cationic UV curing. Macromol. Symp. 2007, 254, 9–15. [Google Scholar] [CrossRef]
- Foix, D.; Ramis, X.; Ferrando, F.; Serra, À. Improvement of epoxy thermosets using a thiol-ene based polyester hyperbranched polymer as modifier. Polym. Int. 2012, 61, 727–734. [Google Scholar] [CrossRef]
- Foix, D.; Yu, Y.; Serra, À.; Ramis, X.; Salla, J.M. Study on the chemical modification of epoxy/anhydride thermosets using a hydroxyl terminated hyperbranched polymer. Eur. Polym. J. 2009, 45, 1454–1466. [Google Scholar] [CrossRef]
- Yang, J.P.; Chen, Z.K.; Yang, G.; Fu, S.Y.; Ye, L. Simultaneous improvements in the cryogenic tensile strength, ductility and impact strength of epoxy resins by a hyperbranched polymer. Polymer 2008, 49, 3168–3175. [Google Scholar] [CrossRef]
- Ferńandez-Francos, X.; Rybak, A.; Sekula, R.; Ramis, X.; Serra, À. Modification of epoxy-anhydride thermosets using a hyperbranched poly(ester-amide): I. Kinetic study. Polym. Int. 2012, 61, 1710–1725. [Google Scholar] [CrossRef]
- Morell, M.; Ramis, X.; Ferrando, F.; Yu, Y.; Serra, À. New improved thermosets obtained from DGEBA and a hyperbranched poly(ester-amide). Polymer 2009, 50, 5374–5383. [Google Scholar] [CrossRef]
- Morell, M.; Fernández-Francos, X.; Ramis, X.; Serra, A. Synthesis of a new hyperbranched polyaminoester and its use as a reactive modifier in anionic curing of DGEBA thermosets. Macromol. Chem. Phys. 2010, 211, 1879–1889. [Google Scholar] [CrossRef]
- Li, S.; Cui, C.; Hou, H. Synthesis and characterization of amino-terminated hyperbranched polymer and its effect on impact resistance of epoxy resin thermosets. Colloid Polym. Sci. 2015, 293, 2681–2688. [Google Scholar] [CrossRef]
- Yan, D.; Gao, C. Hyperbranched polymers made from A2 and BB’2 type monomers. 1. Polyaddition of l-(2-aminoethyl) piperazihe to divinyl sulfone. Macromolecules 2000, 33, 7693–7699. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Y.; Chen, L.; He, C.; Chung, T.S.; Goh, S.H. 2A 2 + BB′B″ approach to hyperbranched poly(amino ester)s. Macromolecules 2005, 38, 5519–5525. [Google Scholar] [CrossRef]
- Park, S.J.; Li, K.; Jin, F.L. Synthesis and characterization of hyper-branched polyimides from 2,4,6-triaminopyrimidine and dianhydrides system. Mater. Chem. Phys. 2008, 108, 214–219. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhuang, L.H.; Sun, J.; Zheng, C.L. Salt-free dyeing of ramie fabric with an amino-terminated hyperbranched polymer. Cellulose 2014, 21, 3725–3736. [Google Scholar] [CrossRef]
- Ping, Y.; Wu, D.; Kumar, J.N.; Cheng, W.; Lay, C.L.; Liu, Y. Redox-responsive hyperbranched poly(amido amine)s with tertiary amino cores for gene delivery. Biomacromolecules 2013, 14, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.-L.; Park, S.-J. Thermal Properties and Toughness Performance of Hyperbranched-Polyimide-Modified Epoxy Resins. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3348–3356. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Santiago, D.; Ferrando, F.; Ramis, X.; Salla, J.M.; Serra, A. Network structure and thermomechanical properties of hybrid DGEBA networks cured with 1-methylimidazole and hyperbranched poly(ethyleneimine)s. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1489–1503. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Zhang, D. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Mezzenga, R.; Månson, J.A.E. Thermo-mechanical properties of hyperbranched polymer modified epoxies. J. Mater. Sci. 2001, 36, 4883–4891. [Google Scholar] [CrossRef]
- DeCarli, M.; Kozielski, K.; Tian, W.; Varley, R. Toughening of a carbon fibre reinforced epoxy anhydride composite using an epoxy terminated hyperbranched modifier. Compos. Sci. Technol. 2005, 65, 2156–2166. [Google Scholar] [CrossRef]
- Fu, J.-F.; Shi, L.-Y.; Yuan, S.; Zhong, Q.-D.; Zhang, D.-S.; Chen, Y.; Wu, J. Morphology, toughness mechanism, and thermal properties of hyperbranched epoxy modified diglycidyl ether of bisphenol A (DGEBA) interpenetrating polymer networks. Polym. Adv. Technol. 2008, 19, 1597–1607. [Google Scholar] [CrossRef]
