Novel Magnetic Polymeric Filters with Laccase-Based Nanoparticles for Improving Congo Red Decolorization in Bioreactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Laccase Production and Purification
2.3. Enzyme Characterization
2.4. Synthesis of Magnetite Nanoparticles
2.5. Laccase Immobilization
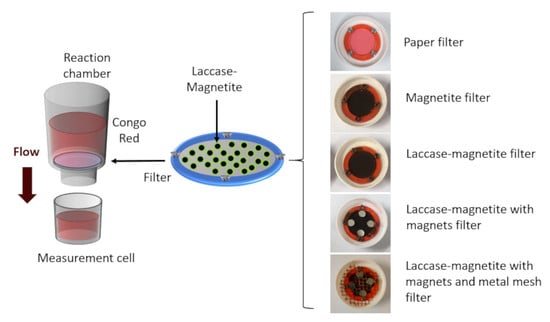
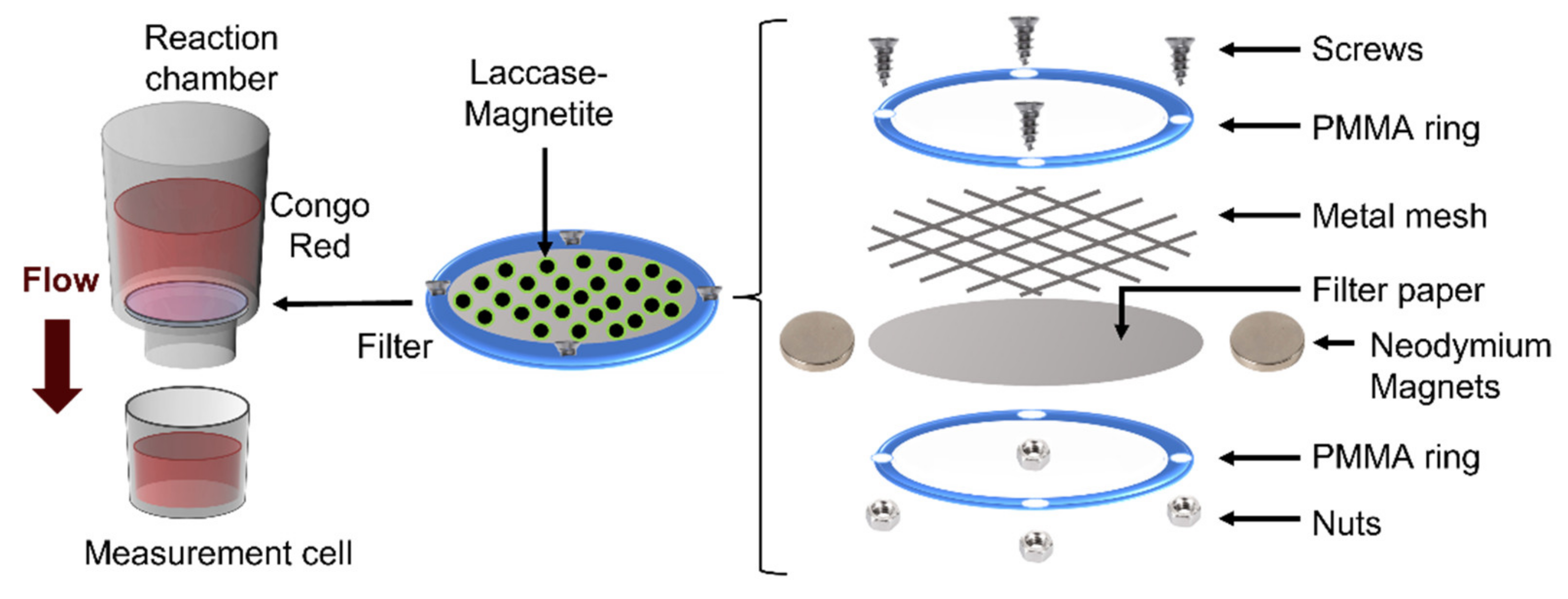
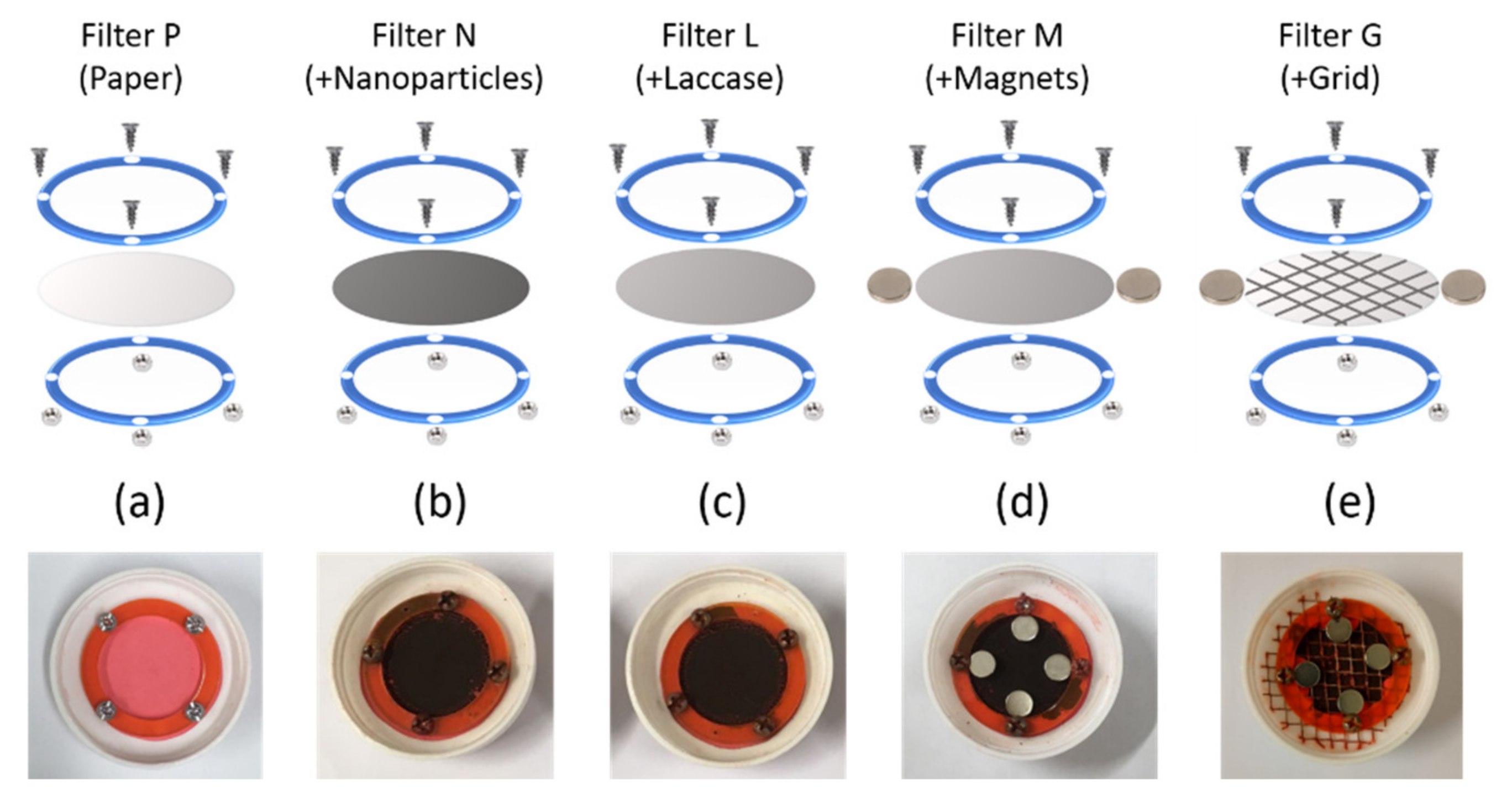
2.6. Nanoparticle Immobilization on Filters
2.7. Bioreactor Setup and Decolorization Measurements
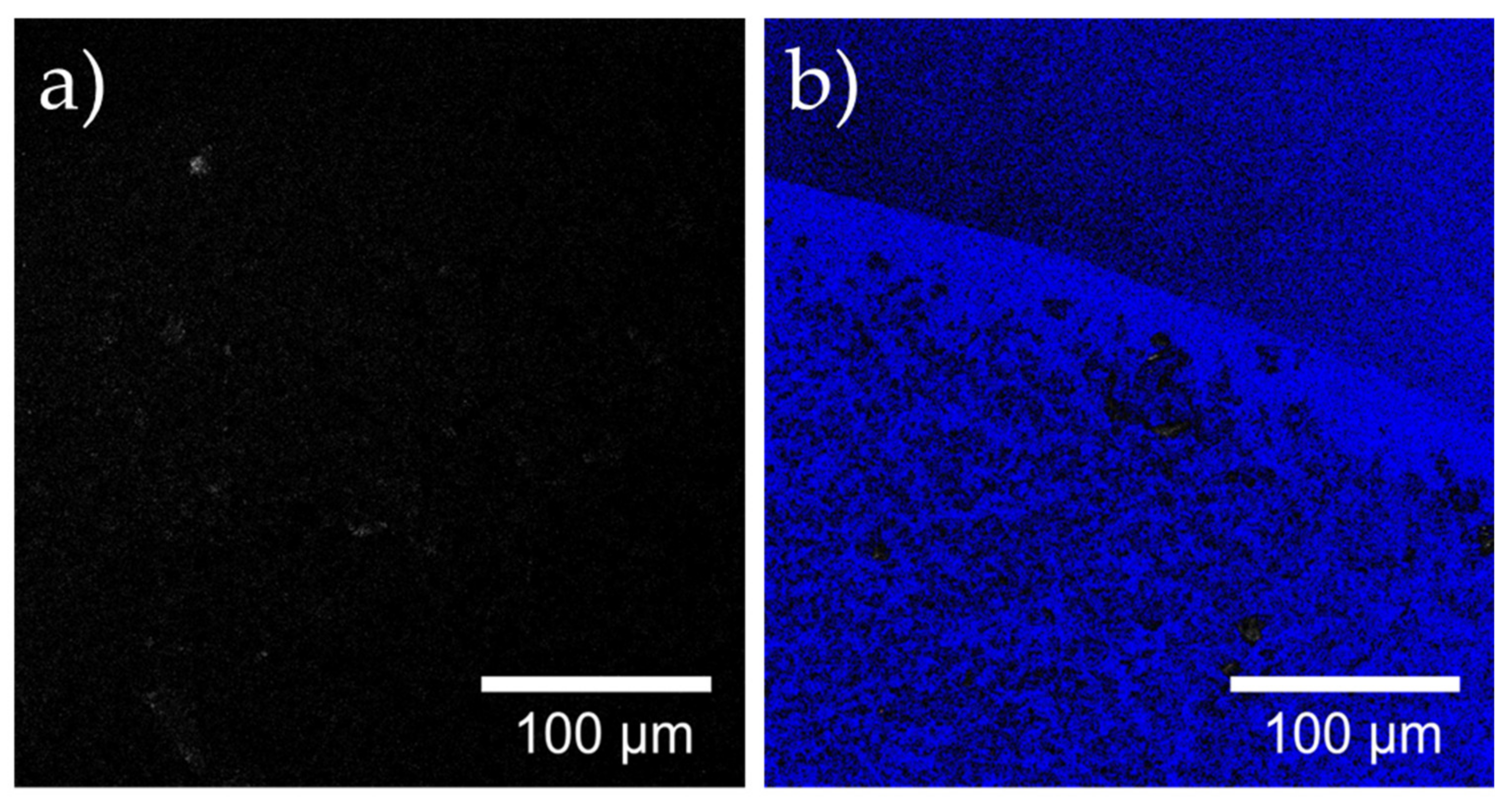
2.8. Confocal Laser Microscopy of the Nanoparticles Added on the Filters
3. Results
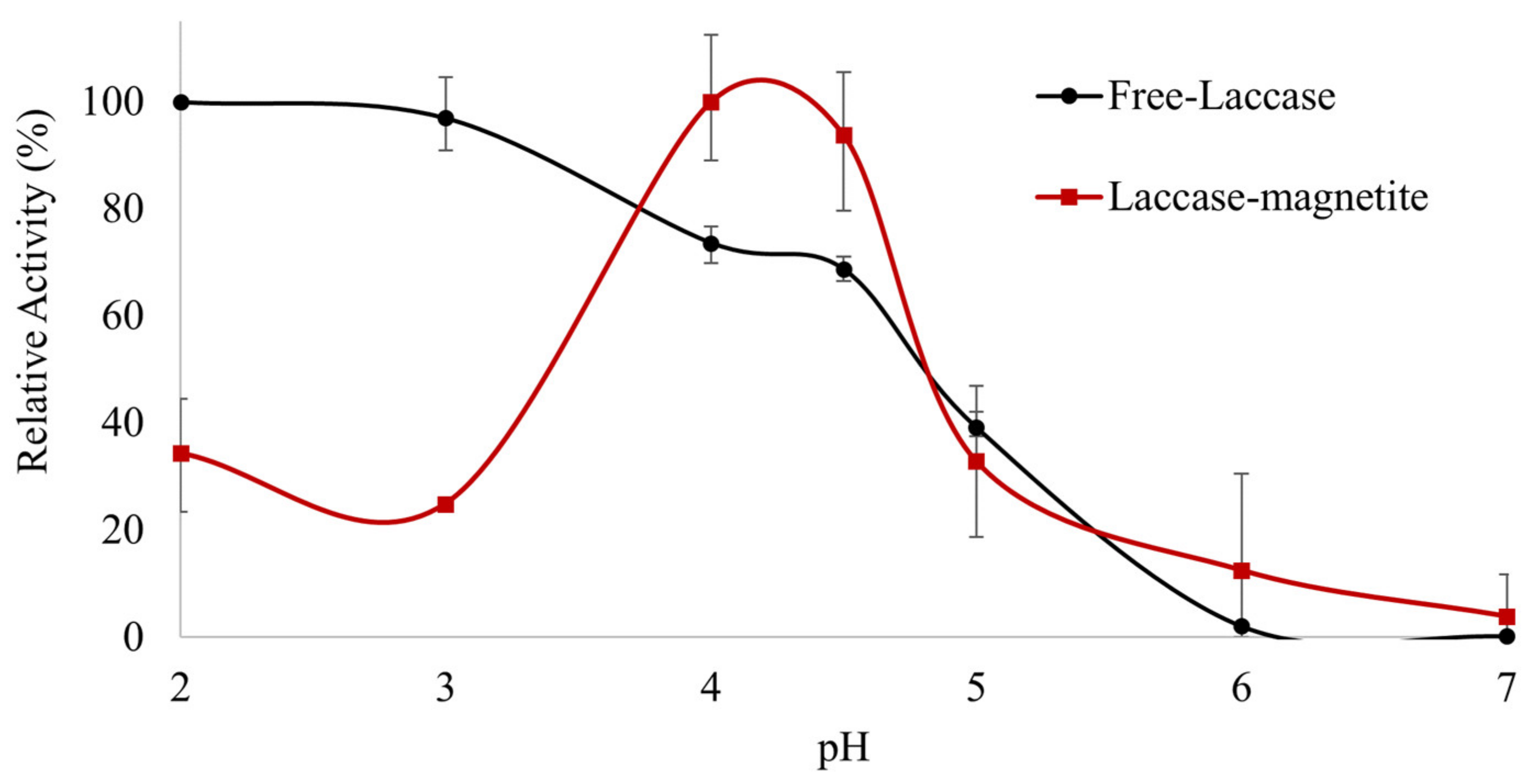
3.1. Impact of pH Changes on the Enzymatic Activity
3.2. Immobilization and Characterization of Magnetite and Laccase-Magnetite
3.3. Nanoparticle Immbolization on Filters
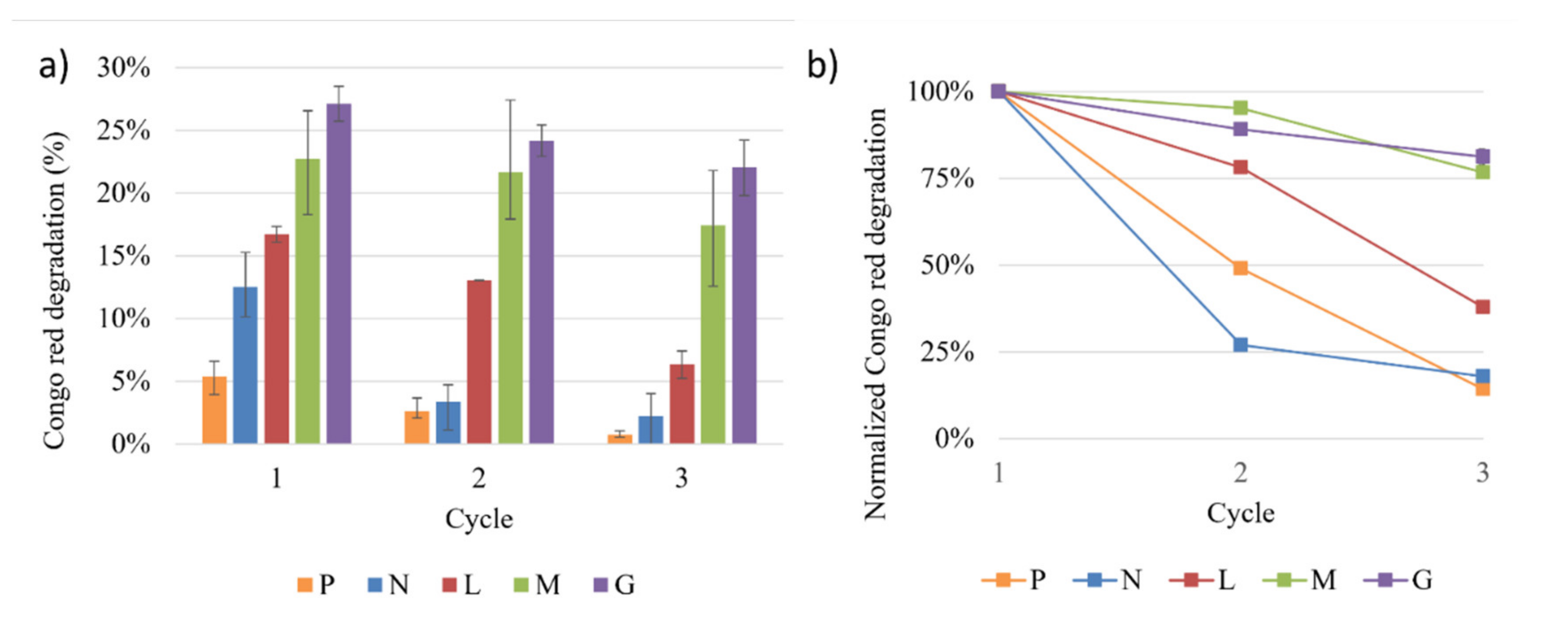
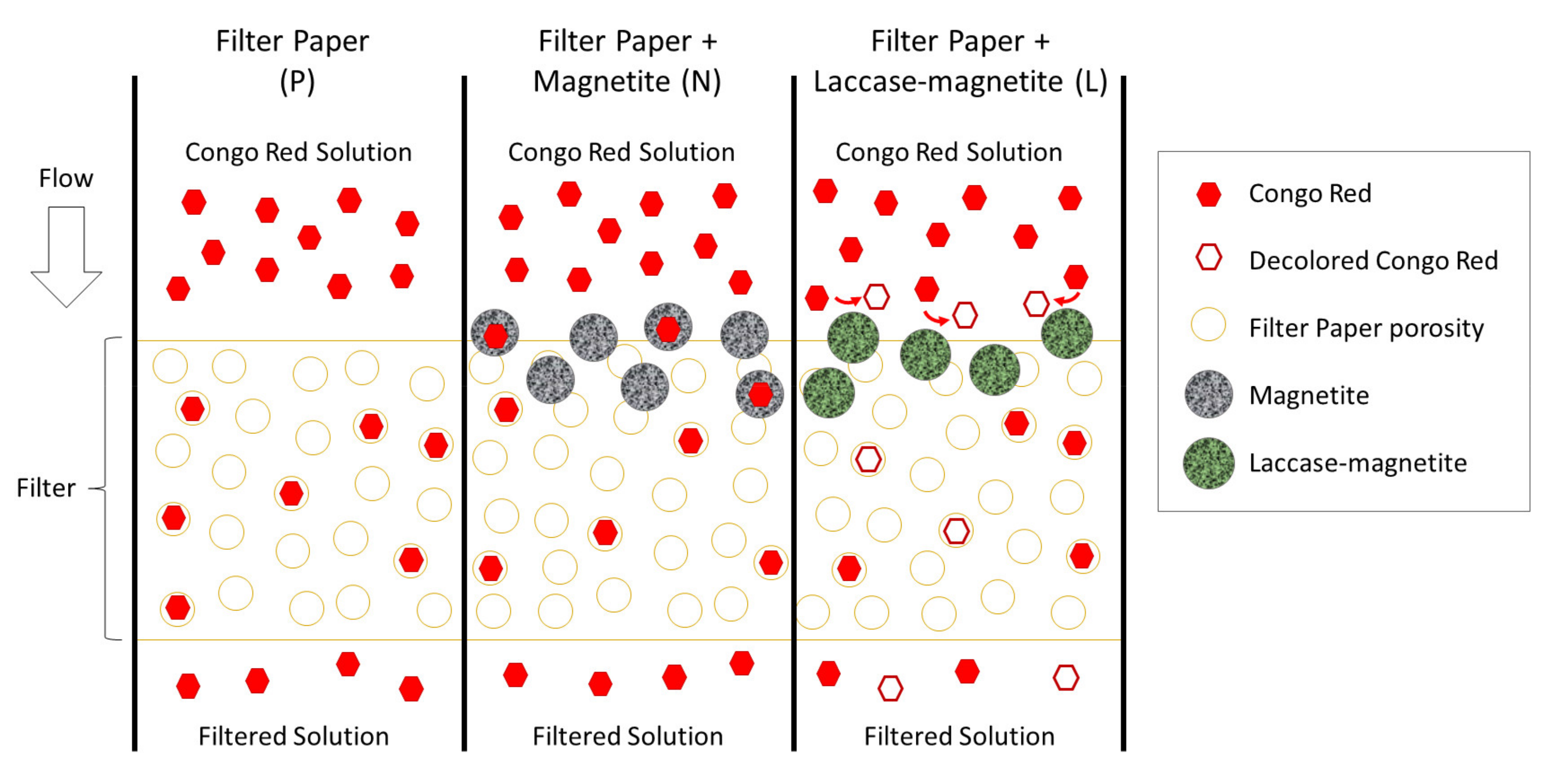
3.4. Bioreactor Setup and Decolorization Measurements
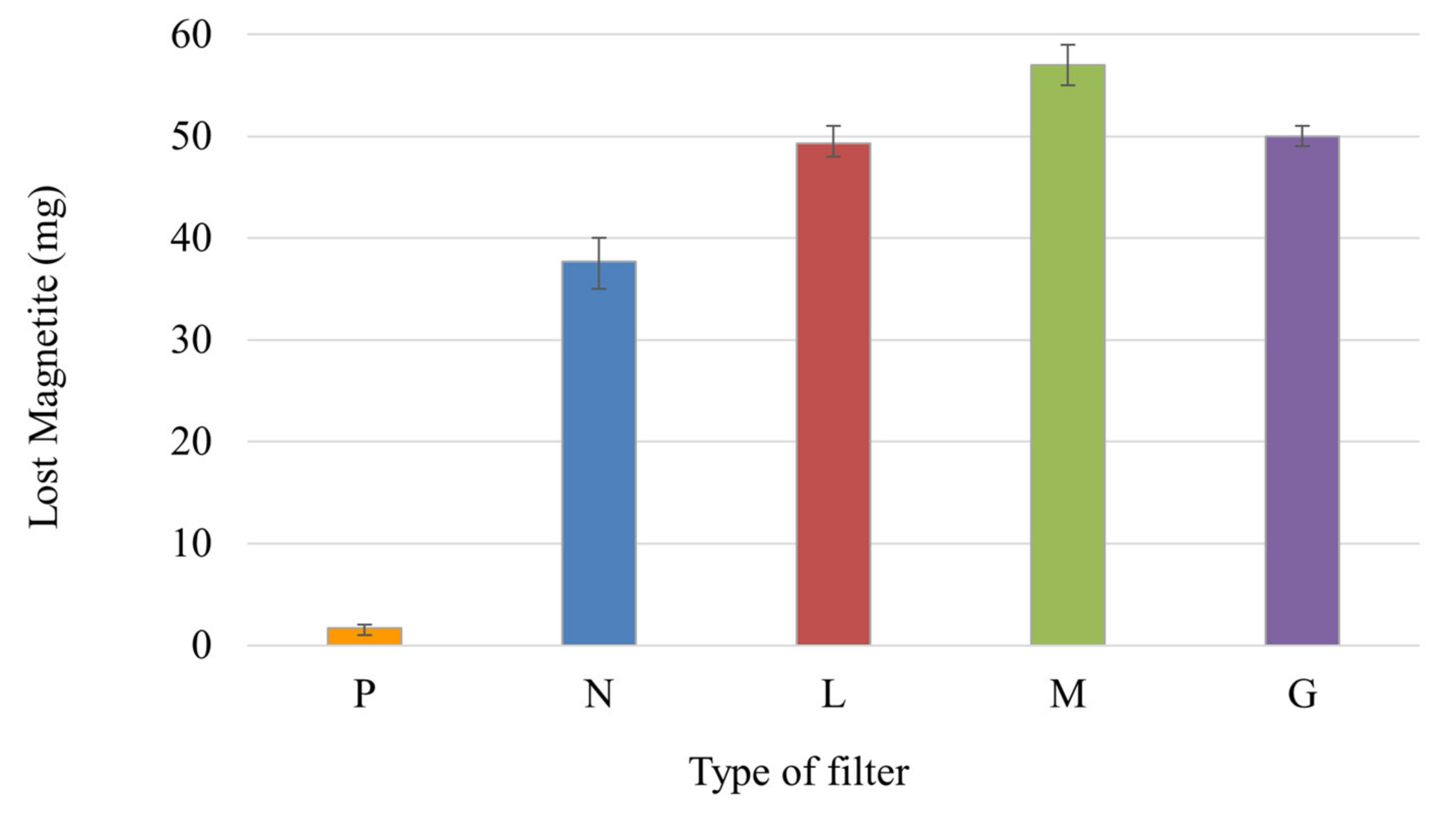
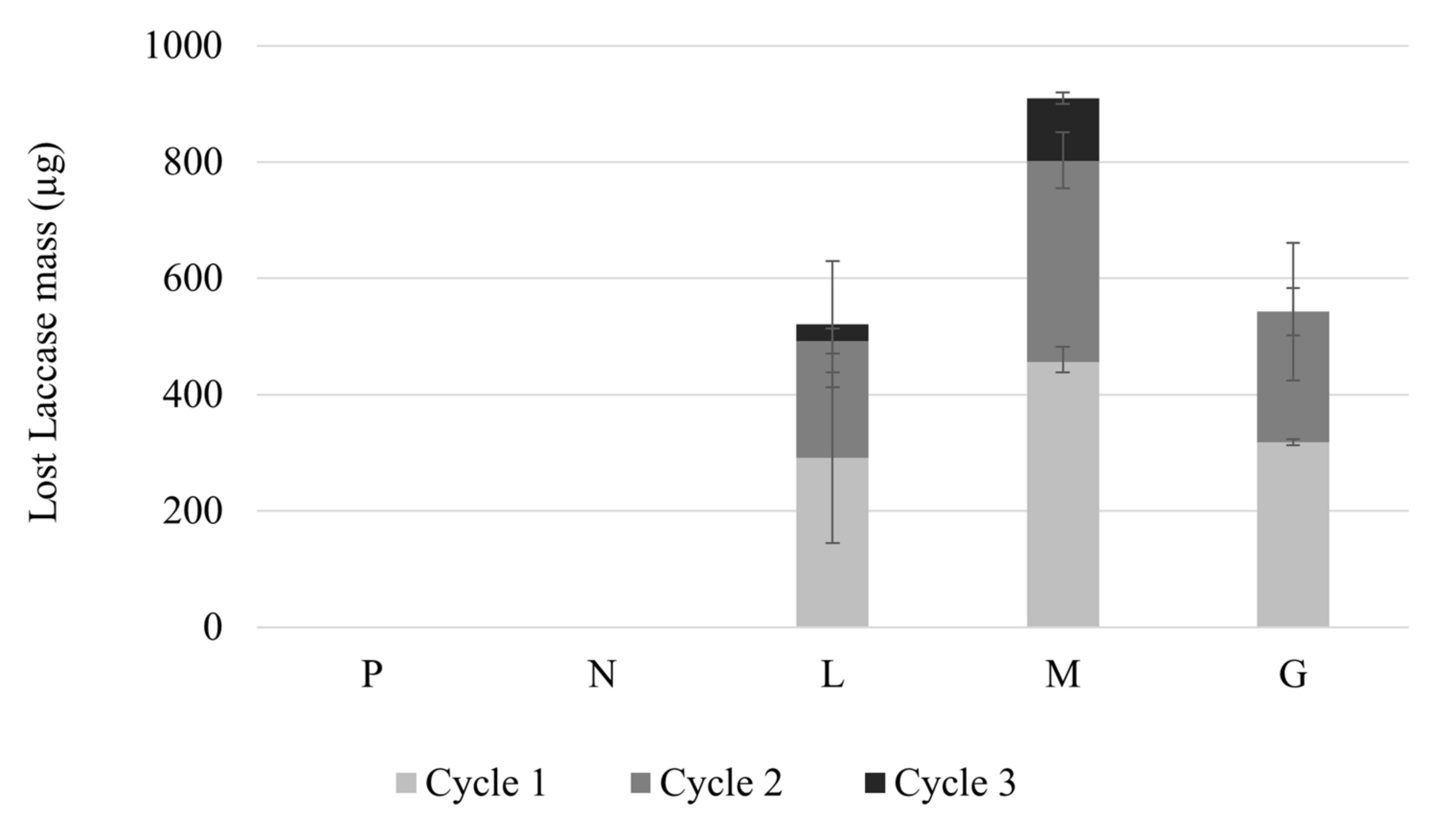
3.5. Loss of Magnetite and Laccase-Magnetite
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.-F.; Meng, G.; Cui, B.; Si, J.; Dai, Y.-C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. Biotechnol. 2020, 95, 481–494. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Barbosa, N.; Florez, S.L.; Cruz, J.C.; Ornelas-Soto, N.; Osma, J.F. Congo Red Decolorization Using Textile Filters and Laccase-Based Nanocomposites in Continuous Flow Bioreactors. Nanomaterials 2020, 10, 1227. [Google Scholar] [CrossRef]
