Laser Sintering Approaches for Bone Tissue Engineering
Abstract
:1. Introduction
1.1. Bone Tissue Regeneration and Engineering
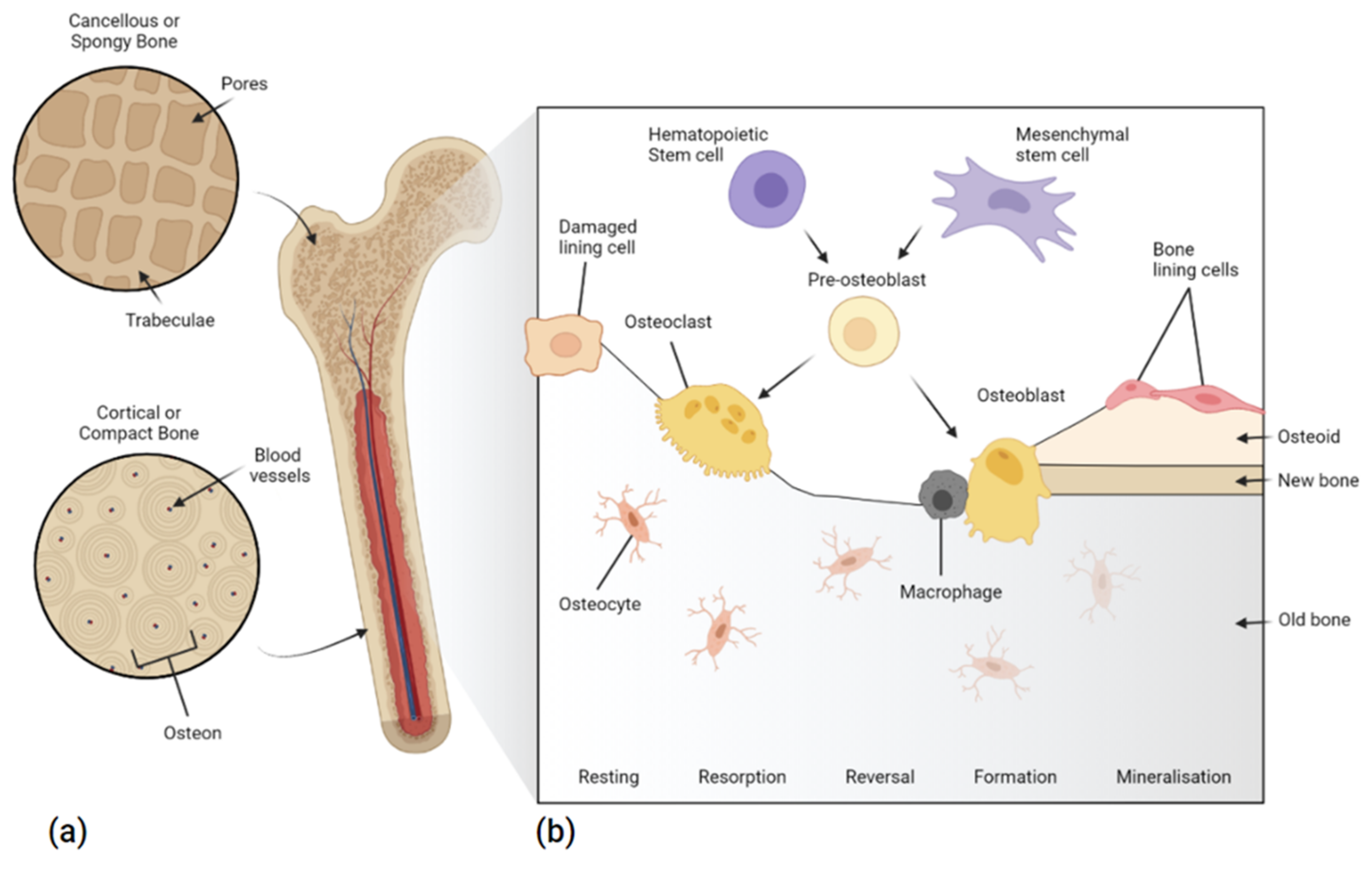
1.1.1. Bone Structure and Repair