- Varley, R.J.; Tian, W. Toughening of an epoxy anhydride resin system using an epoxidized hyperbranched polymer. Polym. Int. 2004, 53, 69–77. [Google Scholar] [CrossRef]
- Ratna, D.; Varley, R.; Simon, G.P. Toughening of trifunctional epoxy using an epoxy-functionalized hyperbranched polymer. J. Appl. Polym. Sci. 2003, 89, 2339–2345. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Malucelli, G.; Sangermano, M.; Priola, A. Preparation of coatings via cationic photopolymerisation: Influence of alcoholic additives. Macromol. Symp. 2002, 187, 481–492. [Google Scholar] [CrossRef]
- Sangermano, M.; Sturari, M.; Chiappone, A.; Roppolo, I. Study of Ink-Jet Printable Vinyl Ether-Graphene UV-Curable Formulations. Macromol. Mater. Eng. 2015, 300, 340–345. [Google Scholar] [CrossRef]
- Nazar, R.; Ronchetti, S.; Roppolo, I.; Sangermano, M.; Bongiovanni, R.M. In situ synthesis of polymer embedded silver nanoparticles via photopolymerization. Macromol. Mater. Eng. 2015, 300, 226–233. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Sangermano, M.; Priola, A.; Malucelli, G.; Bongiavianni, R.; Quaglia, A.; Voit, B.; Ziemer, A. Phenolic hyperbranched polymers as additives in cationic photopolymerization of epoxy systems. Macromol. Mater. Eng. 2004, 289, 442–446. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Bongiovanni, R.; Priola, A.; Harden, A. Investigation on the effect of the presence of hyperbranched polymers on thermal and mechanical properties of an epoxy UV-cured system. Polym. Int. 2005, 54, 917–921. [Google Scholar] [CrossRef]
- Morancho, J.M.; Cadenato, A.; Ramis, X.; Fernández-Francos, X.; Salla, J.M. Thermal curing and photocuring of an epoxy resin modified with a hyperbranched polymer. Thermochim. Acta 2010, 510, 1–8. [Google Scholar] [CrossRef]
- Morancho, J.M.; Cadenato, A.; Ramis, X.; Fernández-Francos, X.; Flores, M.; Salla, J.M. Effect of a hyperbranched polymer over the thermal curing and the photocuring of an epoxy resin. J. Therm. Anal. Calorim. 2011, 105, 479–488. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, D.; Li, Z.; Lin, H.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Toughness modification of cationic UV-cured cycloaliphatic epoxy resin by hydroxyl polymers with different structures. Eur. Polym. J. 2020, 127, 109594. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Foix, D.; Serra, À.; Salla, J.M.; Ramis, X. Novel thermosets based on DGEBA and hyperbranched polymers modified with vinyl and epoxy end groups. React. Funct. Polym. 2010, 70, 798–806. [Google Scholar] [CrossRef]
- Flores, M.; Fernández-Francos, X.; Ramis, X.; Serra, A. Novel epoxy-anhydride thermosets modified with a hyperbranched polyester as toughness enhancer. I. Kinetics study. Thermochim. Acta 2012, 544, 17–26. [Google Scholar] [CrossRef]
- Flores, M.; Morell, M.; Fernández-Francos, X.; Ferrando, F.; Ramis, X.; Serra, À. Enhancement of the impact strength of cationically cured cycloaliphatic diepoxide by adding hyperbranched poly(glycidol) partially modified with 10-undecenoyl chains. Eur. Polym. J. 2013, 49, 1610–1620. [Google Scholar] [CrossRef]
- Tomuta, A.; Ferrando, F.; Serra, À.; Ramis, X. New aromatic-aliphatic hyperbranched polyesters with vinylic end groups of different length as modifiers of epoxy/anhydride thermosets. React. Funct. Polym. 2012, 72, 556–563. [Google Scholar] [CrossRef]
- Tomuta, A.M.; Ramis, X.; De la Flor, S.; Serra, A. Influence of end groups in hyperbranched polyesters used as modifiers in the characteristics of epoxy thermosets cured by adipic dihydrazide. Express Polym. Lett. 2013, 7, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Acebo, C.; Fernández-Francos, X.; De La Flor, S.; Ramis, X.; Serra, À. New anhydride/epoxy thermosets based on diglycidyl ether of bisphenol A and 10-undecenoyl modified poly(ethyleneimine) with improved impact resistance. Prog. Org. Coat. 2015, 85, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Acebo, C.; Fernández-Francos, X.; Ramis, X.; Serra, À. Multifunctional allyl-terminated hyperbranched poly(ethyleneimine) as component of new thiol-ene/thiol-epoxy materials. React. Funct. Polym. 2016, 99, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Badia, J.D.; Teruel-juanes, R.; Acebo, C.; Gil-castell, O.; Serra, A.; Ribes-greus, A. Dielectric spectroscopy of novel thiol-ene/epoxy thermosets obtained from allyl-modi fi ed hyperbranched poly(ethyleneimine) and diglycidylether of bisphenol A. Eur. Polym. J. 2019, 113, 98–106. [Google Scholar] [CrossRef]