- Vallejo, M.; Román, M.F.S.; Ortiz, I.; Irabien, A. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (AOPs) to wastewater treatment. Chemosphere 2015, 118, 44–56. [Google Scholar] [CrossRef]
- Yadav, M.; Gupta, R.; Sharma, R.K. Chapter 14—Green and Sustainable Pathways for Wastewater Purification. In Advances in Water Purification Techniques: Meeting the Needs of Developed and Developing Countries; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Aptitude of Oxidative Enzymes for Treatment of Wastewater Pollutants: A Laccase Perspective. Molecules 2019, 24, 2064. [Google Scholar] [CrossRef] [Green Version]
- Popović, N.; Pržulj, D.; Mladenović, M.; Prodanović, O.; Ece, S.; Đurđić, K.I.; Ostafe, R.; Fischer, R.; Prodanović, R. Immobilization of yeast cell walls with surface displayed laccase from Streptomyces cyaneus within dopamine-alginate beads for dye decolorization. Int. J. Biol. Macromol. 2021, 181, 1072–1080. [Google Scholar] [CrossRef]
- Tiarsa, E.R.; Yandri, Y.; Suhartati, T.; Satria, H.; Irawan, B.; Hadi, S. The Stability Improvement of Aspergillus fumigatus α-Amylase by Immobilization onto Chitin-Bentonite Hybrid. Biochem. Res. Int. 2022, 2022, 5692438. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Zhang, W.; Cai, Y. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Devi, M.K.; Kumar, P.S. Advances in the application of immobilized enzyme for the remediation of hazardous pollutant: A review. Chemosphere 2022, 299, 134390. [Google Scholar] [CrossRef]
- Reungoat, J.; Escher, B.; Macova, M.; Keller, J. Biofiltration of wastewater treatment plant effluent: Effective removal of pharmaceuticals and personal care products and reduction of toxicity. Water Res. 2011, 45, 2751–2762. [Google Scholar] [CrossRef]
- Bhowmik, M.; Kanmani, M.; Debnath, A.; Saha, B. Sono-assisted rapid adsorption of anionic dye onto magnetic CaFe2O4/MnFe2O4 nanocomposite from aqua matrix. Powder Technol. 2019, 354, 496–504. [Google Scholar] [CrossRef]
- Bose, S.; Tripathy, B.K.; Debnath, A.; Kumar, M. Boosted sono-oxidative catalytic degradation of Brilliant green dye by magnetic MgFe2O4 catalyst: Degradation mechanism, assessment of bio-toxicity and cost analysis. Ultrason. Sonochem. 2021, 75, 105592. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.D.; Martins, R.C.; Quinta-Ferreira, R.M.; Castro, L.M. Moving bed biofilm reactor (MBBR) for dairy wastewater treatment. Energy Rep. 2020, 6, 340–344. [Google Scholar] [CrossRef]
- El-Naas, M.; Acio, J.A.; El Telib, A.E. Aerobic biodegradation of BTEX: Progresses and Prospects. J. Environ. Chem. Eng. 2014, 2, 1104–1122. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Barrios, A.F.G.; Batista, C.A.S.; Osma, J.F.; Muñoz-Camargo, C.; Cruz, J.C. Magnetite–OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2020, 6, 415–424. [Google Scholar] [CrossRef]
