Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Microorganisms’ Strains
2.2. Methods
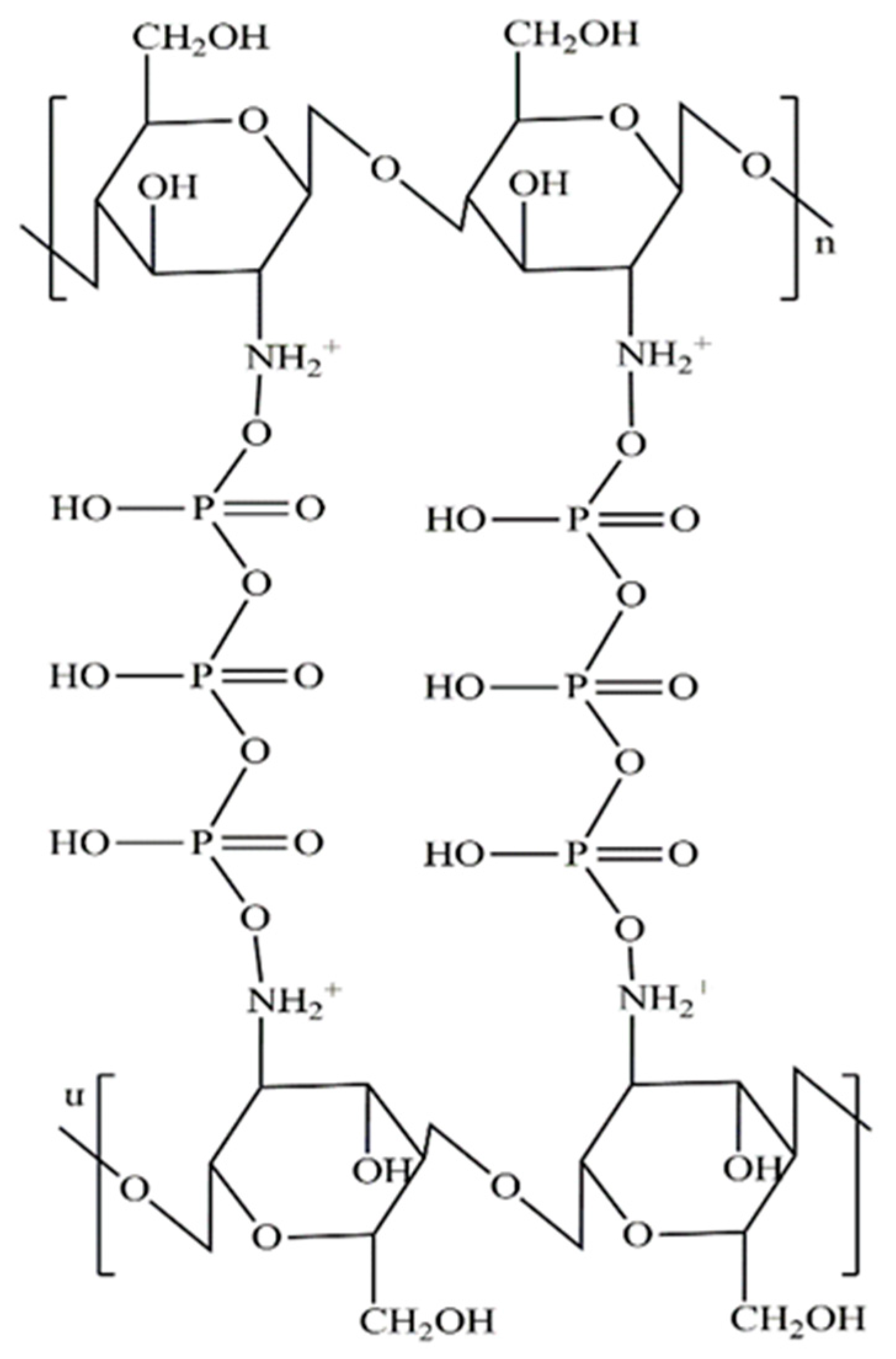
2.2.1. Preparation of Chitosan Nanoparticles (CHNPs)
2.2.2. Treatment Solution Preparation and Application (as Edible Coatings)
2.2.3. Characterization of the Chitosan Nanoparticles
Transmission Electron Microscopic (TEM)
Antimicrobial Activity
2.2.4. Quality Criteria during Storage
3. Results
3.1. Characterization Results
3.1.1. Chitosan Nanoparticle Morphology and Particle Size
3.1.2. Antimicrobial Activity of the Chitosan Nanoparticles
3.2. The Impact of Coatings on Apricot Fruit Quality during Preservation
3.2.1. Weight Losses
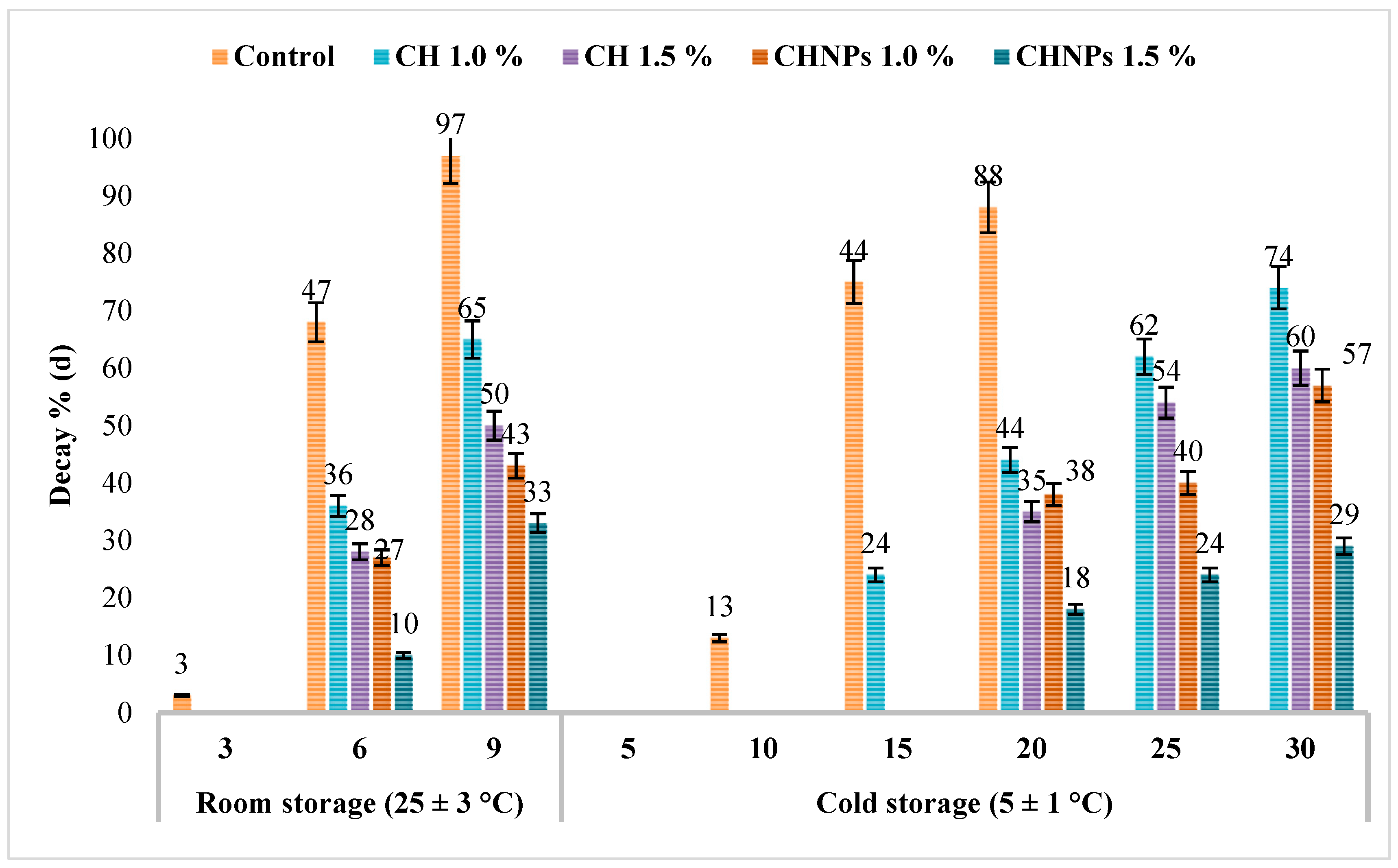
3.2.2. Percentage of Visual Decay
3.2.3. Total Soluble Solids
3.2.4. Total Acidity Content
3.2.5. Content of Ascorbic Acid
3.2.6. Carotenoid Content
3.2.7. Lipid Peroxidation
3.2.8. Sensory Quality Criteria
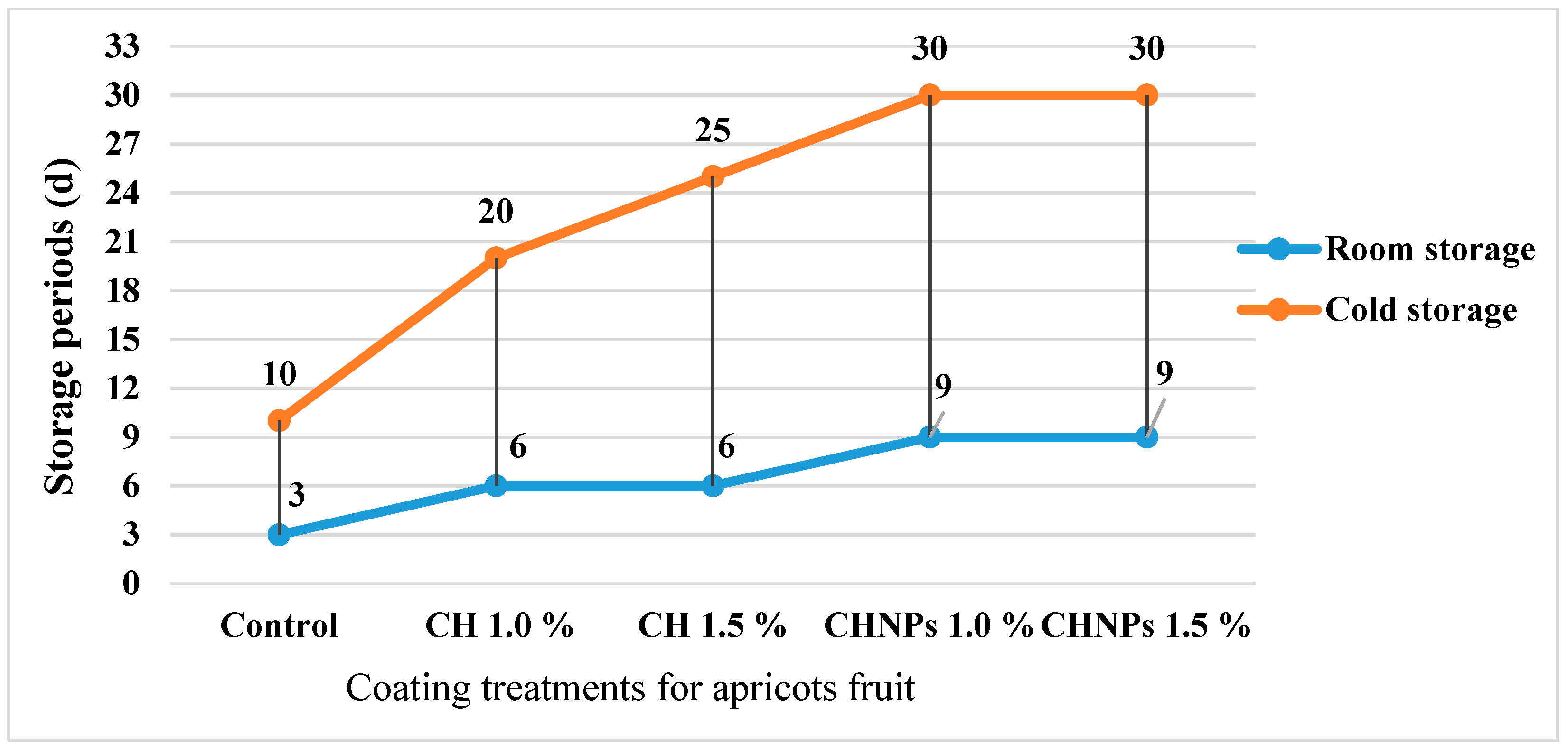
3.2.9. Apricot Shelf-Life
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Deng, J.L.; Tian, Y. Transcriptome Sequencing of the Apricot (Prunus Armeniaca L.) and Identification of Differentially Expressed Genes Involved in Drought Stress. Phytochemistry 2020, 171, 112226. [Google Scholar] [CrossRef] [PubMed]
- Okba, S.K.; Mazrou, Y.; Elmenofy, H.M.; Ezzat, A.; Salama, A.M. New Insights of Potassium Sources Impacts as Foliar Application on ‘Canino’ Apricot Fruit Yield, Fruit Anatomy, Quality and Storability. Plants 2021, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Ahmed Mahmoud, G.; Ahmed Mahmoud, R. Effect of Edible Coating on Storability and Quality of Apricot Fruits. J. Hortic. Sci. Ornam. Plants 2019, 11, 38–51. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving Fresh Apricot (Prunus Armeniaca L.) Quality and Antioxidant Capacity by Storage at near Freezing Temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Srivastav, M.; Varghese, E. Effect of Chitosan Coating on Postharvest Life and Quality of Plum during Storage at Low Temperature. Sci. Hortic. 2017, 226, 104–109. [Google Scholar] [CrossRef]