- Morancho, J.M.; Fernández-Francos, X.; Acebo, C.; Ramis, X.; Salla, J.M.; Serra, A. Thermal curing of an epoxy-anhydride system modified with hyperbranched poly(ethylene imine)s with different terminal groups. J. Therm. Anal. Calorim. 2017, 127, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Fei, X.; Wei, W.; Tang, Y.; Zhu, Y.; Luo, J.; Chen, M.; Liu, X. Simultaneous enhancements in toughness, tensile strength, and thermal properties of epoxy-anhydride thermosets with a carboxyl-terminated hyperbranched polyester. Eur. Polym. J. 2017, 90, 431–441. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; De la Flor, S.; Ramis, X.; Serra, À. The Use of Click-Type Reactions in the Preparation of Thermosets. Polymers 2020, 12, 1084. [Google Scholar] [CrossRef]
- Foix, D.; Ramis, X.; Serra, A.; Sangermano, M. UV generation of a multifunctional hyperbranched thermal crosslinker to cure epoxy resins. Polymer 2011, 52, 3269–3276. [Google Scholar] [CrossRef]
- Flores, M.; Tomuta, A.M.; Fernández-Francos, X.; Ramis, X.; Sangermano, M.; Serra, A. A new two-stage curing system: Thiol-ene/epoxy homopolymerization using an allyl terminated hyperbranched polyester as reactive modifier. Polymer 2013, 54, 5473–5481. [Google Scholar] [CrossRef]
- Acebo, C.; Lederer, A.; Appelhans, D.; Ramis, X.; Serra, À. Synthesis of 1,2,3-triazole functionalized hyperbranched poly(ethyleneimine) and its use as multifunctional anionic macroinitiator for diglycidyl ether of bisphenol A curing. Eur. Polym. J. 2016, 85, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Foix, D.; Khalyavina, A.; Morell, M.; Voit, B.; Lederer, A.; Ramis, X.; Serra, A. The effect of the degree of branching in hyperbranched polyesters used as reactive modifiers in epoxy thermosets. Macromol. Mater. Eng. 2012, 297, 85–94. [Google Scholar] [CrossRef]
- Morell, M.; Ramis, X.; Ferrando, F.; Serra, A. Effect of polymer topology on the curing process and mechanical characteristics of epoxy thermosets modified with linear or multiarm star poly(ε-caprolactone). Polymer 2011, 52, 4694–4702. [Google Scholar] [CrossRef]
- Santiago, D.; Fernández-Francos, X.; Ramis, X.; Salla, J.M.; Sangermano, M. Comparative curing kinetics and thermal-mechanical properties of DGEBA thermosets cured with a hyperbranched poly(ethyleneimine) and an aliphatic triamine. Thermochim. Acta 2011, 526, 9–21. [Google Scholar] [CrossRef]
- Mishra, M.; Kobayashi, S. Star and Hyperbranched Polymers, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Morell, M.; Foix, D.; Lederer, A.; Ramis, X.; Voit, B.; Serra, À. Synthesis of a New Multiarm Star Polymer Based on Hyperbranched Poly(styrene) Core and Poly(e-Caprolactone) Arms and Its Use as Reactive Modifier of Epoxy Thermosets. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4639–4649. [Google Scholar] [CrossRef]
- Tomuta, A.; Fernández-Francos, X.; Ferrando, F.; Serra, À.; Ramis, X. New Epoxy-Anhydride Thermosets Modified with Multiarm Stars with Hyperbranched Polyester Cores and Poly(ε{lunate}-caprolactone) Arms. Polym. Plast. Technol. Eng. 2014, 53, 645–654. [Google Scholar] [CrossRef]
- Morell, M.; Lederer, A.; Ramis, X.; Voit, B.; Serra, À. Multiarm Star Poly(glycidol)-block-Poly(e-caprolactone) of Different Arm Lengths and Their Use as Modifiers of Diglycidylether of Bisphenol A Thermosets. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2395–2406. [Google Scholar] [CrossRef]
- Acebo, C.; Fernández-Francos, X.; Ferrando, F.; Serra, À.; Salla, J.M.; Ramis, X. Multiarm star with poly(ethyleneimine) core and poly(ε-caprolactone) arms as modifiers of diglycidylether of bisphenol A thermosets cured by 1-methylimidazole. React. Funct. Polym. 2013, 73, 431–441. [Google Scholar] [CrossRef]