- Muñoz, N.R.; Suarez-Arnedo, A.; Anguita, R.; Prats-Ejarque, G.; Osma, J.F.; Muñoz-Camargo, C.; Boix, E.; Cruz, J.C.; Salazar, V.A. Magnetite Nanoparticles Functionalized with RNases against Intracellular Infection of Pseudomonas aeruginosa. Pharmaceutics 2020, 12, 631. [Google Scholar] [CrossRef]
- Rangel-Muñoz, N.; González-Barrios, A.; Pradilla, D.; Osma, J.; Cruz, J. Novel Bionanocompounds: Outer Membrane Protein A and Lacasse Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment. Nanomaterials 2020, 10, 2278. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Zhu, Q.; Song, J.; Li, S.; Liu, X. Improved performance of immobilized laccase on Fe3O4@C-Cu2+ nanoparticles and its application for biodegradation of dyes. J. Hazard. Mater. 2020, 399, 123088. [Google Scholar] [CrossRef]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Luna, C.E.H.; Demarche, P.; Enaud, E.; García-Morales, R.; Agathos, S.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Morales, R.; Rodriguez, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Luna, C.E.H.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus sanguineus CS43. Water. Air. Soil Pollut. 2015, 226, 251. [Google Scholar] [CrossRef] [Green Version]