- Cui, K.; Zhao, H.; Sun, L.; Yang, L.; Cao, J.; Jiang, W. Impact of near Freezing Temperature Storage on Postharvest Quality and Antioxidant Capacity of Two Apricot (Prunus Armeniaca L.) Cultivars. J. Food Biochem. 2019, 43, e12857. [Google Scholar] [CrossRef] [PubMed]
- Arnon-Rips, H.; Poverenov, E. Improving Food Products’ Quality and Storability by Using Layer by Layer Edible Coatings. Trends Food Sci. Technol. 2018, 75, 81–92. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Pérez-Larios, A.; Ruvalcaba-Gómez, J.M.; Sánchez-Burgos, J.A.; Romero-Toledo, R.; Montalvo-González, E. Funcionalización de Los Recubrimientos a Base de Quitosano Para La Conservación Postcosecha de Frutas y Hortalizas. TIP Revista Especializada en Ciencias Químico-Biológicas 2020, 23, 1–14. Available online: http://Creativecommons.Org/Licenses/by-Nc-Nd/4.0/ (accessed on 25 February 2022). [CrossRef]
- Montaser, A.S.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, Characterization and Antimicrobial Activity of Schiff Bases from Chitosan and Salicylaldehyde/TiO2 Nanocomposite Membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf Life Extension of Apricot Fruit by Application of Nanochitosan Emulsion Coatings Containing Pomegranate Peel Extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef]
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; del Río-García, J.C.; Hernández-López, M.; Guillén-Sánchez, D.; Salazar-Piña, D.A.; Ramos-García, M.D.; Bautista-Baños, S. Edible Chitosan/Propolis Coatings and Their Effect on Ripening, Development of Aspergillus Flavus, and Sensory Quality in Fig Fruit, during Controlled Storage. Plants 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Singh, M.; Sureshkumar, S.; Anand, M.; Ramakritinan, C.M.; Quero, F.; Kumaraguru, A.K. Chitosan Mediated Gold Nanoparticles against Pathogenic Bacteria, Fungal Strains and MCF-7 Cancer Cells. Int. J. Biol. Macromol. 2020, 146, 560–568. [Google Scholar] [CrossRef]
- Heena, J.; Salahuddin, M.; Gazalli, H. Nanotechnology in Food Packaging. Int. J. Food Nutr. Saf. 2013, 3, 111–118. [Google Scholar]
