The Properties of Poly(ester amide)s Based on Dimethyl 2,5-Furanedicarboxylate as a Function of Methylene Sequence Length in Polymer Backbone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Copoly(amide-esters)
2.2. Characterization Methods
3. Results
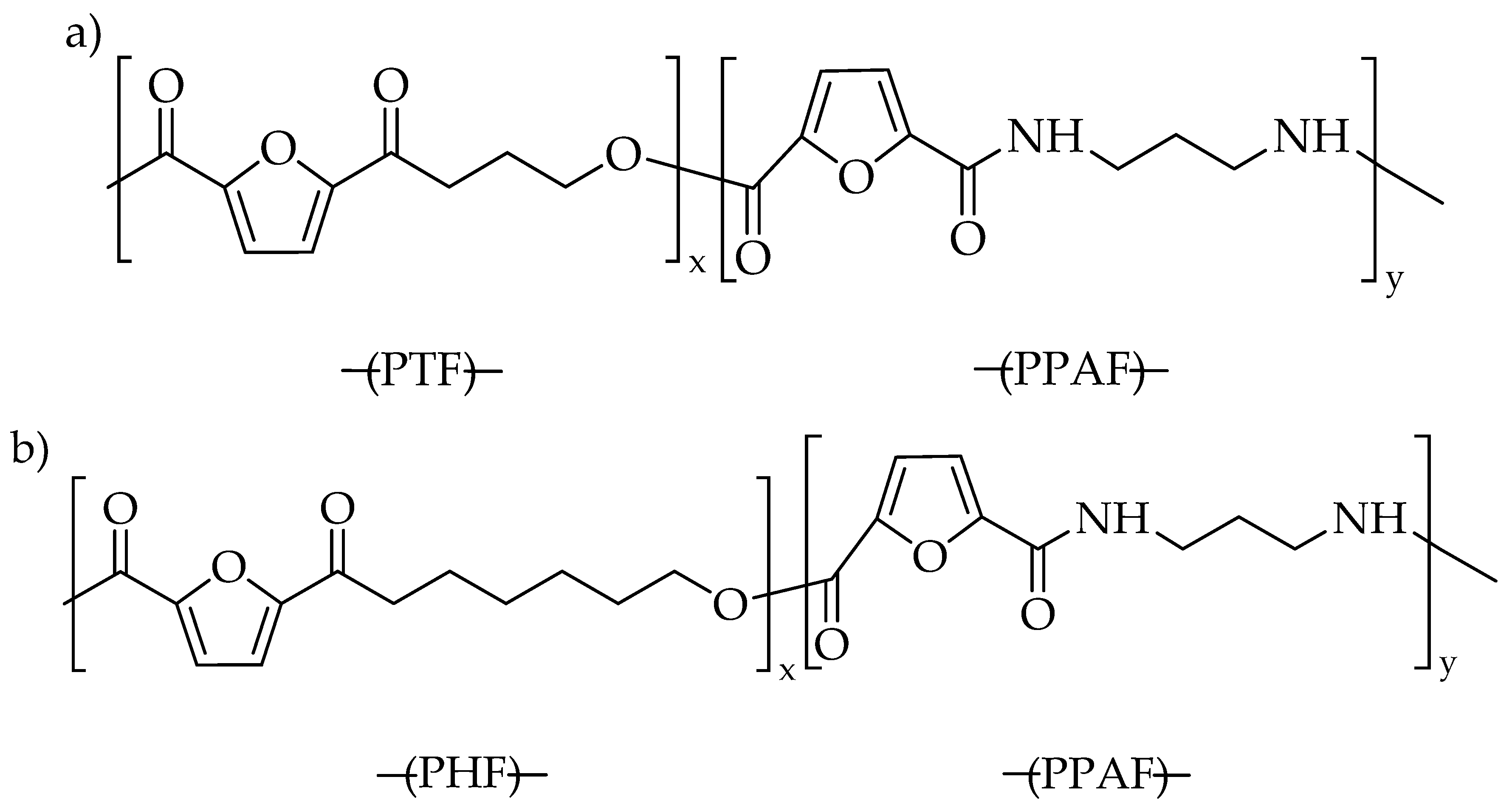
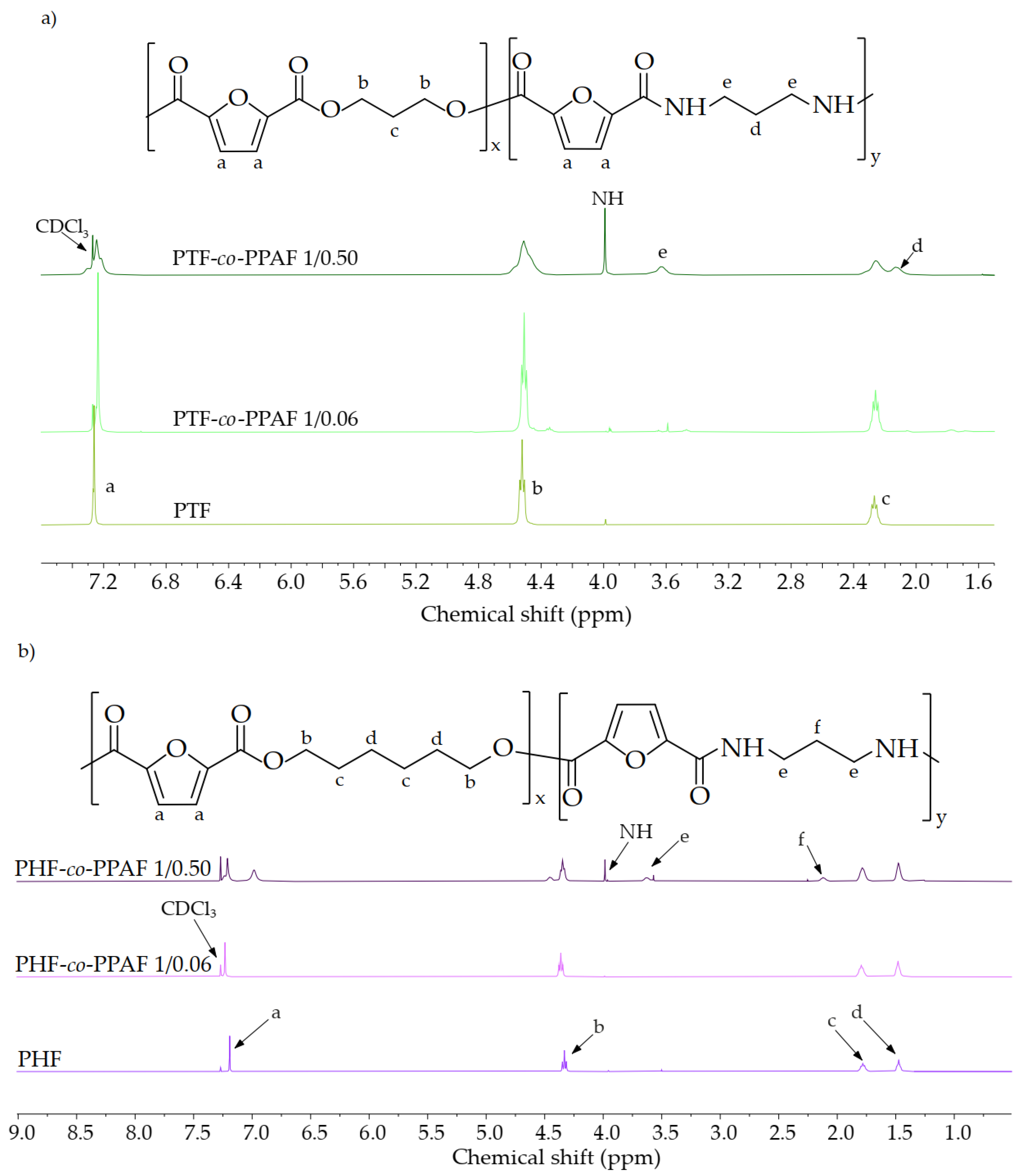
3.1. Structure and Composition
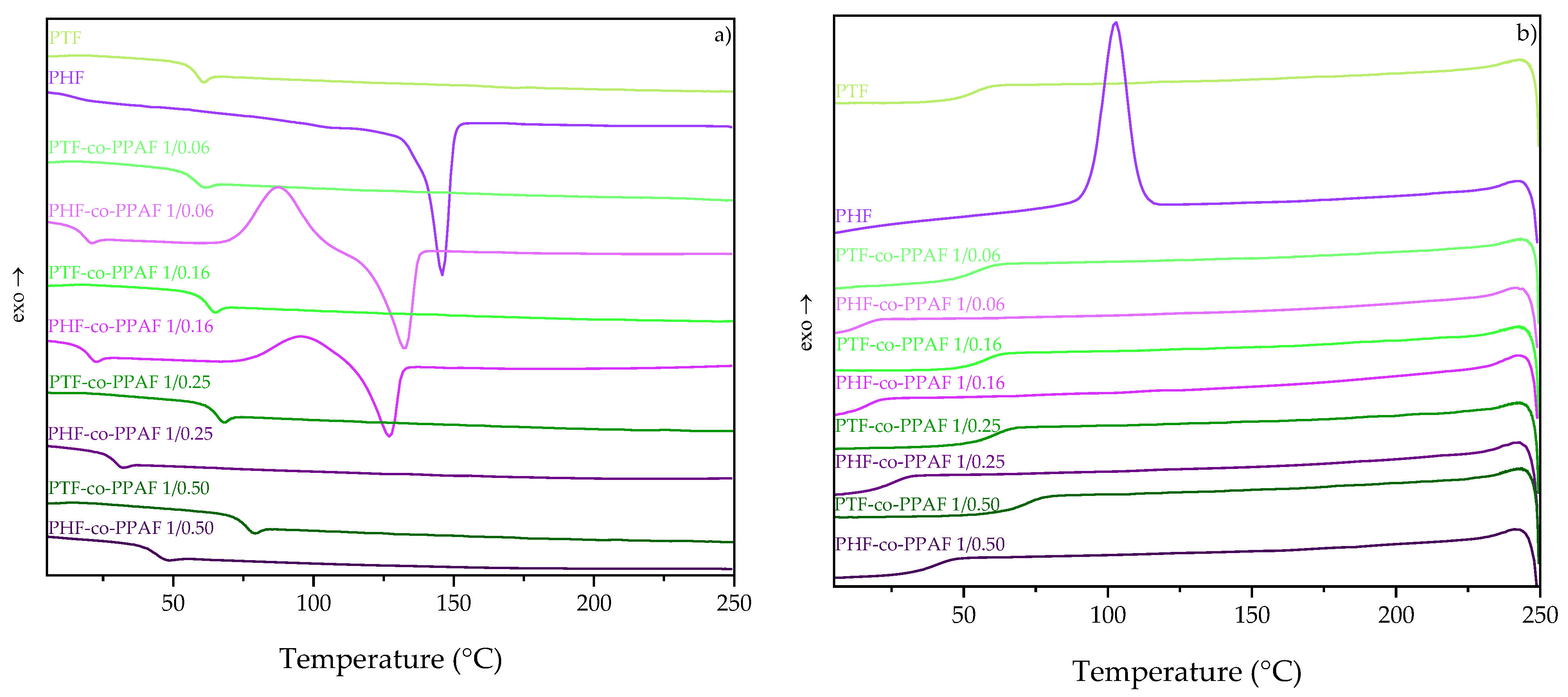
3.2. Thermal Properties
3.3. Positron Annihilation Lifetime Spectroscopy
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papadopoulos, L.; Klonos, P.A.; Kluge, M.; Zamboulis, A.; Terzopoulou, Z.; Kourtidou, D.; Magaziotis, A.; Chrissafis, K.; Kyritsis, A.; Bikiaris, D.N.; et al. Unlocking the Potential of Furan-Based Poly(Ester Amide)s: An Investigation of Crystallization, Molecular Dynamics and Degradation Kinetics of Novel Poly(Ester Amide)s Based on Renewable Poly(Propylene Furanoate). Polym. Chem. 2021, 12, 5518–5534. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I; US National Renewable Energy Lab: Golden, CO, USA, 2004; pp. 1–76. [CrossRef] [Green Version]
- Papamokos, G.; Dimitriadis, T.; Bikiaris, D.N.; Papageorgiou, G.Z.; Floudas, G. Chain Conformation, Molecular Dynamics, and Thermal Properties of Poly(n-Methylene 2,5-Furanoates) as a Function of Methylene Unit Sequence Length. Macromolecules 2019, 52, 6533–6546. [Google