- Morell, M.; Fernández-Francos, X.; Gombau, J.; Ferrando, F.; Lederer, A.; Ramis, X.; Voit, B.; Serra, A. Multiarm star poly(glycidol)-block-poly(styrene) as modifier of anionically cured diglycidylether of bisphenol A thermosetting coatings. Prog. Org. Coat. 2012, 73, 62–69. [Google Scholar] [CrossRef]
- Morell, M.; Ramis, X.; Ferrando, F.; Serra, A. New improved thermosets obtained from diglycidylether of bisphenol A and a multiarm star copolymer based on hyperbranched poly(glycidol) core and poly(methyl methacrylate) arms. Macromol. Chem. Phys. 2012, 213, 335–343. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, D. Toughness and strength improvement of diglycidyl ether of bisphenol-A by low viscosity liquid hyperbranched epoxy resin. J. Appl. Polym. Sci. 2006, 101, 2504–2511. [Google Scholar] [CrossRef]
- Cicala, G.; Recca, A.; Restuccia, C. Influence of hydroxyl functionalized hyperbranched polymers on the thermomechanical and morphological properties of epoxy resins. Polym. Eng. Sci. 2005, 45, 225–237. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Fox, B. Thermal and Mechanical Properties of a Dendritic Hydroxyl-Functional Hyperbranched Polymer and Tetrafunctional Epoxy Resin Blends. J. Poym. Sci. Part B Polym. Phys. 2010, 48, 417–424. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Gao, X.; Huang, G. Simultaneous reinforcement and toughness improvement of an epoxy-phenolic network with a hyperbranched polysiloxane modifier. RSC. Adv. 2018, 8, 17606–17615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicala, G.; Recca, G. Thermomechanical and Morphological Properties of Epoxy Blends with Hyperbranched Polyester: Effect of the Pseudo-Generation Number. J. Appl. Polym. Sci. 2010, 115, 1395–1406. [Google Scholar] [CrossRef]
- Manjula Dhevi, D.; Jaisankar, S.N.; Pathak, M. Effect of new hyperbranched polyester of varying generations on toughening of epoxy resin through interpenetrating polymer networks using urethane linkages. Eur. Polym. J. 2013, 49, 3561–3572. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wong, C.P. Syntheses and characterizations of thermally reworkable epoxy resins II. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3771–3782. [Google Scholar] [CrossRef]
- Arasa, M.; Ramis, X.; Salla, J.M.; Mantecón, A.; Serra, A. A study of the degradation of ester-modified epoxy resins obtained by cationic copolymerization of DGEBA with γ-lactones initiated by rare earth triflates. Polym. Degrad. Stab. 2007, 92, 2214–2222. [Google Scholar] [CrossRef]
- Giménez, R.; Fernández-Francos, X.; Salla, J.M.; Serra, A.; Mantecón, A.; Ramis, X. New degradable thermosets obtained by cationic copolymerization of DGEBA with an s(γ-butyrolactone). Polymer 2005, 46, 10637–10647. [Google Scholar] [CrossRef]
- González, L.; Ramis, X.; Salla, J.M.; Mantecón, A.; Serra, A. The degradation of new thermally degradable thermosets obtained by cationic curing of mixtures of DGEBA and 6,6-dimethyl (4,8-dioxaspiro[2.5]octane-5,7-dione). Polym. Degrad. Stab. 2007, 92, 596–604. [Google Scholar] [CrossRef]
- Morell, M.; Erber, M.; Ramis, X.; Ferrando, F.; Voit, B.; Serra, A. New epoxy thermosets modified with hyperbranched poly(ester-amide) of different molecular weight. Eur. Polym. J. 2010, 46, 1498–1509. [Google Scholar] [CrossRef]
- Chen, J.-S.; Ober, C.K.; Poliks, M.D. Characterization of thermally reworkable thermosets: Materials for environmentally friendly processing and reuse. Polymer 2002, 43, 131–139. [Google Scholar] [CrossRef]
- Foix, D.; Erber, M.; Voit, B.; Lederer, A.; Ramis, X.; Mantecón, A.; Serera, A. New hyperbranched polyester modified DGEBA thermosets with improved chemical reworkability. Polym. Degrad. Stab. 2010, 95, 445–452. [Google Scholar] [CrossRef]
- Tomuta, A.M.; Fernández-Francos, X.; Ferrando, F.; Ramis, X.; Serra, À. Enhanced chemical reworkability of DGEBA thermosets cured with rare earth triflates using aromatic hyperbranched polyesters (HBP) and multiarm star HBP-b-poly(ε-caprolactone) as modifiers. Polym. Adv. Technol. 2013, 24, 962–970. [Google Scholar] [CrossRef]