- Niku-Paavola, M.; Raaska, L.; Itävaara, M. Detection of white-rot fungi by a non-toxic stain. Mycol. Res. 1990, 94, 27–31. [Google Scholar] [CrossRef]
- Souza, C.; Garcia-Rojas, E.E.; Souza, C.S.; Vriesmann, L.C.; Vicente, J.; de Carvalho, M.G.; Petkowicz, C.L.; Favaro-Trindade, C.S. Immobilization of β-galactosidase by complexation: Effect of interaction on the properties of the enzyme. Int. J. Biol. Macromol. 2019, 122, 594–602. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R. Pinewood nanobiochar: A unique carrier for the immobilization of crude laccase by covalent bonding. Int. J. Biol. Macromol. 2018, 115, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Echen, L.; Ezou, M.; Hong, F.F. Evaluation of Fungal Laccase Immobilized on Natural Nanostructured Bacterial Cellulose. Front. Microbiol. 2015, 6, 1245. [Google Scholar] [CrossRef] [Green Version]
- Grimmer, A.; Chen, X.; Hamidović, M.; Haselmayr, W.; Ren, C.L.; Wille, R. Simulation before fabrication: A case study on the utilization of simulators for the design of droplet microfluidic networks. RSC Adv. 2018, 8, 34733–34742. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, N.; Chen, J.; Rodgers, V.G.J.; Brisk, P.; Grover, W.H. Finding the optimal design of a passive microfluidic mixer. Lab a Chip 2019, 19, 3618–3627. [Google Scholar] [CrossRef]
- Nor, W.F.K.W.; Soh, S.K.C.; Azmi, A.A.A.R.; Yusof, M.S.M.; Shamsuddin, M. Synthesis and physicochemical properties of magnetite nanoparticles (Fe3O4) as potential solid support for homogeneous catalysts. Malays. J. Anal. Sci. 2018, 22, 768–774. [Google Scholar] [CrossRef]