1.1.2. Scaffold Design
1.1.3. Regulatory Requirements for the Future of BTE Scaffolds
1.1.4. Advancing the Field of BTE
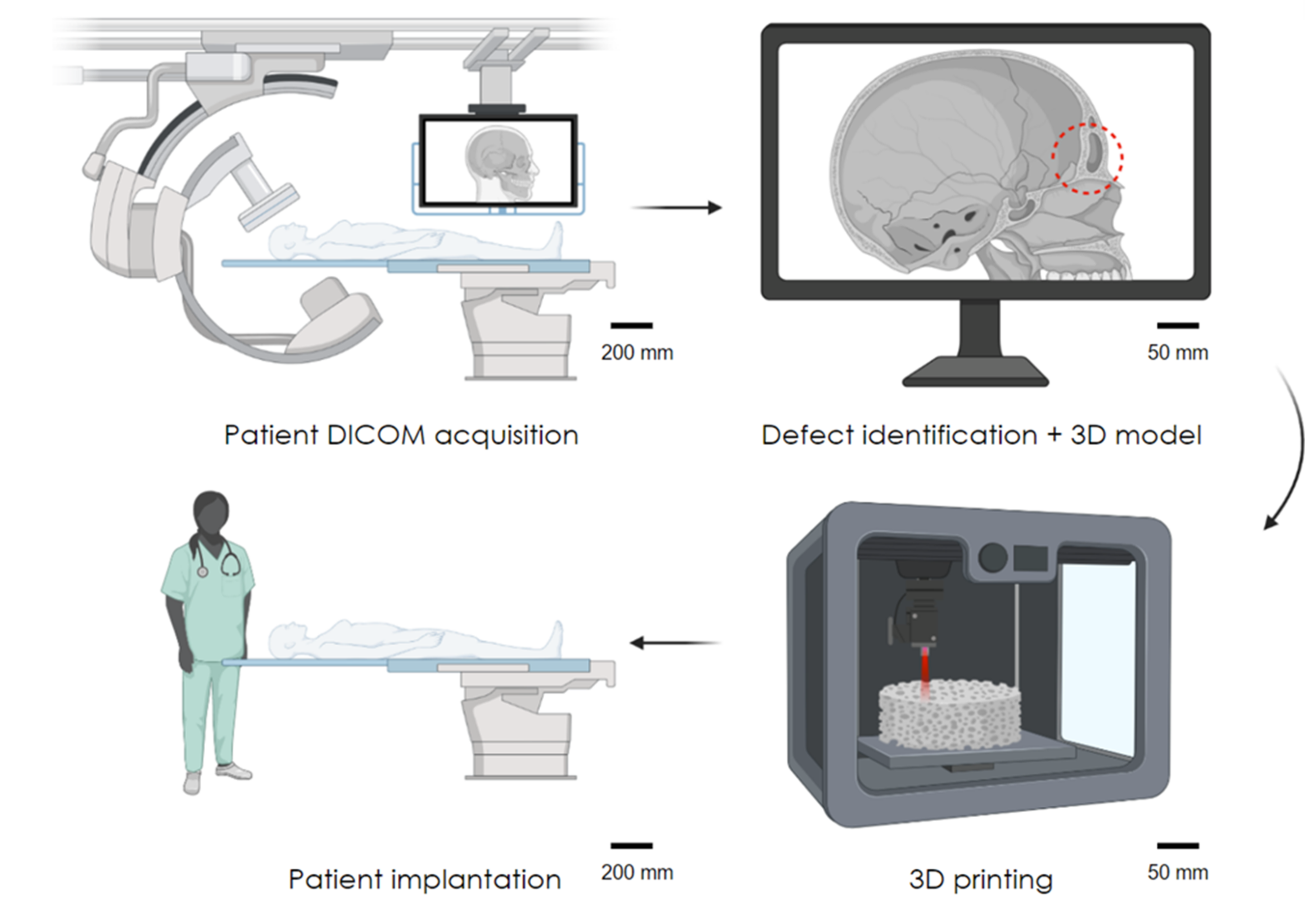
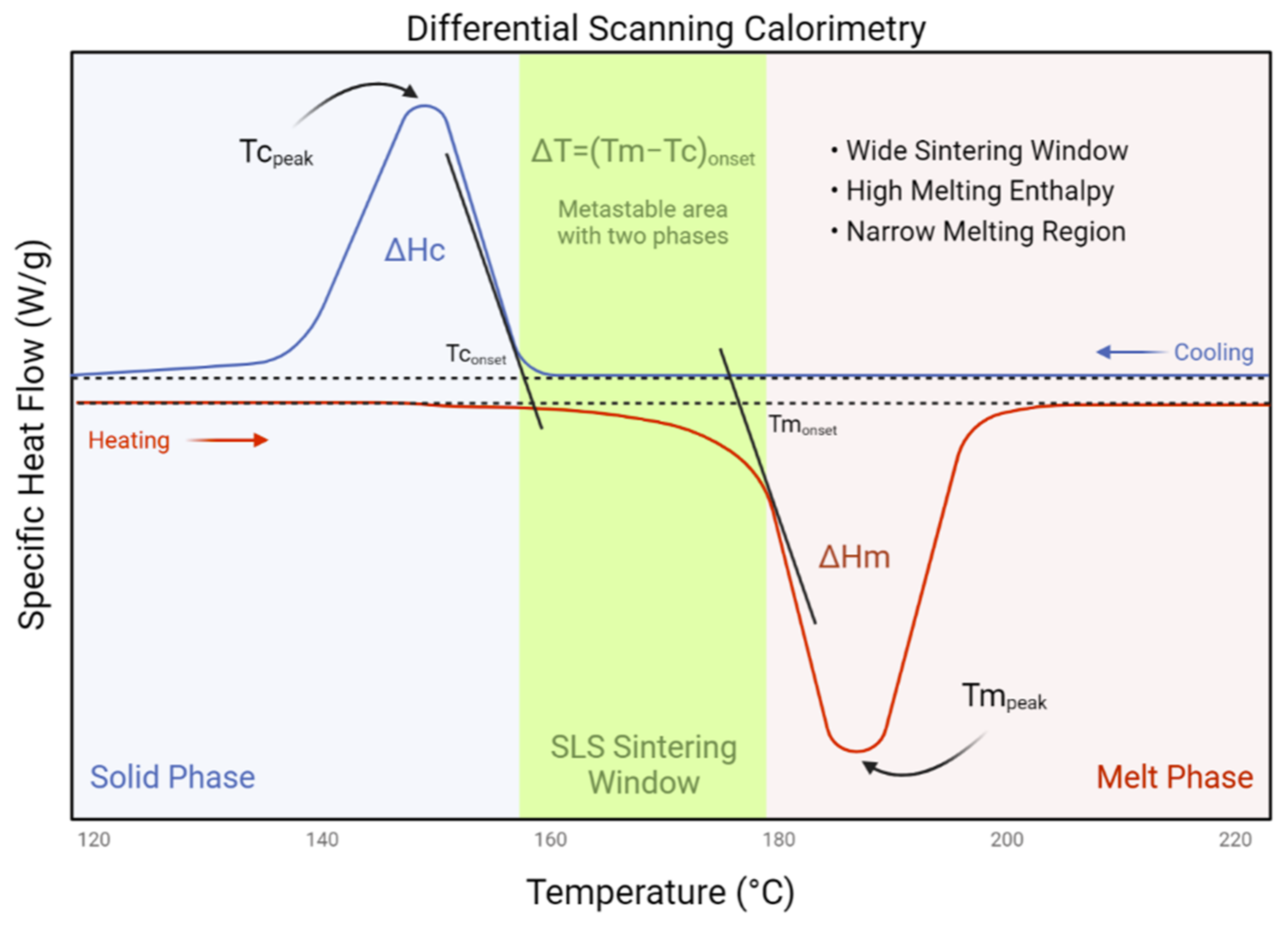