- Acebo, C.; Fernández-Francos, X.; Ferrando, F.; Serra, À.; Ramis, X. New epoxy thermosets modified with multiarm star poly(lactide) with poly(ethyleneimine) as core of different molecular weight. Eur. Polym. J. 2013, 49, 2316–2326. [Google Scholar] [CrossRef]
- Pearson, R.A.; Yee, A.F. Toughening mechanisms in thermoplastic-modified epoxies: 1. Modification using poly(phenylene oxide). Polymer 1993, 34, 3658–3670. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, R.; Marouf, B.T.; Pearson, R.A. Rubber-Toughened Epoxies: A Critical Review. Polym. Rev. 2009, 49, 201–225. [Google Scholar] [CrossRef]
- Brooker, R.D.; Kinloch, A.J.; Taylor, A.C. The Morphology and Fracture Properties of Thermoplastic-Toughened Epoxy Polymers. J. Adhes. 2010, 86, 726–741. [Google Scholar] [CrossRef]
- Ruiz-Pérez, L.; Royston, G.J.; Fairclough, J.P.A.; Ryan, A.J. Toughening by nanostructure. Polymer 2008, 49, 4475–4488. [Google Scholar] [CrossRef] [Green Version]
- Pascault, J.P.; Williams, R.J.J. Epoxy Polymers: New Materials and Innovations, 1st ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Mezzenga, R.; Boogh, L.; Pettersson, B.; Månson, J.-A.E. Chemically induced phase separated morphologies in epoxy resin-hyperbranched polymer blends. Macromol. Symp. 2000, 149, 17–22. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Shaw, S.J.; Tod, D.A.; Hunston, D.L. Deformation and fracture behaviour of a rubber-toughened epoxy: 1. Microstructure and fracture studies. Polymer 1983, 24, 1341–1354. [Google Scholar] [CrossRef]
- Bagheri, R.; Pearson, R.A. Role of particle cavitation in rubber-toughened epoxies: 1. Microvoid toughening. Polymer 1996, 37, 4529–4538. [Google Scholar] [CrossRef]
- Cardwell, B.J.; Yee, A.F. Toughening of epoxies through thermoplastic crack bridging. J. Mater. Sci. 1998, 33, 5473–5484. [Google Scholar] [CrossRef]
- Ratna, D.; Simon, G.P. Thermal and Mechanical Properties of a Hydroxyl-functional Dendritic Hyperbranched Polymer and Trifunctional Epoxy Resin Blends. Polym. Eng. Sci. 2001, 41, 1815–1822. [Google Scholar] [CrossRef]
- Liu, T.; Nie, Y.; Chen, R.; Zhang, L.; Meng, Y.; Li, X. Hyperbranched polyether as an all-purpose epoxy modifier: Controlled synthesis and toughening mechanisms. J. Mater. Chem. A 2015, 3, 1188–1198. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Jia, D. Toughness and Reinforcement of Diglycidyl Ether of Bisphenol-A by Hyperbranched Poly(trimellitic anhydride-butanediol glycol) Ester Epoxy Resin. Polym. Compos. 2009, 30, 918–925. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Y.; Yu, Q.; Chen, S.; Zhang, J.; Miao, M.; Zhang, D. Synthesis of Epoxy-Ended Hyperbranched Polyesters with Reinforcing and Toughening Function for Diglycidyl Ether of Bisphenol-A. Polym. Compos. 2018, 39, E2046–E2055. [Google Scholar] [CrossRef]
- Luo, L.; Meng, Y.; Qiu, T.; Li, X. An epoxy-ended hyperbranched polymer as a new modifier for toughening and reinforcing in epoxy resin. J. Appl. Polym. Sci. 2013, 130, 1064–1073. [Google Scholar] [CrossRef]
- Zhang, J.; Chunhai, L.; Cheng, J.; Miao, M.; Zhang, D. Simultaneous Toughening and Strengthening of Diglycidyl Ether of Bisphenol-A Using Epoxy-Ended Hyperbranched Polymers Obtained From Thiol-Ene Click Reaction. Polym. Eng. Sci. 2018, 58, 1703–1709. [Google Scholar] [CrossRef]
- Miao, X.; Meng, Y.; Li, X. A novel all-purpose epoxy-terminated hyperbranched polyether sulphone toughener for an epoxy/amine system. Polymer 2015, 60, 88–95. [Google Scholar] [CrossRef]
- Miao, X.; Meng, Y.; Li, X. Epoxide-terminated hyperbranched polyether sulphone as triple enhancement modifier for DGEBA. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Zhu, T.; Lu, C.; Lu, X.; Zhi, J.; Song, Y. Curing process optimization and mechanical properties improvement of epoxy resin copolymer modified by epoxy-terminated hyperbranched polyether sulfone. Polymer 2022, 241, 124535. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Deng, B.; Huang, G. Simultaneously reinforcing and toughening epoxy network with a novel hyperbranched polysiloxane modifier. J. Appl. Polym. Sci. 2018, 135, 46340. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Chen, S.; Zhang, J.; Cheng, J.; Miao, M.; Zhang, D. Preparation of Epoxy Resins with Excellent Comprehensive Performance by Thiol-Epoxy Click Reaction. Prog. Org. Coat. 2019, 139, 105436. [Google Scholar] [CrossRef]