- Božič, M.; Gorgieva, S.; Kokol, V. Homogeneous and heterogeneous methods for laccase-mediated functionalization of chitosan by tannic acid and quercetin. Carbohydr. Polym. 2012, 89, 854–864. [Google Scholar] [CrossRef]
- Kalaichelvi, K.; Dhivya, S.M. Screening of phytoconstituents, UV-VIS Spectrum and FTIR analysis of Micrococca mercurialis (L.) Benth. Int. J. Herb. Med. 2017, 5, 40–44. [Google Scholar]
- Iark, D.; Buzzo, A.J.D.R.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Moreira, R.D.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar] [CrossRef]
- Kadam, A.A.; Jang, J.; Lee, D.S. Supermagnetically Tuned Halloysite Nanotubes Functionalized with Aminosilane for Covalent Laccase Immobilization. ACS Appl. Mater. Interfaces 2017, 9, 15492–15501. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Nejad, Z.G.; Ghasemi, S.; Khafaji, M.; Borghei, S.M. Removal of bisphenol A in aqueous solution using magnetic cross-linked laccase aggregates from Trametes hirsuta. Bioresour. Technol. 2020, 306, 123169. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Stanišić, M.; Đurđić, K.I.; Prodanović, O.; Polović, N.; Prodanović, R. Dopamine-modified pectin for a Streptomyces cyaneus laccase induced microbeads formation, immobilization, and textile dyes decolorization. Environ. Technol. Innov. 2021, 22, 101399. [Google Scholar] [CrossRef]