- Shahat, M.; Mohamed, M.I.; Osheba, A.S.; Taha, I.M. Improving the Quality and Shelf-Life of Strawberries as Coated with Nano-Edible Films during Storage. J. Agric. Res. 2020, 45, 1–14. [Google Scholar]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.V.C.; Kalia, A.; Gill, K.S. Influence of Carboxy Methylcellulose, Chitosan and Beeswax Coatings on Cold Storage Life and Quality of Kinnow Mandarin Fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- Elmenofy, H.M.; Okba, S.K.; Salama, A.M.; Alam-Eldein, S.M. Yield, Fruit Quality, and Storability of ‘Canino’ Apricot in Response to Aminoethoxyvinylglycine, Salicylic Acid, and Chitosan. Plants 2021, 10, 1838. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, Z.; Wu, J.; Zhu, S. Effects of Pre-Treatment with S-Nitrosoglutathione-Chitosan Nanoparticles on Quality and Antioxidant Systems of Fresh-Cut Apple Slices. LWT 2021, 139, 110565. [Google Scholar] [CrossRef]
- Elmenofy, H.M.; Mark, C. Effect of Natural Antimicrobial Substances with Packaging System on Improving Quality of ‘ETMANI’ Guava (Psidium Guajava L.) Fruit during Cold Storage. J. Plant Prod. 2021, 12, 527–540. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Electrospun Alginate Nanofibres Impregnated with Silver Nanoparticles: Preparation, Morphology and Antibacterial Properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some Compositional Properties of Main Malatya Apricot (Prunus Armeniaca L.) Varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Fan, X.J.; Zhang, B.; Yan, H.; Feng, J.T.; Ma, Z.Q.; Zhang, X. Effect of Lotus Leaf Extract Incorporated Composite Coating on the Postharvest Quality of Fresh Goji (Lycium Barbarum L.) Fruit. Postharvest Biol. Technol. 2019, 148, 132–140. [Google Scholar] [CrossRef]
- Ezzat, A.; Ammar, A.; Szabó, Z.; Nyéki, J.; Holb, I.J. Postharvest Treatments with Methyl Jasmonate and Salicylic Acid for Maintaining Physico-Chemical Characteristics and Sensory Quality Properties of Apricot Fruit during Cold Storage and Shelf-Life. Pol. J. Food Nutr. Sci. 2017, 67, 159–166. [Google Scholar] [CrossRef]