1.2. Laser Sintering Bone Tissue Engineering Scaffolds
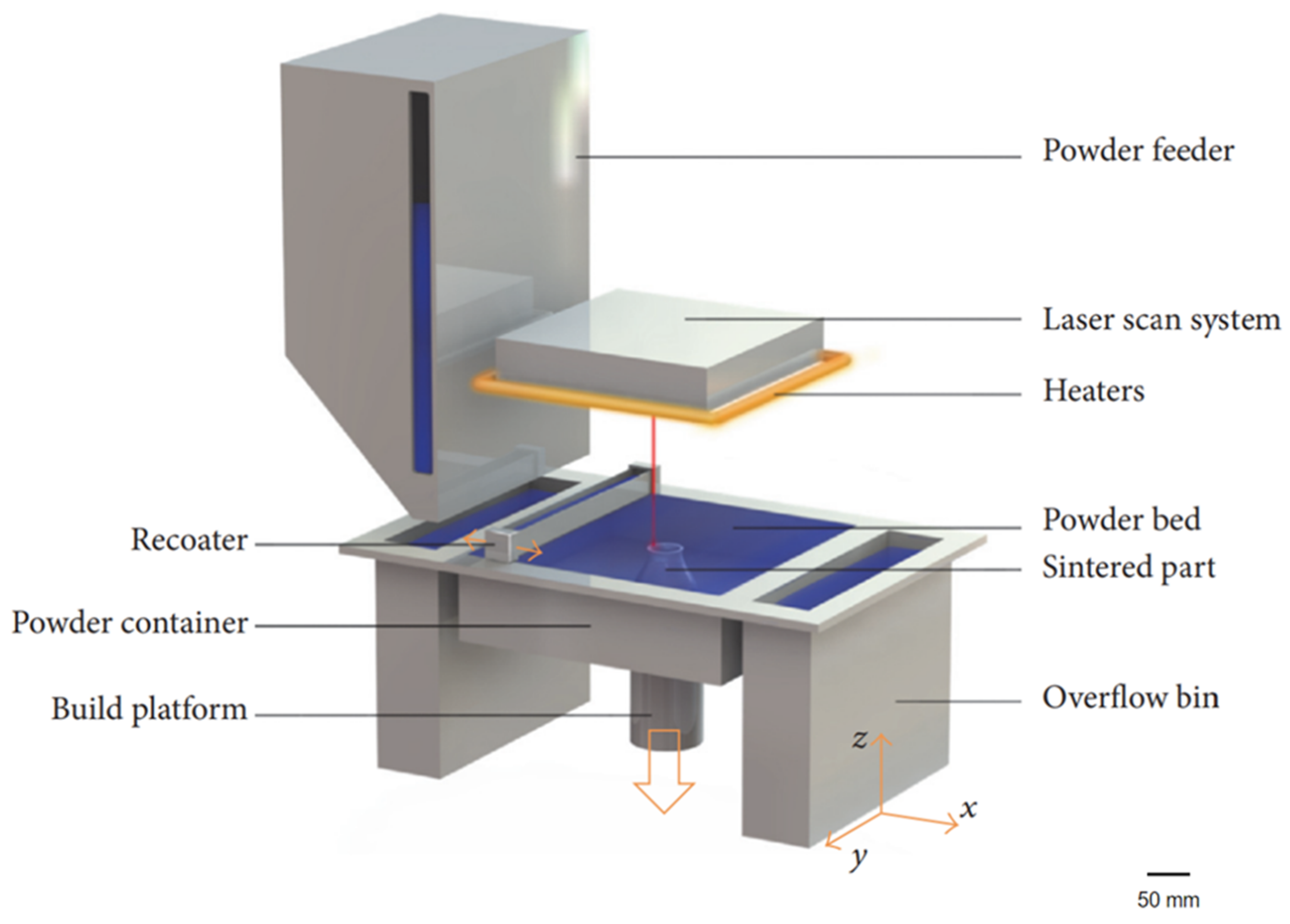
2. Materials for Laser Sintering
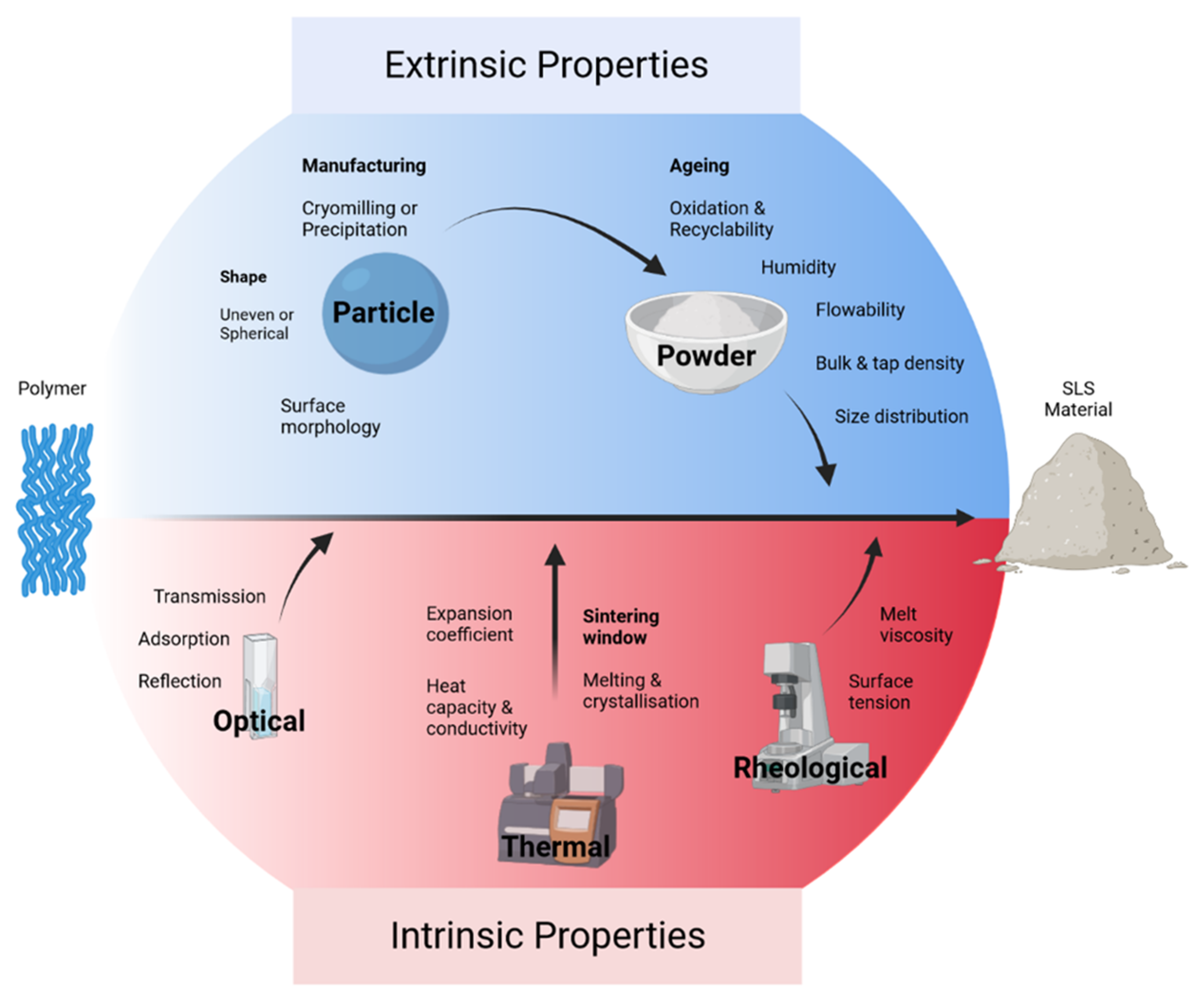