Scholar] [CrossRef]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(Ethylene Furanoate) Compared to Poly(Ethylene Terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of Bio-Based 2,5-Furan Dicarboxylate Polyesters: Recent Progress and Critical Aspects in Their Synthesis and Thermal Properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Piesowicz, E. Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials 2020, 13, 2673. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen Sorption and Transport in Amorphous Poly(Ethylene Furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Walkowiak, K.; Irska, I.; Zubkiewicz, A.; Rozwadowski, Z.; Paszkiewicz, S. Influence of Rigid Segment Type on Copoly(Ether-Ester) Properties. Materials 2021, 14, 4614. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Irska, I.; Miadlicki, P.; Walkowiak, K.; Rozwadowski, Z.; Paszkiewicz, S. Structure, Thermal and Mechanical Properties of Copoly(Ester Amide)s Based on 2,5-furandicarboxylic Acid. J. Mater. Sci. 2021, 56, 19296–19309. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Loos, K. Enzymatic Synthesis of 2,5-Furandicarboxylic Acid-Based Semi-Aromatic Polyamides: Enzymatic Polymerization Kinetics, Effect of Diamine Chain Length and Thermal Properties. RSC Adv. 2016, 6, 67941–67953. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Zhang, C.; He, B.; Huang, M.; Jiang, S. Synthesis of 2,5-Furandicarboxylic Acid-Based Heat-Resistant Polyamides under Existing Industrialization Process. Macromol. Res. 2017, 25, 722–729. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Semi-Bio-Based Aromatic Polyamides from 2,5-Furandicarboxylic Acid: Toward High-Performance Polymers from Renewable Resources. RSC Adv. 2016, 6, 87013–87020. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Alberda Van Ekenstein, G.O.R.; Loos, K. Enzymatic Polymerization of Furan-2,5-Dicarboxylic Acid-Based Furanic-Aliphatic Polyamides as Sustainable Alternatives to Polyphthalamides. Biomacromolecules 2015, 16, 3674–3685. [Google Scholar] [CrossRef]
- Maniar, D.; Silvianti, F.; Ospina, V.M.; Woortman, A.J.J.; van Dijken, J.; Loos, K. On the Way to Greener Furanic-Aliphatic Poly(Ester Amide)s: Enzymatic Polymerization in Ionic Liquid. Polymer 2020, 205, 122662. [Google Scholar] [CrossRef]
- Mitiakoudis, A.; Gandini, A. Characterization of Furanic. Macromolecules 1991, 24, 830–835. [Google Scholar] [CrossRef]
- Endah, Y.K.; Han, S.H.; Kim, J.H.; Kim, N.K.; Kim, W.N.; Lee, H.S.; Lee, H. Solid-State Polymerization and Characterization of a Copolyamide Based on Adipic Acid, 1,4-Butanediamine, and 2,5-Furandicarboxylic Acid. J. Appl. Polym. Sci. 2016, 133, 4–11. [Google Scholar] [CrossRef]
- Huang, W.; Hu, X.; Zhai, J.; Zhu, N.; Guo, K. Biorenewable Furan-Containing Polyamides. Mater. Today Sustain. 2020, 10, 100049. [Google Scholar] [CrossRef]