- Jatoi, M.A.; Jurić, S.; Vidrih, R.; Vinceković, M.; Vuković, M.; Jemrić, T. The Effects of Postharvest Application of Lecithin to Improve Storage Potential and Quality of Fresh Goji (Lycium Barbarum L.) Berries. Food Chem. 2017, 230, 241–249. [Google Scholar] [CrossRef]
- Mondal, M.F. Production and Storage of Fruit; BAU Campus: Mymsningh, Bangladesh, 2000. (In Bangla) [Google Scholar]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and Characterization of Chitin and Chitosan with Antimicrobial and Antioxidant Activities from Cosmopolitan Orthoptera Species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol. 2009, 69, 500–506. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure–property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Carlos-Salazar, M.J.; Valderrama-Negron, A.C. Release of anthocyanins from chitosan films cross-linked with sodium tripolyphosphate. Rev. Soc. Química Del Perú 2017, 83, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2021, 172, 108367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of Soybean Protein Isolate-Chitosan Edible Coating on the Softening of Apricot Fruit during Storage. LWT 2018, 96, 604–611. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf Life Extension of White Mushrooms (Agaricus Bisporus) by Low Temperatures Conditioning, Modified Atmosphere, and Nanocomposite Packaging Material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Göttingerová, M.; Kumšta, M.; Rampáčková, E.; Kiss, T.; Nečas, T. Analysis of Phenolic Compounds and Some Important Analytical Properties in Selected Apricot Genotypes. HortScience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical Characteristics of Wild and Cultivated Apricots (Prunus Armeniaca L.) from Aras Valley in Turkey. Genet. Resour. Crop Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.B.; Palou, L.; Maraschin, M.; Monteiro, A.R. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Xue, J. The quality evaluation of postharvest strawberries stored in nano-Ag packages at refrigeration temperature. Polymers 2018, 10, 894. [Google Scholar] [CrossRef] [Green Version]