2.1. Polymers
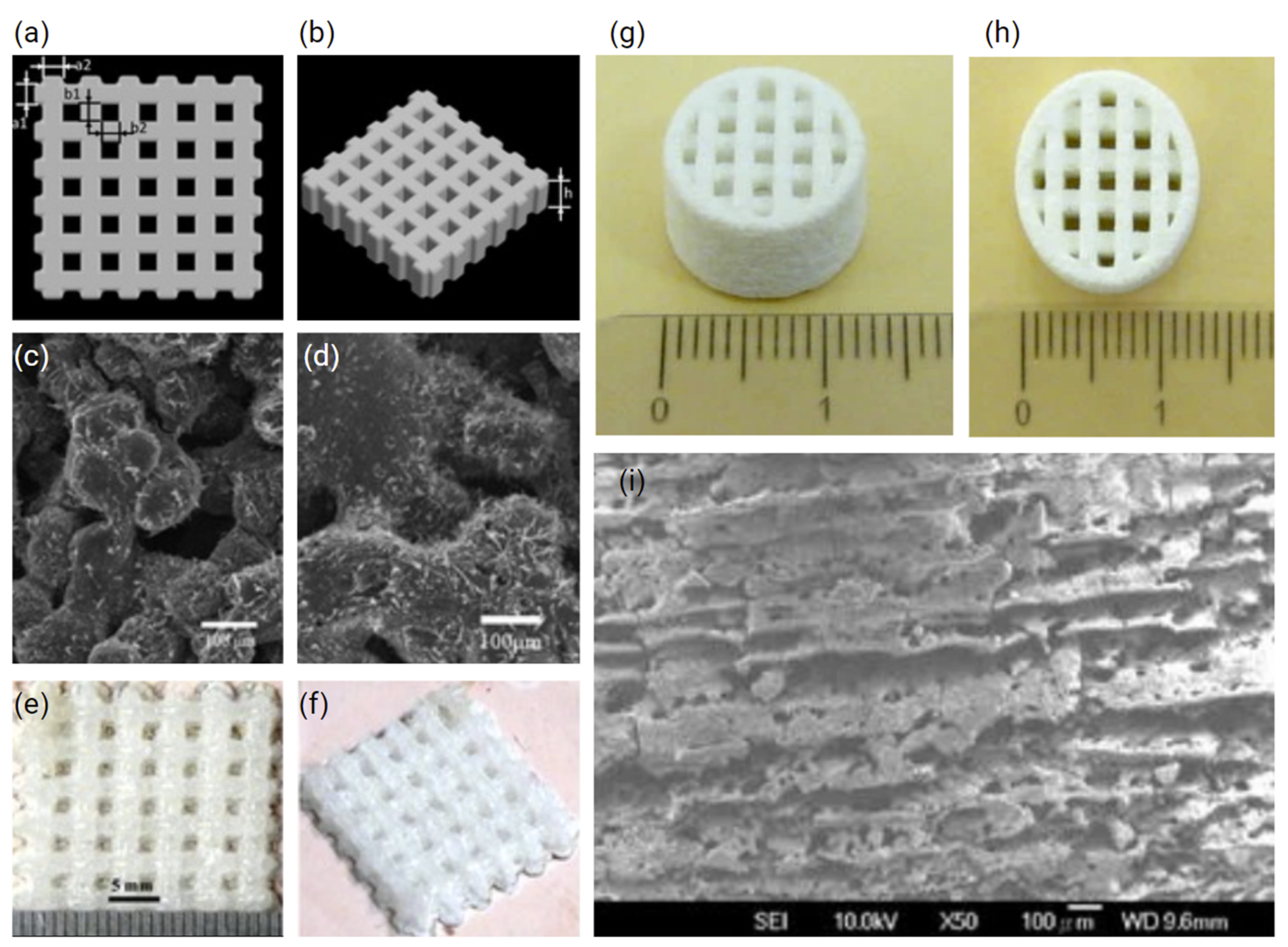
2.1.1. Polyamide
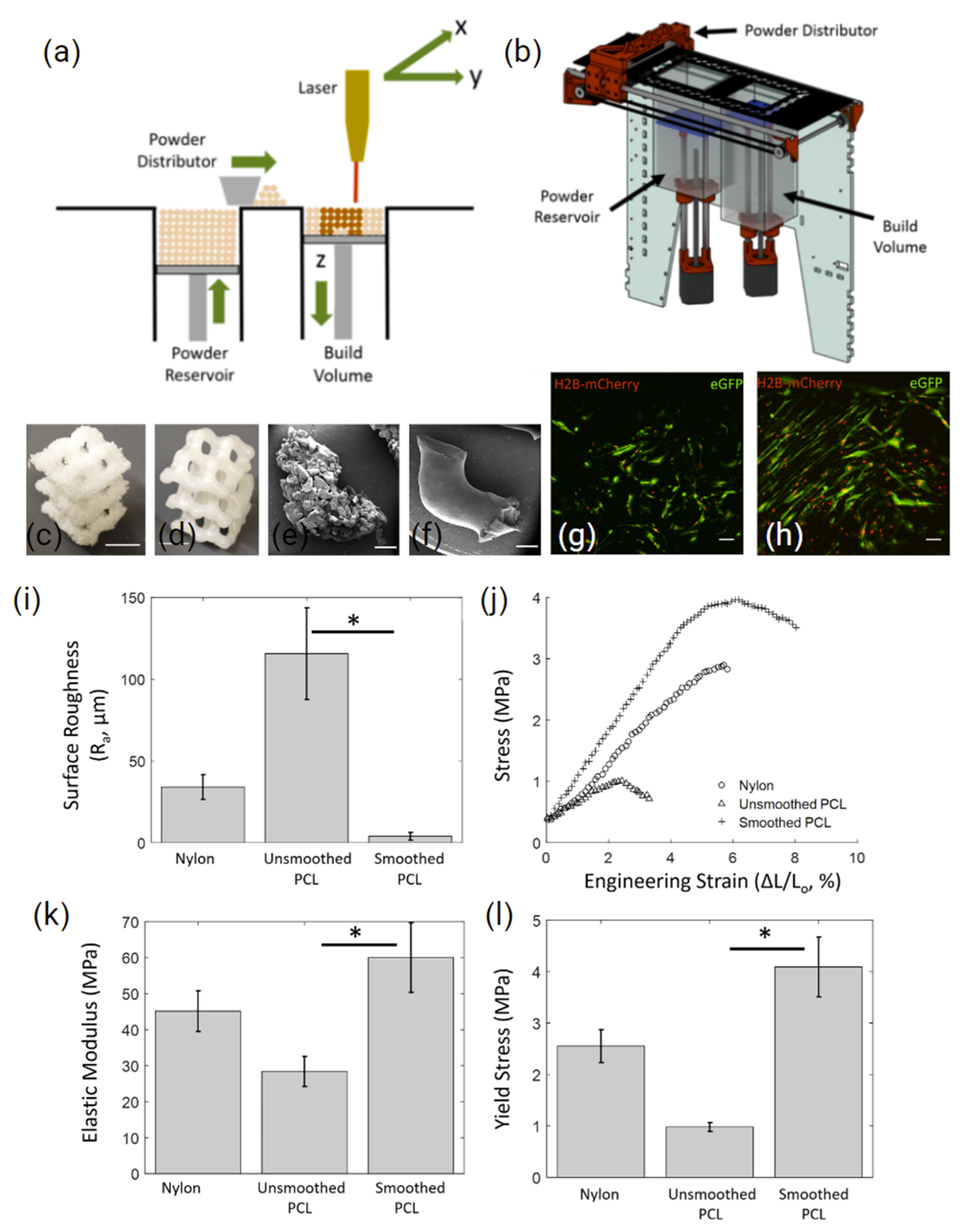
2.1.2. Polycaprolactone
2.1.3. Polyethylene
2.1.4. Polyetheretherketone