- Cao-Milán, R.; He, L.D.; Shorkey, S.; Tonga, G.Y.; Wang, L.-S.; Zhang, X.; Uddin, I.; Das, R.; Sulak, M.; Rotello, V.M. Modulating the catalytic activity of enzyme-like nanoparticles through their surface functionalization. Mol. Syst. Des. Eng. 2017, 2, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Fu, C.-C.; Juang, R.-S. Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads. Chem. Eng. J. 2016, 304, 313–324. [Google Scholar] [CrossRef]
- Meerbergen, K.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Isolation and screening of bacterial isolates from wastewater treatment plants to decolorize azo dyes. J. Biosci. Bioeng. 2018, 125, 448–456. [Google Scholar] [CrossRef]
- Chen, K.-C.; Wu, J.-Y.; Liou, D.-J.; Hwang, S.-C.J. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003, 101, 57–68. [Google Scholar] [CrossRef]
- Abo-State, M.A.; Hazaa, H.; Abo-State, M.A.M.; Saleh, Y.E.; Hazaa, H.A. Decolorization of Congo Red dye by bacterial isolates. J. Ecol. Health Environ. An. Int. J. 2017, 5, 41–48. [Google Scholar]
- Mazumdar, A.; Chen, Y.; Waanders, B.G.V.B.; Brooks, C.F.; Kuehl, M.A.; Nemer, M.B. Wireless Temperature Sensing Using Permanent Magnets for Nonlinear Feedback Control of Exothermic Polymers. IEEE Sens. J. 2018, 18, 7970–7979. [Google Scholar] [CrossRef]