- Taha, I.M.; Zaghlool, A.; Nasr, A.; Nagib, A.; El Azab, I.H.; Mersal, G.A.M.; Ibrahim, M.M.; Fahmy, A. Impact of Starch Coating. Embedded with Silver Nanoparticles on Strawberry Storage Time. Polymers 2022, 14, 1439. [Google Scholar] [CrossRef]
- Abd El-Khalek, A.F.; El-Abbasy, U.K.; Ismail, M.I. Postharvest Applications of 1-Methylcyclopropene and Salicylic Acid for Maintaining Quality and Enhancing Antioxidant Enzyme Activity of Apricot Fruits Cv. ‘Canino’ During Cold Storage. Egypt. J. Hortic. 2018, 45, 1–23. [Google Scholar] [CrossRef]
- Ishaq, S.; Rathore, H.A.; Majeed, S.; Awan, S.; Ali Shah, S.Z. The Studies on the Physico-Chemical and Organoleptic Characteristics of Apricot (Prunus Armeniaca L.) Produced in Rawalakot, Azad Jammu and Kashmir during Storage. Pak. J. Nutr. 2009, 8, 856–860. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, A.; Niro, S.; Messia, M.C.; Cinquanta, L.; Panfili, G.; Albanese, D.; Di Matteo, M. Kinetics of Carotenoids Degradation and Furosine Formation in Dried Apricots (Prunus Armeniaca L.). Food Res. Int. 2017, 99, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Masud, T.; Abbasi, K.S. Physico-Chemical Characteristics of Apricot (Prunus Armeniaca L.) Grown in Northern Areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S. Influence of collapsed structure on stability of β-carotene in freeze-dried mangoes. Food Res. Int. 2011, 44, 3188–3194. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin Treatment Delays Postharvest Senescence and Regulates Reactive Oxygen Species Metabolism in Peach Fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Adiletta, G.; Pasquariello, M.S.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Petriccione, M. Chitosan Coating: A Postharvest Treatment to Delay Oxidative Stress in Loquat Fruits during Cold Storage. Agronomy 2018, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of Chitosan-Beeswax Edible Coatings on the Quality of Fresh Strawberries (Fragaria Ananassa Cv Camarosa) under Commercial Storage Conditions. LWT—Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Manoj, H.; Sreenivas, K.; Shankarappa, T.; Krishna, H. Studies