- Kluge, M.; Papadopoulos, L.; Magaziotis, A.; Tzetzis, D.; Zamboulis, A.; Bikiaris, D.N.; Robert, T. A Facile Method to Synthesize Semicrystalline Poly(Ester Amide)s from 2,5-Furandicarboxylic Acid, 1,10-Decanediol, and Crystallizable Amido Diols. ACS Sustain. Chem. Eng. 2020, 8, 10812–10821. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Irska, I.; Walkowiak, K.; Dryzek, J.; Paszkiewicz, S. Bio-Renewable Furan-Based Poly(Ester Amide)s: Synthesis, Structure, Spectroscopic and Mechanical Properties of Poly(Hexylene 2,5- Furandicarboxylate)-Co-Poly(Propylene Furanamide)s (PHF-Co-PPAF). Express Polym. Lett. 2022, in press. [Google Scholar]
- Zhang, Q.; Song, M.; Xu, Y.; Wang, W.; Wang, Z.; Zhang, L. Bio-Based Polyesters: Recent Progress and Future Prospects. Prog. Polym. Sci. 2021, 120, 101430. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the Bio-Based Polyester Poly(Propylene 2,5-Furan Dicarboxylate). Comparison of Thermal Behavior and Solid State Structure with Its Terephthalate and Naphthalate Homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-Based Polyesters from Renewable Resources: Crystallization and Thermal Degradation Behavior of Poly(Hexamethylene 2,5-Furan-Dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Knasy, J. LT Programs. Available online: https://prac.us.edu.pl/~kansy/index.php?id=ltpolym (accessed on 16 November 2010).