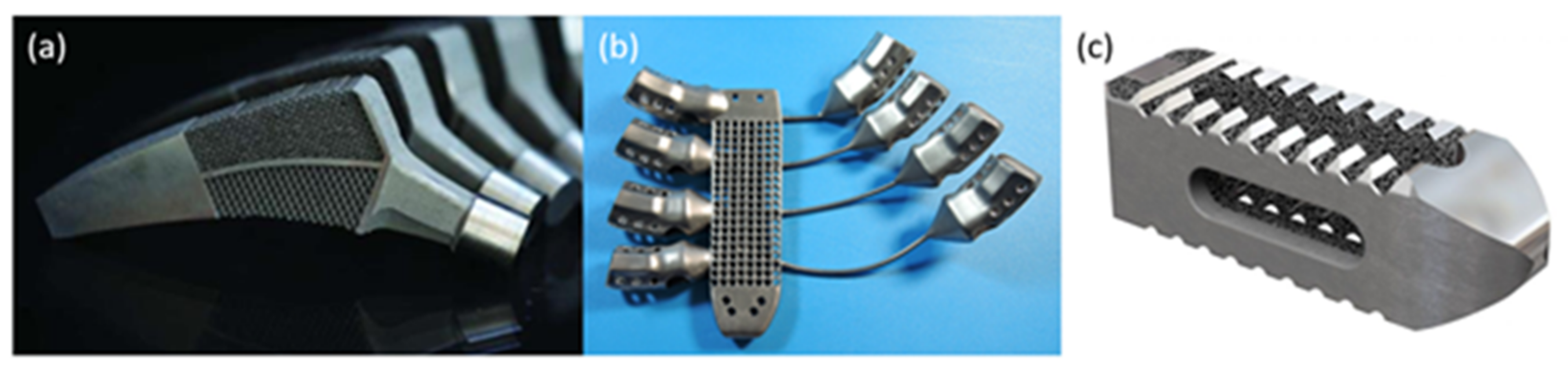
2.2. Metals
2.3. Ceramics
2.4. Composites
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Thayaparan, G.K.; Lewis, P.M.; Thompson, R.G.; D’Urso, P.S. Patient-specific implants for craniomaxillofacial surgery: A manufacturer’s experience. Ann. Med. Surg. 2021, 66, 102420. [Google Scholar] [CrossRef]
- Bilezikian, J.; Raisz, L.; Martin, T.J. (Eds.) Principles of Bone Biology, 3rd ed.; Elsevier: Singapore, 2019; Available online: https://www.elsevier.com/books/principles-of-bone-biology/bilezikian/978-0-12-373884-4 (accessed on 9 October 2019).