on Chitosan and Aloe Vera Gel Coatings on Biochemical Parameters and Microbial Population of Bell Pepper (Capsicum Annuum L.) under Ambient Condition. Available online: https://www.researchgate.net/profile/Shankarappa-Hanumaiah/publication/289882301_Studies_on_Chitosan_and_Aloe_vera_Gel_Coatings_on_Biochemical_Parameters_and_Microbial_Population_of_Bell_Pepper_Capsicum_annuum_L_Under_Ambient_Condition/links/56934c9b08aed (accessed on 4 April 2022).
- Moradinezhad, F.; Jahani, M. Effect of Potassium Permanganate, 1-Methylcyclopropene and Modified Atmosphere Packaging on Postharvest Losses and Quality of Fresh Apricot Cv. Shahroudi. J. Hortic. Postharvest Res. 2019, 2, 39–48. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly (Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]



| Treatment | Storage Period (Day) at 25 ± 3 °C | Storage Period (Day) at 5 ± 1 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Control | 1.58 Ca | 8.00 Ba | 13.96 Aa | 1.30 Da | 3.97 Ca | 6.40 Ba | 8.86 Aa | ND | ND | |
| CH % | 1.0 | 1.48 Ca | 5.55 Bb | 8.81 Ab | 1.22 Fab | 2.89 Eb | 4.55 Db | 5.90 Cb | 7.15 Ba | 10.30 Aa |
| 1.5 | 1.42 Ca | 5.04 Bc | 7.73 Ac | 1.06 Fb | 2.78 Ebc | 4.33 Dc | 5.71 Cc | 6.87 Bb | 9.50 Ab | |
| CHNPs % | 1.0 | 1.45 Ca | 4.91 Bc | 7.64 Ac | 1.14 Fab | 2.81 Ebc | 4.29 Dc | 5.47 Cd | 6.72 Bb | 9.06 Ab |
| 1.5 | 1.50 Ca | 4.72 Bc | 6.75 Ad | 1.08 Fb | 2.62 Ec | 4.18 Dc | 5.04 Ce | 5.97 Bc | 8.48 Ac | |
| Treatments | Storage Period (Day) at 25 ± 3 °C | Storage Period (Day) at 5 ± 1 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Control | 10.39 Ba | 12.91 Aa | ND | ND | 10.39 Ca | 12.28 Ba | 14.41 Aa | 14.61 Aa | ND | ND | ND | |
| CH % | 1.0 | 10.49 Ca | 12.00 Bb | 15.09 Aa | 14.82 Aa | 10.49 Ca | 12.19 Ba | 13.96 Aab | 14.44 Aab | 14.52 Aa | ND | ND |
| 1.5 | 10.48 Ca | 11.79 Bb | 14.88 Aa | 15.16 Aa | 10.48 Ea | 11.95 Da | 13.68 Cb | 14.27 ABab | 14.39 ABa | 14.50 Aa | 14.09 BCb | |
| CHNPs % | 1.0 | 10.50 Ca | 11.86 Bb | 14.90 Aa | 15.35 Aa | 10.50 Da | 11.99 Ca | 13.85 Bab | 14.34 ABab | 14.48 Aa | 14.59 Aa | 14.75 Aa |