- Hoffman, J.D.; Weeks, J.J. Melting Process and the Equilibrium Melting Temperature of Polychlorotrifluoroethylene. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1962, 66, 13–28. [Google Scholar] [CrossRef]
- Irska, I.; Paszkiewicz, S.; Pawlikowska, D.; Dryzek, J.; Linares, A.; Nogales, A.; Ezquerra, T.A.; Piesowicz, E. Relaxation Behaviour and Free Volume of Bio-Based Poly(Trimethylene Terephthalate)-Block-Poly(Caprolactone) Copolymers as Revealed by Broadband Dielectric and Positron Annihilation Lifetime Spectroscopies. Polymer 2021, 229, 123949. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Nakanishi, H.; Jean, Y.C.; Sandreczki, T.C.; Douglas, M. Positron Annihilation in Amine-Cured Epoxy Polymers- Pressure Dependence. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1431–1441. [Google Scholar] [CrossRef]
- Gao, H.; Bai, Y.; Liu, H.; He, J. Mechanical and Gas Barrier Properties of Structurally Enhanced Poly(Ethylene Terephthalate) by Introducing 1,6-Hexylenediamine Unit. Ind. Eng. Chem. Res. 2019, 58, 21872–21880. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Chou, Y.L.; Yang, H.C.; Chen, C.W.; Rwei, S.P. Synthesis and Characterization of Thermoplastic Poly(Ester Amide)s Elastomer (Tpeae) Obtained from Recycled Pet. J. Renew. Mater. 2021, 9, 867–880. [Google Scholar] [CrossRef]

| Series | Ti [°C] | Pi [MPa] | Tf [°C] | Pd [Mpa] | ti [s] | tc [s] |
|---|---|---|---|---|---|---|
| PTF-co-PPAF | 195 | 85 | 30 | 30 | 6 | 15 |
| PHF-co-PPAF | 180 | 40 | 30 | 25 | 5 | 30 |
| Material | Wpolyester (mol.%) | WPPAF (mol.%) | WPPAF NMR (mol.%) |
|---|---|---|---|
| PTF | 100 | 0 | 0 |
| PTF-co-PPAF 1/0.06 | 94 | 6 | 4.76 |
| PTF-co-PPAF 1/0.16 | 84 | 16 | 16.67 |
| PTF-co-PPAF 1/0.25 | 75 | 25 | 25.37 |
| PTF-co-PPAF 1/0.50 | 50 | 50 | 45.03 |
| PHF | 100 | 0 | 0 |
| PHF-co-PPAF 1/0.06 | 94 | 6 | 7.47 |
| PHF-co-PPAF 1/0.16 | 84 | 16 | 15.25 |
| PHF-co-PPAF 1/0.25 | 75 | 25 | 24.24 |
| PHF-co-PPAF 1/0.50 | 50 | 50 | 45.05 |
| Sample | Tg (°C) | ΔCp (J/g °C) | Tcc (°C) | ΔHcc (J/g) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) | T5% (°C) | T50% (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTF | 56.6 | 0.40 | - | - | - | - | - | - | - | 364 A | 392 A |