- Park, H.; Choe, Y. Enhancing Toughness and Impact Strength of Epoxy Resins by Using Hyperbranched Polymers. Int. J. Polym. Sci. 2021, 2021, 9984174. [Google Scholar] [CrossRef]
- Flores, M.; Fernández-Francos, X.; Ferrando, F.; Ramis, X.; Serra, À. Efficient impact resistance improvement of epoxy/anhydride thermosets by adding hyperbranched polyesters partially modified with undecenoyl chains. Polymer 2012, 53, 5232–5241. [Google Scholar] [CrossRef]
- Ren, Q.; Xiang, Y.; Huang, C.; Li, J.; Wang, C. Epoxy-functionalized star-shaped polymers as novel tougheners for epoxy resin. Polym. Bull. 2015, 72, 2949–2965. [Google Scholar] [CrossRef]
- Acebo, C.; Picardi, A.; Fernández-Francos, X.; De La Flor, S.; Ramis, X.; Serra, À. Effect of hydroxyl ended and end-capped multiarm star polymers on the curing process and mechanical characteristics of epoxy/anhydride thermosets. Prog. Org. Coat. 2014, 77, 1288–1298. [Google Scholar] [CrossRef]
- Rodlert, M.; Plummer, C.J.G.; Leterrier, Y.; Månson, J.-A.E.; Grünbauer, H.J.M. Rheological behavior of hyperbranched polymer/montmorillonite clay nanocomposites. J. Rheol. 2004, 48, 1049–1065. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Chen, X.; Lu, Y.; Miao, M.; Zhang, D. Controllability of epoxy equivalent weight and performance of hyperbranched epoxy resins. Compos. Part B 2018, 160, 615–625. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, D.; Zhou, Z. Synthesis and Characterization of Low Viscosity Aromatic Hyperbranched Polyester Epoxy Resin. Macromol. Res. 2009, 17, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Corcione, C.E.; Frigione, M.; Acierno, D. Rheological Characterization of UV-Curable Epoxy Systems: Effects of o-Boehmite Nanofillers and a Hyperbranched Polymeric Modifier. J. Appl. Polym. Sci. 2009, 112, 1302–1310. [Google Scholar] [CrossRef]
- Hsieh, T.; Tiu, C.; Simon, G.P. Rheological behaviour of polymer blends containing only hyperbranched polyesters of varying generation number. Polymer 2001, 42, 7635–7638. [Google Scholar] [CrossRef]
- Eloundou, J.; Harran, D. Temperature Dependence of the Behavior of a Reactive Epoxy-Amine System by Means of Dynamic Rheology. 2. High-Tg Epoxy-Amine System. Macromolecules 1996, 9297, 6917–6927. [Google Scholar] [CrossRef]
- Ratna, D.; Varley, R.; Simon, G.P. Processing and chemorheology of epoxy resins and their blends with dendritic hyperbranched polymers. J. Appl. Polym. Sci. 2004, 92, 1604–1610. [Google Scholar] [CrossRef]
- Morell, M.; Voit, B.; Ramis, X.; Serra, À.; Lederer, A. Synthesis, characterization, and rheological properties of multiarm stars with poly(glycidol) core and poly(methyl methacrylate) arms by AGET ATRP. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3138–3151. [Google Scholar] [CrossRef]
- Alcón, M.J.; Ribera, G.; Galià, M.; Cádiz, V. Synthesis, characterization and polymerization of isobutylbis (glycidylpropylether) phosphine oxide. Polymer 2003, 44, 7291–7298. [Google Scholar] [CrossRef]
- Martín, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Synthesis of Novel Boron-Containing Epoxy–Novolac Resins and Properties of Cured Products. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6332–6344. [Google Scholar] [CrossRef]
- Chen, M.; Lin, X.; Liu, C.; Zhang, H. An effective strategy to enhance the flame retardancy and mechanical properties of epoxy resin by using hyperbranched flame retardant. J. Mater. Sci. 2021, 56, 5956–5974. [Google Scholar] [CrossRef]
- Ye, G.; Huo, S.; Wang, C.; Shi, Q.; Yu, L.; Liu, Z.; Fang, Z.; Wang, H. A novel hyperbranched phosphorus-boron polymer for transparent, flame-retardant, smoke-suppressive, robust yet tough epoxy resins. Compos. Part B Eng. 2021, 227, 109395. [Google Scholar] [CrossRef]