| 1.5 | 10.49 Da | 11.65 Cb | 14.75 Ba | 15.22 Aa | 10.49 Da | 11.82 Ca | 13.77 Bb | 13.82 Bb | 14.15 Ba | 14.53 ABa | 14.72 Aa | |
| Treatments | Storage Period (Day) at 25 ± 3 °C | Storage Period (Day) at 5 ± 1 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Control | 1.04 Aa | 0.86 Ba | ND | ND | 1.04 Aa | 0.90 Aa | 0.75 Bc | 0.57 Cb | ND | ND | ND | |
| CH % | 1.0 | 1.06 Aa | 0.95 Aa | 0.69 Ba | 0.46 Cb | 1.06 Aa | 0.94 ABa | 0.90 Bab | 0.79 BCa | 0.69 Ca | ND | ND |
| 1.5 | 1.06 Aa | 0.94 Aa | 0.74 Ba | 0.58 Ca | 1.06 Aa | 0.97 ABa | 0.96 ABa | 0.83 BCa | 0.69 CDa | 0.60 DEa | 0.50 Ea | |
| CHNPs % | 1.0 | 1.06 Aa | 0.94 Aa | 0.71 Ba | 0.61 Ba | 1.06 Aa | 0.99 ABa | 0.96 ABa | 0.85 BCa | 0.74 CDa | 0.63 DEa | 0.48 Ea |
| 1.5 | 1.06 Aa | 0.98 Aa | 0.77 Ba | 0.68 Ba | 1.06 Aa | 1.03 Aa | 1.00 Aa | 0.92 ABa | 0.80 BCa | 0.70 CDa | 0.59 Da | |
| Treatments | Storage Period (Day) at 25 ± 3 °C | Storage Period (Day) at 5 ± 1 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Control | 113.8 Aa | 98.2 Bb | ND | ND | 113.8 Aa | 101.0 Ba | 82.3 Cc | 61.1 Dc | ND | ND | ND | |
| CH % | 1.0 | 113.8 Aa | 99.3 Bab | 76.1 Cc | 60.8 Dc | 113.8 Aa | 102.3 Ba | 92.8 Cb | 81.9 Db | 70.7 Ec | ND | ND |
| 1.5 | 113.8 Aa | 101.1 Ba | 78.8 Cb | 64.1 Db | 113.8 Aa | 102.9 Ba | 94.7 Ca | 83.8 Db | 74.8 Eb | 63.0 Fb | 51.2 Gb | |
| CHNPs % | 1.0 | 113.8 Aa | 102.6 Ba | 77.4 Cbc | 65.4 Db | 113.8 Aa | 101.8 Ba | 94.4 Ca | 85.5 Db | 74.3 Eb | 64.9 Fb | 53.1 Gb |
| 1.5 | 113.8 Aa | 101.9 Ba | 82.0 Ca | 68.7 Da | 113.8 Aa | 102.4 Ba | 96.0 Ca | 88.6 Da | 80.5 Ea | 75.1 Fa | 64.5 Ga | |
| Treatments | Room Temperature Storage at 25 ± 3 °C | Cold Storage at 5 ± 1 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Control | 111.6 Ba | 119.6 Aa | ND | ND | 111.6 Ca | 115.9 Ba | 128.5 Aa | 118.8 Bc | ND | ND | ND | |
| CH (%) | 1.0 | 111.6 Ca | 116.8 Ba | 129.0 Aab | 115.1 Bc | 111.6 Ca | 115.0 Ca | 122.8 Bbc | 134.2 Aa | 119.7 Bb | ND | ND |
| 1.5 | 111.6 Da | 115.0 Ca | 131.7 Aa | 124.3 Bb | 111.6 Ea | 116.6 DEa | 121.3 Dbc | 129.1 Cab | 138.3 Ba | 147.3 Aa | 137.5 Bc | |