- Ghernaout, D.; Elboughdiri, N. Magnetic Field Application: An Underappreciated Outstanding Technology. OALib 2020, 7, 1. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpää, M. Magnetic Field Application and its Potential in Water and Wastewater Treatment Systems. Sep. Purif. Rev. 2014, 43, 206–240. [Google Scholar] [CrossRef]



| Dye | λmax (nm) | Color Index Number | Color Index Name | Structure |
|---|---|---|---|---|
| Congo Red | 495 | 22120 | Direct Red 28 | 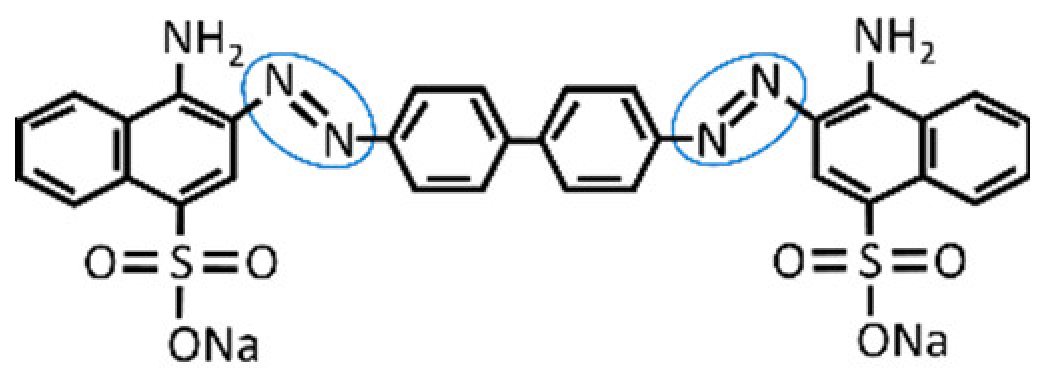 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotelo, D.C.; Ornelas-Soto, N.; Osma, J.F. Novel Magnetic Polymeric Filters with Laccase-Based Nanoparticles for Improving Congo Red Decolorization in Bioreactors. Polymers 2022, 14, 2328. https://doi.org/10.3390/polym14122328
Sotelo DC, Ornelas-Soto N, Osma JF. Novel Magnetic Polymeric Filters with Laccase-Based Nanoparticles for Improving Congo Red Decolorization in Bioreactors. Polymers. 2022; 14(12):2328. https://doi.org/10.3390/polym14122328
Chicago/Turabian StyleSotelo, Diana C., Nancy Ornelas-Soto, and Johann F. Osma. 2022. "Novel Magnetic Polymeric Filters with Laccase-Based Nanoparticles for Improving Congo Red Decolorization in Bioreactors" Polymers 14, no. 12: 2328. https://doi.org/10.3390/polym14122328
APA StyleSotelo, D. C., Ornelas-Soto, N., & Osma, J. F. (2022). Novel Magnetic Polymeric Filters with Laccase-Based Nanoparticles for Improving Congo Red Decolorization in Bioreactors. Polymers, 14(12), 2328. https://doi.org/10.3390/polym14122328