- Huo, S.; Sai, T.; Ran, S.; Guo, Z.; Fang, Z.; Song, P.; Wang, H. A hyperbranched P/N/B-containing oligomer as multifunctional flame retardant for epoxy resins. Compos. Part B Eng. 2022, 234, 109701. [Google Scholar] [CrossRef]
- Sun, D.; Yao, Y. Synthesis of three novel phosphorus-containing flame retardants and their application in epoxy resins. Polym. Degrad. Stab. 2011, 96, 1720–1724. [Google Scholar] [CrossRef]
- Pan, M.; Huang, R.; Wang, T.; Huang, D.; Mu, J.; Zhang, C. Preparation and properties of epoxy resin composites containing hexaphenoxycyclotriphosphazene. High Perform. Polym. 2014, 26, 114–121. [Google Scholar] [CrossRef]
- Carja, I.D.; Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Coroaba, A.; Lisa, G.; Guillem, C.; Fuensanta, M.; Forrat, V.; Romero, M.D. A straightforward, eco-friendly and cost-effective approach towards flame retardant epoxy resins. J. Mater. Chem. A 2014, 2, 16230–16241. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.; Wang, S.; Liu, X.; Hu, J.; Yi, X.; Liu, X. Hyperbranched flame retardant to simultaneously improve the fire-safety, toughness and glass transition temperature of epoxy resin. Eur. Polym. J. 2021, 157, 110638. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.; Wang, S.; Liu, X.; Hu, J.; Yi, X.; Liu, X. Hyperbranched flame retardant for epoxy resin modification: Simultaneously improved flame retardancy, toughness and strength as well as glass transition temperature. Chem. Eng. J. 2021, 428, 131226. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, S.; Shi, W. Effect of molecular chain structure of the cured epoxy resin containing hyperbranched (3-hydroxyphenyl) phosphate on expansion and flame retardance. J. Appl. Polym. Sci. 2004, 94, 2065–2070. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, W. Synthesis and thermal decomposition of a novel hyperbranched polyphosphate ester used for flame retardant systems. Polym. Degrad. Stab. 2006, 91, 1289–1294. [Google Scholar] [CrossRef]
- Zang, L.; Wagner, S.; Ciesielski, M.; Müller, P.; Döring, M. Novel star-shaped and hyperbranched phosphorus-containing flame retardants in epoxy resins. Polym. Adv. Technol. 2001, 22, 1182–1191. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Li, S.; Sun, J. Flame retardant epoxy resins from bisphenol-A epoxy cured with hyperbranched polyphosphate ester. J. Polym. Res. 2011, 18, 2229–2237. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Yu, B.; Wang, J.; Wang, C.; Zeng, W.; Hu, Y. Economical and environment-friendly synthesis of a novel hyperbranched poly(aminomethylphosphine oxide-amine) as co-curing agent for simultaneous improvement of fire safety, glass transition temperature and toughness of epoxy resins. Chem. Eng. J. 2017, 322, 618–631. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Wang, J.; Sheng, H.; Zhang, Y.; Hu, W. Facile synthesis of a novel hyperbranched poly(urethane-phosphine oxide) as an effective modifier for epoxy resin. Polym. Degrad. Stab. 2018, 154, 157–169. [Google Scholar] [CrossRef]
- Täuber, K.; Marsico, F.; Wurm, F.R.; Schartel, B. Hyperbranched poly(phosphoester)s as flame retardants for technical and high performance polymers. Polym. Chem. 2014, 5, 7042–7053. [Google Scholar] [CrossRef]
- Markwart, J.C.; Battig, A.; Zimmermann, L.; Wagner, M.; Fischer, J.; Schartel, B.; Wurm, F.R. Systematically Controlled Decomposition Mechanism in Phosphorus Flame Retardants by Precise Molecular Architecture: P–O vs P–N. ACS. Appl. Polym. Mater. 2019, 1, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Batting, A.; Müller, P.; Bertin, A.; Schartel, B. Hyperbranched Rigid Aromatic Phosphorus-Containing Flame Retardants for Epoxy Resins. Macromol. Mater. Eng. 2021, 306, 2000731. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Matrix matters: Hyperbranched flame retardants in aliphatic and aromatic epoxy resins. Polym. Degrad. Stab. 2019, 170, 108986. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Hyperbranched phosphorus flame retardants: Multifunctional additives for epoxy resins. Polym. Chem. 2019, 10, 4346–4358. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yang, H.; Jiang, Y.; He, H.; Liu, H.; Huang, H.; Wan, C. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef]