| PTF-co-PPAF 1/0.06 | 55.9 | 0.39 | - | - | - | - | - | - | - | 366 A | 397 A |
| PTF-co-PPAF 1/0.16 | 60.1 | 0.47 | - | - | - | - | - | - | - | 367 A | 400 A |
| PTF-co-PPAF 1/0.25 | 63.7 | 0.41 | - | - | - | - | - | - | - | 367 A | 408 A |
| PTF-co-PPAF 1/0.50 | 73.9 | 0.41 | - | - | - | - | - | - | - | 360 A | 401 A |
| PHF | 15.0 | 0.12 | - | - | 103.0 | 41.9 | 146.0 | 36.3 | 25.4 | 354 B | 388 B |
| PHF-co-PPAF 1/0.06 | 17.0 | 0.41 | 87.0 | 32.5 | - | - | 133.0 | 34.8 | 1.6 | 349 B | 386 B |
| PHF-co-PPAF 1/0.16 | 19.0 | 0.33 | 96.0 | 18.1 | - | - | 127.0 | 19.0 | 0.7 | 352 B | 388 B |
| PHF-co-PPAF 1/0.25 | 28.0 | 0.32 | - | - | - | - | - | - | - | 350 B | 388 B |
| PHF-co-PPAF 1/0.50 | 43.0 | 0.30 | - | - | - | - | - | - | - | 334 B | 383 B |
| Sample | τ1 (ps) | I1 (%) | τ2 (ps) | I2 (%) | τ3 (ns) | I3 (%) | R (Å) | Vv (Å3) | fv (%) |
|---|---|---|---|---|---|---|---|---|---|
| PTF | 206.5 ± 6.7 | 34.7 ± 2.0 | 418.6 ± 10.3 | 49.7 ± 1.8 | 1.54 ± 0.01 | 15.6 ± 0.61 | 0.24 ± 0.01 | 0.06 ± 0.01 | 1.59 ± 0.02 |
| PTF-co-PPAF 1/0.06 | 207.8 ± 7.3 | 35.1 ± 1.9 | 424.5 ± 9.4 | 50.5 ± 1.8 | 1.56 ± 0.02 | 14.5 ± 0.56 | 0.24 ± 0.01 | 0.06 ± 0.01 | 1.51 ± 0.04 |
| PTF-co-PPAF 1/0.16 | 200.4 ± 5.4 | 31.5 ± 1.4 | 406.3 ± 6.2 | 54.0 ± 1.3 | 1.51 ± 0.01 | 14.5 ± 0.41 | 0.24 ± 0.01 | 0.05 ± 0.01 | 1.4 ± 0.04 |
| PTF-co-PPAF 1/0.25 | 192.4 ± 8.0 | 31.4 ± 1.9 | 407.4 ± 9.0 | 54.9 ± 1.8 | 1.53 ± 0.02 | 13.6 ± 0.54 | 0.24 ± 0.01 | 0.06 ± 0.01 | 1.37 ± 0.03 |
| PTF-co-PPAF 1/0.50 | 188.7 ± 7.6 | 30.8 ± 1.8 | 403.7 ± 8.1 | 56.6 ± 1.7 | 1.50 ± 0.01 | 12.6 ± 0.46 | 0.24 ± 0.01 | 0.05 ± 0.01 | 1.21 ± 0.03 |
| PHF | 192.3 ± 11.9 | 27.0 ± 2.7 | 395.1 ± 11.6 | 52.6 ± 2.5 | 1.72 ± 0.01 | 20.4 ± 1.02 | 0.26 ± 0.01 | 0.07 ± 0.01 | 2.64 ± 0.03 |
| PHF-co-PPAF 1/0.06 | 184.0 ± 6.0 | 26.3 ± 1.4 | 381.5 ± 5.3 | 54.3 ± 1.2 | 1.67 ± 0.01 | 19.4 ± 0.49 | 0.25 ± 0.01 | 0.07 ± 0.01 | 2.34 ± 0.03 |
| PHF-co-PPAF 1/0.16 | 193.0 ± 9.2 | 29.1 ± 1.9 | 389.2 ± 7.1 | 50.8 ± 1.8 | 1.68 ± 0.01 | 20.1 ± 0.71 | 0.25 ± 0.01 | 0.07 ± 0.01 | 2.49 ± 0.03 |
| PHF-co-PPAF 1/0.25 | 191.3 ± 9.2 | 31.3 ± 2.3 | 399.5 ± 11.4 | 49.9 ± 2.1 | 1.67 ± 0.01 | 18.8 ± 0.84 | 0.25 ± 0.01 | 0.07 ± 0.01 | 2.27 ± 0.03 |