| CHNPs (%) | 1.0 | 111.6 Ba | 115.5 Ba | 124.5 Ab | 128.5 Ab | 111.6 Da | 115.8 Da | 126.4 Cab | 131.6 Cab | 142.6 Ba | 154.4 Aa | 152.3 Ab |
| 1.5 | 111.6 Ca | 114.3 Ca | 125.6 Bab | 144.7 Aa | 111.6 Fa | 114.8 EFa | 119.1 Ec | 125.8 Dbc | 136.2 Ca | 148.7 Ba | 161.46 Aa | |
| Treatments | Storage Periods (Day) at 25 ± 3 °C | Storage Periods (Day) at 5 ± 1 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Control | 8.46 Ba | 8.95 Aa | 3.87 Cd | ND | 8.46 Ba | 8.93 Aa | 6.38 Cb | 4.25 Dd | 2.22 Ee | ND | ND | |
| CH (%) | 1.0 | 8.59 Aa | 8.91 Aa | 6.63 Bc | 4.21 Cd | 8.59 Ba | 8.92 Aa | 8.89 ABa | 7.11 Cc | 5.62 Dd | 3.38 Ed | ND |
| 1.5 | 8.58 Aa | 8.88 Aa | 6.94 Bc | 4.94 Cc | 8.58 Aa | 8.76 Aa | 8.84 Aa | 7.38 Bc | 6.27 Cc | 5.10 Dc | 4.03 Ec | |
| CHNPs (%) | 1.0 | 8.58 Aa | 8.90 Aa | 7.27 Bb | 5.44 Cb | 8.58 Aa | 8.81 Aa | 8.91 Aa | 7.78 Bb | 6.80 Cb | 5.83 Db | 5.05 Eb |
| 1.5 | 8.59 ABa | 8.84 Aa | 8.31 Ba | 6.26 Ca | 8.59 Aa | 8.71 Aa | 8.85 Aa | 8.26 Aa | 7.33 Ba | 6.49 Ca | 5.53 Da | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarni, E.H.A.; Elnaggar, I.A.; Abd El-wahed, A.E.-w.N.; Taha, I.M.; AL-Jumayi, H.A.; Elhamamsy, S.M.; Mahmoud, S.F.; Fahmy, A. Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits. Polymers 2022, 14, 2227. https://doi.org/10.3390/polym14112227
Algarni EHA, Elnaggar IA, Abd El-wahed AE-wN, Taha IM, AL-Jumayi HA, Elhamamsy SM, Mahmoud SF, Fahmy A. Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits. Polymers. 2022; 14(11):2227. https://doi.org/10.3390/polym14112227
Chicago/Turabian StyleAlgarni, Eman H. A., Ibrahim A. Elnaggar, Abd El-wahed N. Abd El-wahed, Ibrahim M. Taha, Huda A. AL-Jumayi, Sam M. Elhamamsy, Samy F. Mahmoud, and Alaa Fahmy. 2022. "Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits" Polymers 14, no. 11: 2227. https://doi.org/10.3390/polym14112227
APA StyleAlgarni, E. H. A., Elnaggar, I. A., Abd El-wahed, A. E.-w. N., Taha, I. M., AL-Jumayi, H. A., Elhamamsy, S. M., Mahmoud, S. F., & Fahmy, A. (2022). Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits. Polymers, 14(11), 2227. https://doi.org/10.3390/polym14112227