- Müller, P.; Bykov, Y.; Döring, M. New star-shaped phosphorus-containing flame retardants based on acrylates for epoxy resins. Polym. Adv. Technol. 2013, 24, 834–840. [Google Scholar] [CrossRef]

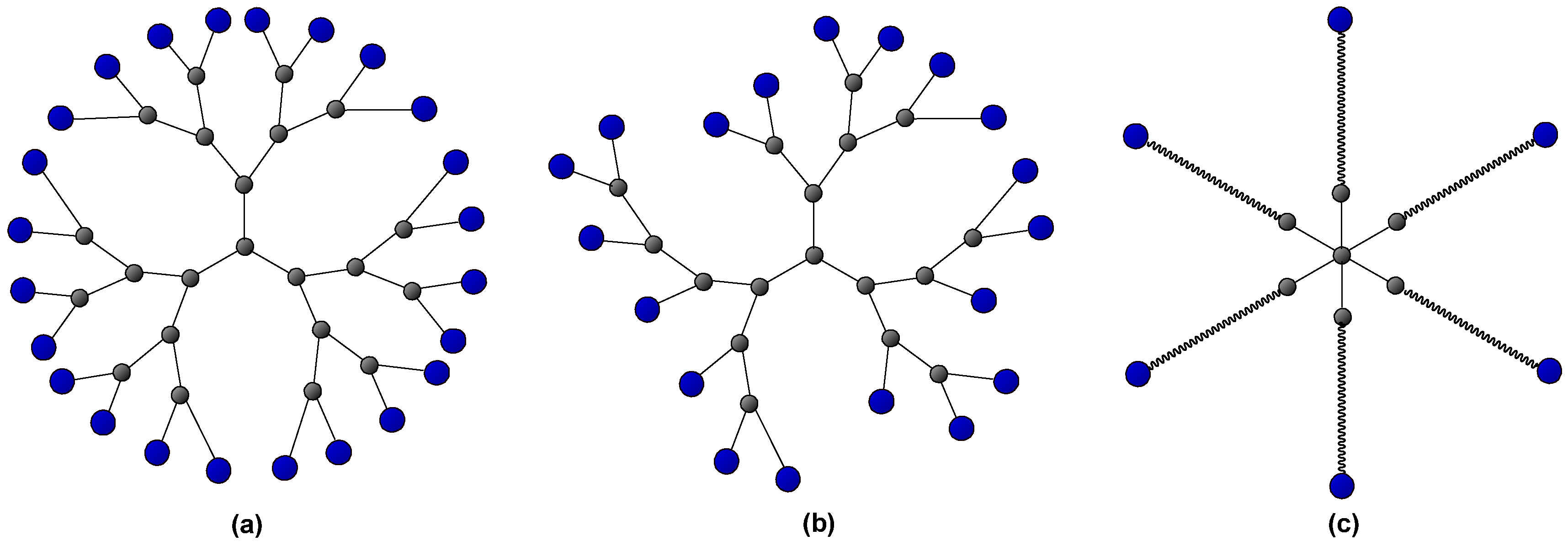


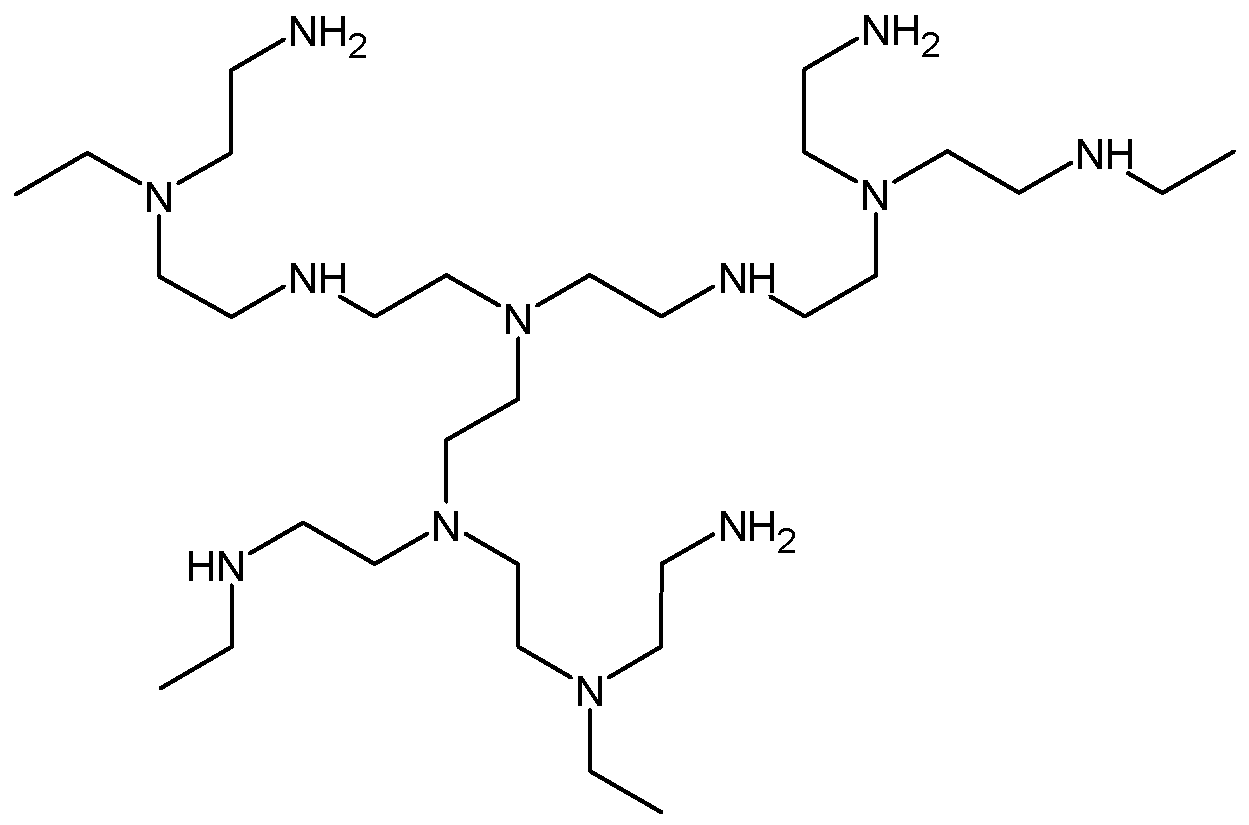
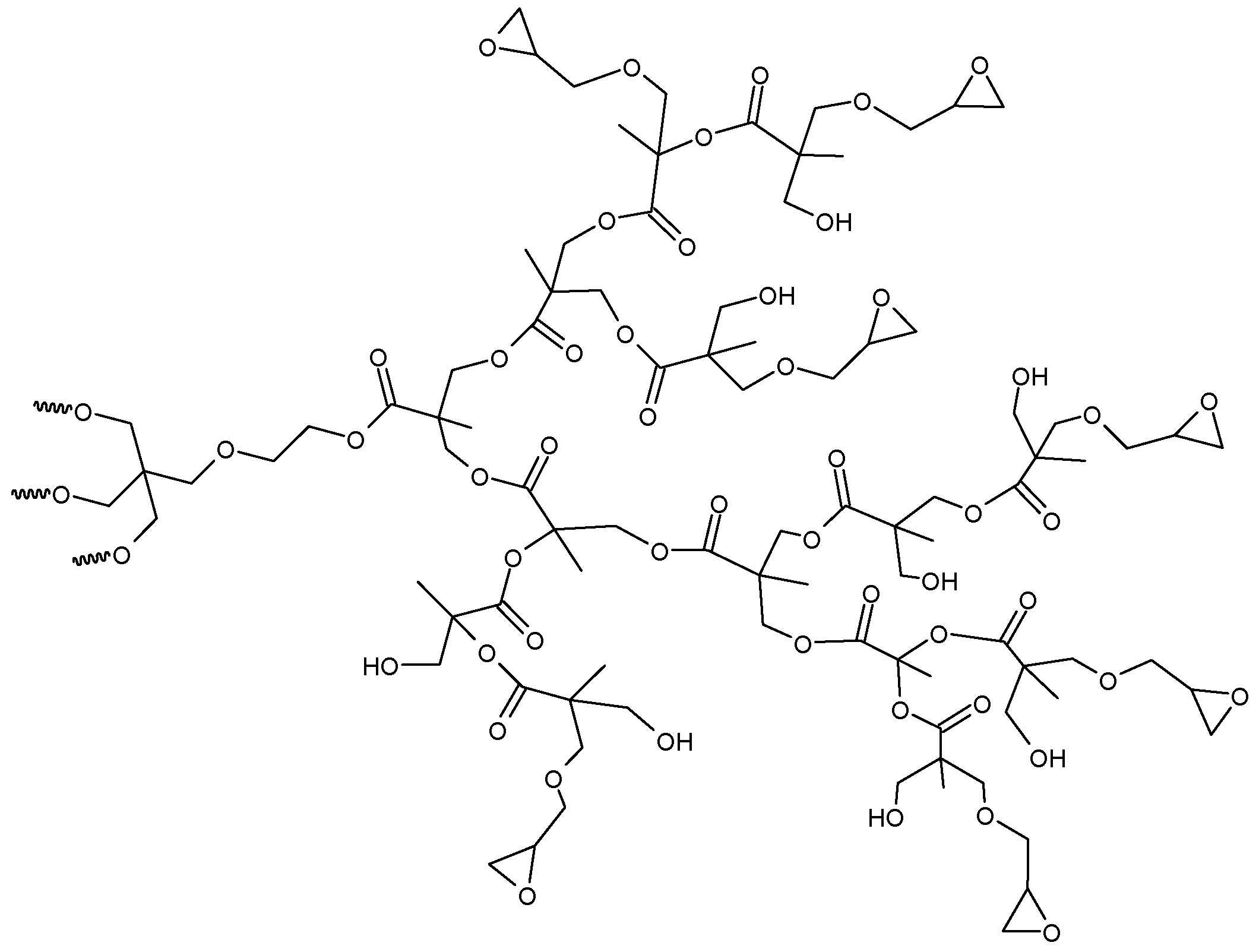
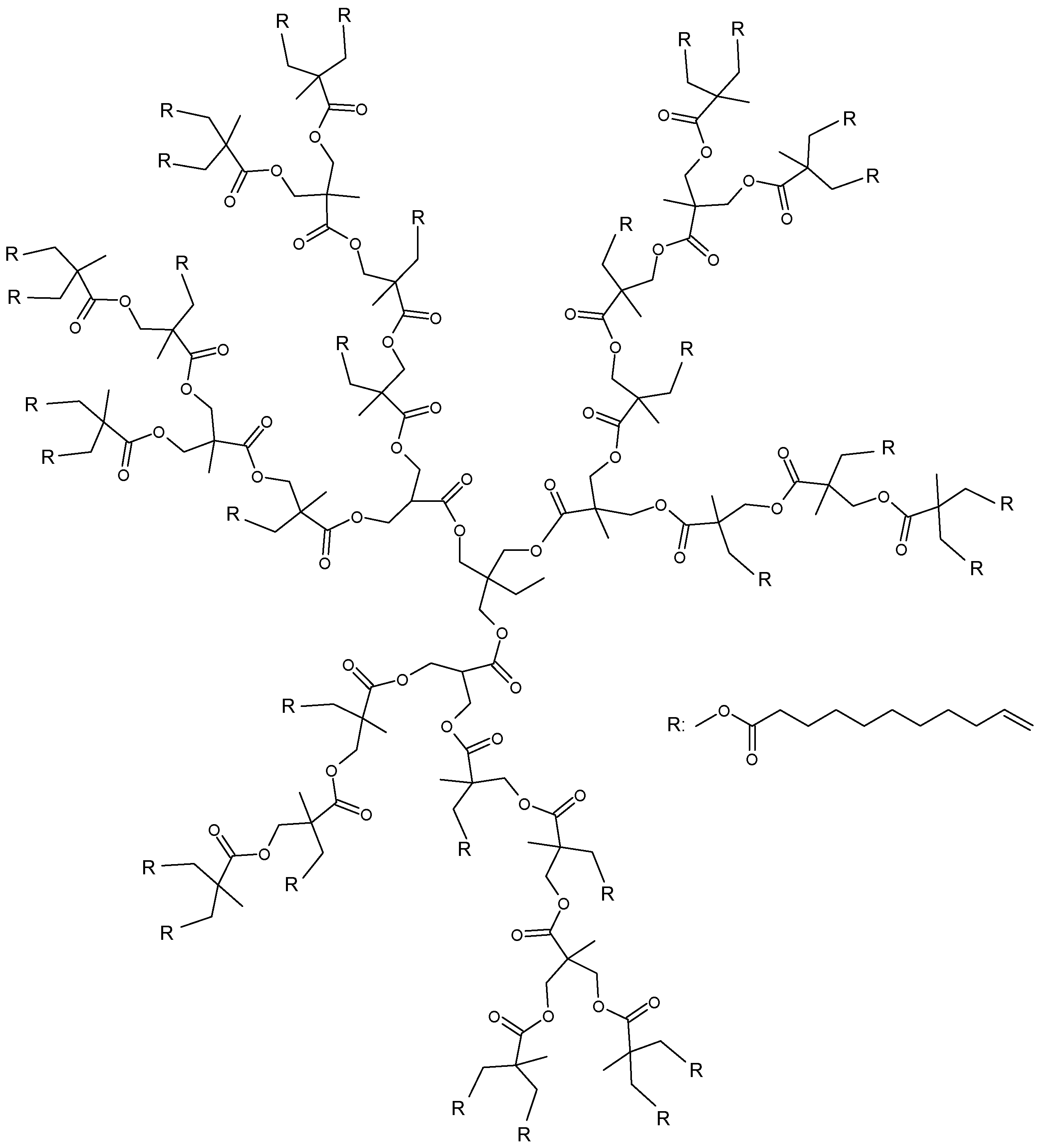




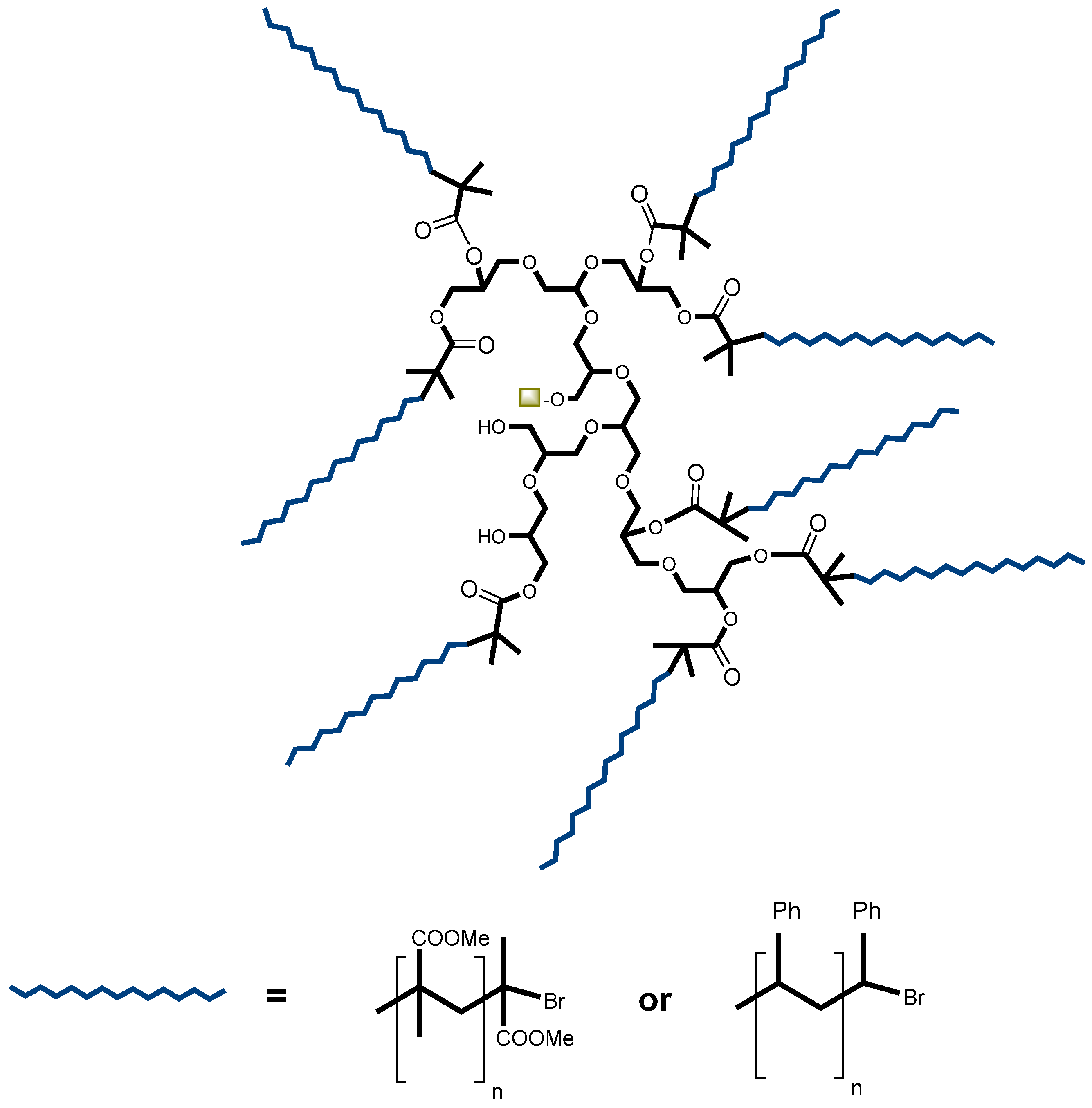
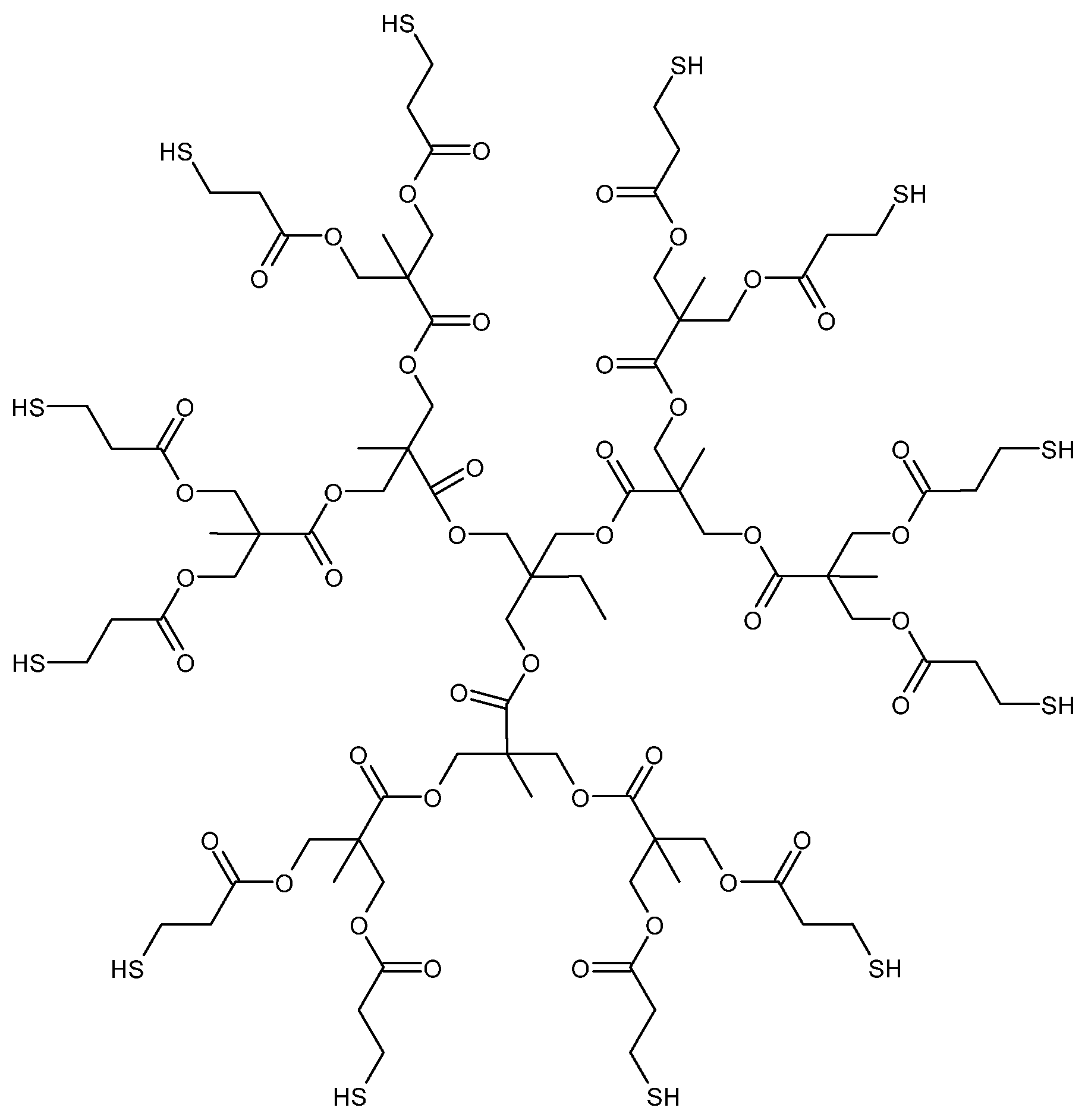
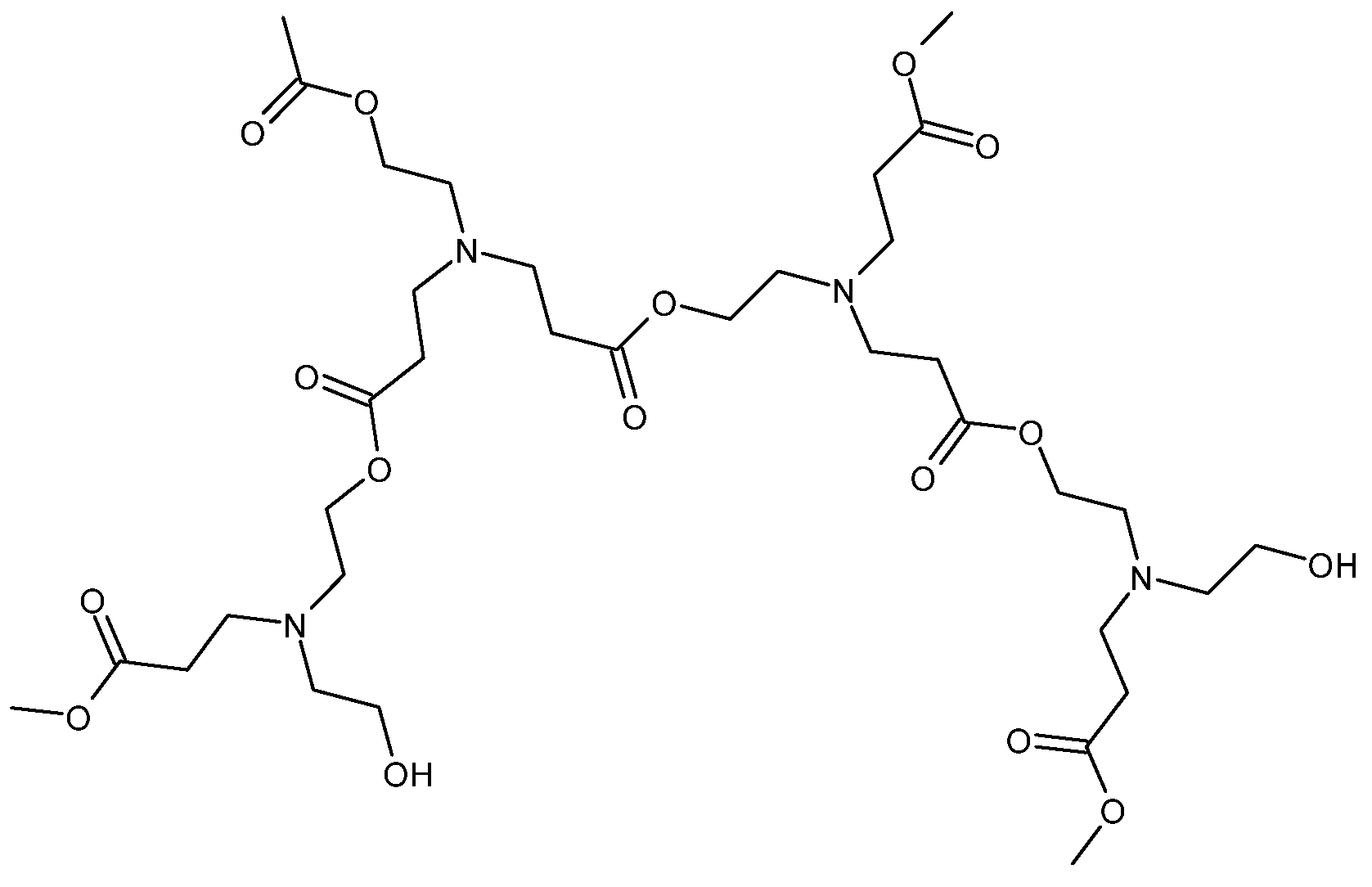
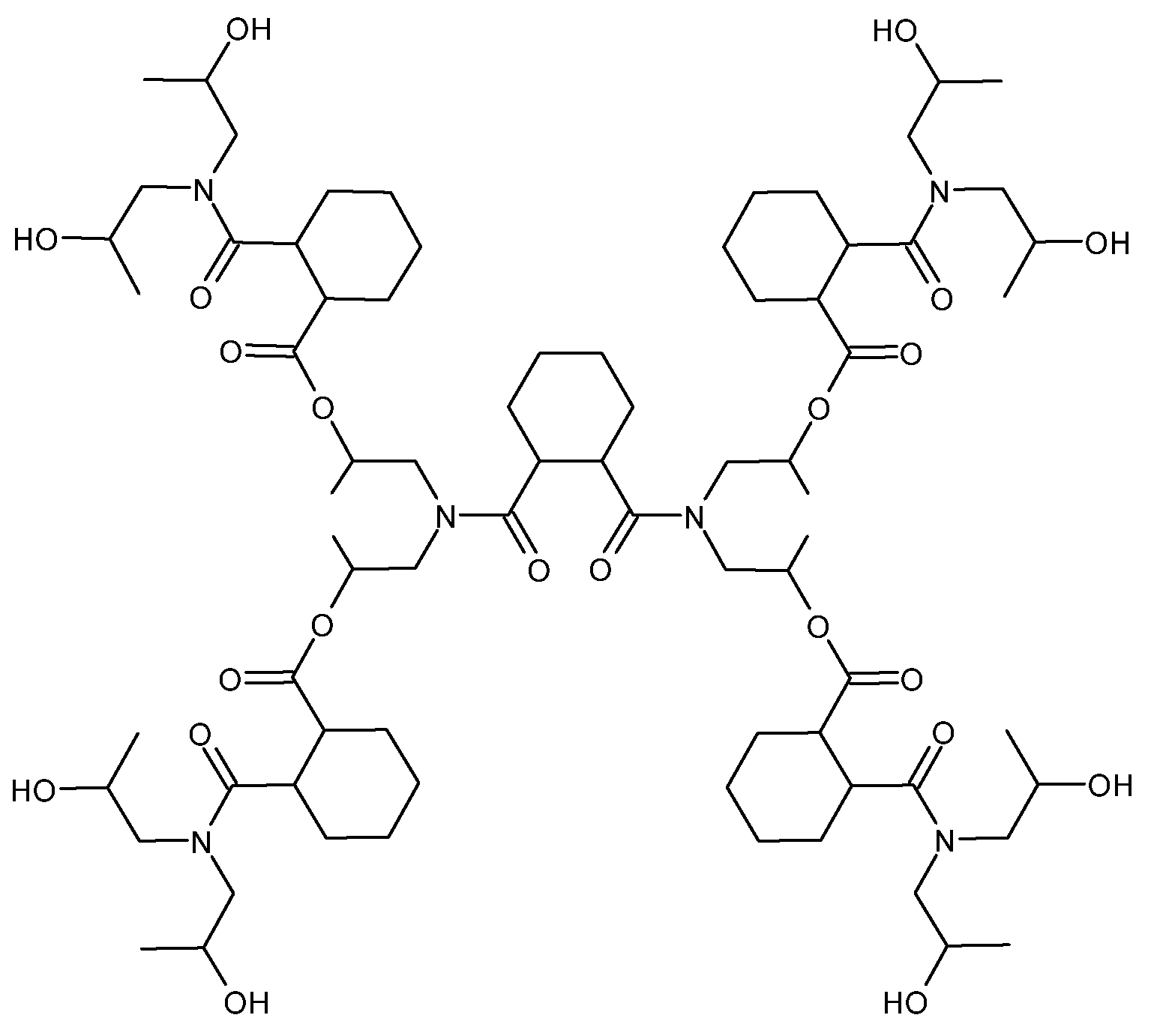

| Effect on the curing process | Hydroxyl-ended HBPs | Epoxy/amine | [46,47,48] | |
| Epoxy/anhydride | Acceleration due to the presence of OH groups | [53,55,56] | ||
| UV-polymerization | [76,77,78,79,80] | |||
| Amino-ended HBPs | Increase in reaction rate | [64,65] | ||
| Epoxy-ended HBPs | No modification | [44] | ||
| Allyl-modified HBPs | Epoxy/anhydride | Opposite effects from OH/increased viscosity | [81,82] | |
| Deceleration | [83] | |||
| Acceleration due the presence of residual COOH groups and tertiary amines | [84] | |||
| Acceleration due to the presence of tertiary amines | [86,87,88] | |||
| Other functional groups | Benzoylated HBPs | [43] | ||
| t-butyl, phenyl HBPs | [89] | |||
| Carboxyl HBPs | [90] | |||
| HBPs as macroinitiators | [93,94] | |||
| Comparison between HBP and linear analogous | [65,96] | |||
| MASP with ε-polycaprolactone arms | High amounts of MASP cause a deceleration due to the increased viscosity | [98,99,100,101] | ||
| MASP with poly(styrene) arms | [103] | |||
| MASP with poly(methyl methacrylate) arms | [104] | |||
| Thermal properties | Reduction in the Tg due to the decrease in crosslinked density (low % of HBP or molar mass) Little reduction in the Tg due to the phase segregation (high % of HBP or molar mass) | [106,109] | ||
| Enhanced reworkability | Hyperbranched poly(ester amide)s | [56,115] | ||
| MASP with ε-polycaprolactone arms | [98,99,102,119] | |||
| Chemical reworkability | [117,118] | |||
| Mechanical properties | Epoxy-ended HBPs | Mechanical properties | HBP does not form a segregate phase: toughening effect moderate HBP forms a segregate phase: high toughening effect. | [26,27,44,67,68,69,70] |
| HBP does not form a segregate phase, but the toughening effect is good due to the good compatibility. | [105,130,131,132,133,134,135,136,137] | |||
| Hydroxyl-ended HBPs | Mechanical properties | HBP forms a segregate phase, but the toughening effect is moderate due to poor compatibility. | [48,129] | |
| HBP does not form a segregate phase, but the toughening effect is good due to good compatibility. | [56,115] | |||
| Shrinkage reduction | [50,53,56] | |||
| Allyl-modified HBPs | Mechanical properties | Enhanced compatibility between matrix and HBPs. | [83,86,141] | |
| Other functional groups | Mechanical properties | [90,108,138,139,140] | ||
| MASP | Mechanical properties | [17,98,142] | ||
| Rheological properties | HBP increases the viscosity of the formulation | [52,95,144] | ||
| HBP decreases the viscosity of the formulation | [108,145,146] | |||
| Decreases gelation time and increases conversion at the gelation | [52,55,86,96] | |||
| Effect of MASPs on the viscosity of the formulation | [102,142,151] | |||
| Fire retardancy | Phosphorus-containing HBPs | [162,163,164,165,166,167,168,169,170,171,172,173,174] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, D.; Serra, À. Enhancement of Epoxy Thermosets with Hyperbranched and Multiarm Star Polymers: A Review. Polymers 2022, 14, 2228. https://doi.org/10.3390/polym14112228
Santiago D, Serra À. Enhancement of Epoxy Thermosets with Hyperbranched and Multiarm Star Polymers: A Review. Polymers. 2022; 14(11):2228. https://doi.org/10.3390/polym14112228
Chicago/Turabian StyleSantiago, David, and Àngels Serra. 2022. "Enhancement of Epoxy Thermosets with Hyperbranched and Multiarm Star Polymers: A Review" Polymers 14, no. 11: 2228. https://doi.org/10.3390/polym14112228