| PHF-co-PPAF 1/0.50 | 186.5 ± 4.9 | 27.9 ± 1.1 | 393.4 ± 4.2 | 53.0 ± 1.0 | 1.65 ± 0.01 | 19.1 ± 0.38 | 0.25 ± 0.01 | 0.07 ± 0.01 | 2.28 ± 0.03 |
| Sample | E (GPa) | σy (MPa) | εy (%) | σb (MPa) | εb (%) |
|---|---|---|---|---|---|
| PTF | 2.5 ± 0.4 | - | - | 81.6 ± 1.6 | 3.1 ± 0.2 |
| PTF-co-PPAF 1/0.06 | 2.2 ± 0.2 | - | - | 68.5 ± 1.0 | 3.0 ± 0.1 |
| PTF-co-PPAF 1/0.16 | 2.6 ± 0.2 | - | - | 64.7 ± 0.6 | 1.6 ± 0.1 |
| PTF-co-PPAF 1/0.25 | 3.0 ± 0.2 | - | - | 72.8 ± 0.7 | 1.4 ± 0.1 |
| PTF-co-PPAF 1/0.50 | 3.6 ± 0.1 | - | - | 75.6 ± 0.9 | 0.7 ± 0.1 |
| PHF | 0.4 ± 0.1 | 23.6 ± 0.9 | 8.8 ± 1.0 | 45.3 ± 2.7 | 319.2 ± 13.3 |
| PHF-co-PPAF 1/0.06 | 0.8 ± 0.1 | 42.4 ± 1.8 | 6.1 ± 0.9 | 39.1 ± 4.1 | 146.3 ± 14.9 |
| PHF-co-PPAF 1/0.16 | 1.7 ± 0.2 | 43.3 ± 2.6 | 5.0 ± 0.4 | 38.8 ± 3.6 | 106.6 ± 13.7 |
| PHF-co-PPAF 1/0.25 | 1.3 ± 0.2 | 43.7 ± 1.0 | 2.1 ± 0.2 | 39.4 ± 2.8 | 147.1 ± 24.6 |
| PHF-co-PPAF 1/0.50 | 1.0 ± 0.2 | 38.4 ± 2.2 | 2.5 ± 0.7 | 25.8 ± 3.1 | 75.3 ± 10.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowiak, K.; Irska, I.; Zubkiewicz, A.; Dryzek, J.; Paszkiewicz, S. The Properties of Poly(ester amide)s Based on Dimethyl 2,5-Furanedicarboxylate as a Function of Methylene Sequence Length in Polymer Backbone. Polymers 2022, 14, 2295. https://doi.org/10.3390/polym14112295
Walkowiak K, Irska I, Zubkiewicz A, Dryzek J, Paszkiewicz S. The Properties of Poly(ester amide)s Based on Dimethyl 2,5-Furanedicarboxylate as a Function of Methylene Sequence Length in Polymer Backbone. Polymers. 2022; 14(11):2295. https://doi.org/10.3390/polym14112295
Chicago/Turabian StyleWalkowiak, Konrad, Izabela Irska, Agata Zubkiewicz, Jerzy Dryzek, and Sandra Paszkiewicz. 2022. "The Properties of Poly(ester amide)s Based on Dimethyl 2,5-Furanedicarboxylate as a Function of Methylene Sequence Length in Polymer Backbone" Polymers 14, no. 11: 2295. https://doi.org/10.3390/polym14112295
APA StyleWalkowiak, K., Irska, I., Zubkiewicz, A., Dryzek, J., & Paszkiewicz, S. (2022). The Properties of Poly(ester amide)s Based on Dimethyl 2,5-Furanedicarboxylate as a Function of Methylene Sequence Length in Polymer Backbone. Polymers, 14(11), 2295. https://doi.org/10.3390/polym14112295