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Caplan, A.I. Tissue engineering designs for the future: New logics, old molecules. Tissue Eng. 2000, 6, 1–8. [Google Scholar] [CrossRef]
- Reddi, A.H. Morphogenetic messages are in the extracellular matrix: Biotechnology from bench to bedside. In Biochemical Society Transactions; Portland Press Ltd.: London, UK, 2000; pp. 345–349. [Google Scholar]
- WHO Scientific Group. The Burden of Musculoskeletal Conditions at the Start of the New Millennium; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; Volume 919, pp. 1–218. [Google Scholar]
- Yunus Basha, R.; Sampath, S.K.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Kumar, G.; Narayan, B. Morbidity at bone graft donor sites. In Classic Papers in Orthopaedics; Springer: London, UK, 2014; pp. 503–505. [Google Scholar]
- Behnam Manesh, S.; Omani Samani, R.; Behnam Manesh, S. Ethical issues of transplanting organs from transgenic animals into human beings. Cell J. 2014, 16, 353–360. [Google Scholar]
- Griesemer, A.; Yamada, K.; Sykes, M. Xenotransplantation: Immunological hurdles and progress toward tolerance. Immunol. Rev. 2014, 258, 241–258. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.; Janbaz, P.; Rayati, F. Maxillofacial reconstruction with Medpor porous polyethylene implant: A case series study. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Paxton, N.C.; Allenby, M.C.; Lewis, P.M.; Woodruff, M.A. Biomedical applications of polyethylene. Eur. Polym. J. 2019, 118, 412–428. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, W.-C.; Huang, C.-H.; Cheng, C.-K.; Chan, K.-K.; Chang, T.-K. The effect of graft strength on knee laxity and graft in-situ forces after posterior cruciate ligament reconstruction. PLoS ONE 2015, 10, e0127293. [Google Scholar] [CrossRef] [Green Version]
- Palágyi, K.; Németh, G.; Kardos, P. Topology Preserving Parallel 3D Thinning Algorithms. In Digital Geometry Algorithms: Theoretical Foundations and Applications to Computational Imaging; Brimkov, V.E., Barneva, R.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 165–188. [Google Scholar]
- Wang, X.; Nyman, J.S.; Dong, X.; Leng, H.; Reyes, M. Fundamental Biomechanics in Bone Tissue Engineering. Synth. Lect. Tissue Eng. 2010, 2, 1–225. [Google Scholar] [CrossRef]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, eaao2189. [Google Scholar] [CrossRef] [Green Version]
- Galante, J.; Rostoker, W.; Ray, R.D. Physical properties of trabecular bone. Calcif. Tissue Res. 1970, 5, 236–246. [Google Scholar] [CrossRef]
- Roesler, H. The history of some fundamental concepts in bone biomechanics. J. Biomech. 1987, 20, 1025–1034. [Google Scholar] [CrossRef]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.J.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K. Fabrication of 3D Printed PCL/PEG Polyblend Scaffold Using Rapid Prototyping System for Bone Tissue Engineering Application. J. Bionic. Eng. 2018, 15, 435–442. [Google Scholar] [CrossRef]
- Le Huec, J.C.; Schaeverbeke, T.; Clement, D.; Faber, J.; Le Rebeller, A. Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials 1995, 16, 113–118. [Google Scholar] [CrossRef]
- Blokhuis, T.J.; Termaat, M.F.; den Boer, F.C.; Patka, P.; Bakker, F.C.; Haarman, H.J. Properties of calcium phosphate ceramics in relation to their in vivo behavior. J. Trauma 2000, 48, 179–186. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Rustom, L.E.; Boudou, T.; Lou, S.; Pignot-Paintrand, I.; Nemke, B.W.; Lu, Y.; Markel, M.D.; Picart, C.; Johnson, A.J.W. Micropore-induced capillarity enhances bone distribution in vivo in biphasic calcium phosphate scaffolds. Acta Biomater. 2016, 44, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohner, M.; Baroud, G.; Bernstein, A.; Döbelin, N.; Galea, L.; Hesse, B.; Heuberger, R.; Meille, S.; Michel, P.; von Rechenberg, B.; et al. Characterization and distribution of mechanically competent mineralized tissue in micropores of β-tricalcium phosphate bone substitutes. Mater. Today 2017, 20, 106–115. [Google Scholar] [CrossRef]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Bréchet, Y.J.M.; Fratzl, P.; Dunlop, J.W.C. How linear tension converts to curvature: Geometric control of bone tissue growth. PLoS ONE 2012, 7, e36336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidan, C.M.; Kollmannsberger, P.; Gering, V.; Ehrig, S.; Joly, P.; Petersen, A.; Vogel, V.; Fratzl, P.; Dunlop, J.W. Gradual conversion of cellular stress patterns into pre-stressed matrix architecture during in vitro tissue growth. J. R. Soc. Interface 2016, 13, 20160136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; OuYang, J.; Deng, T.; Liu, X.; Feng, Y.; Zhao, N.; Mao, C.; Wang, Y. 3D-plotted beta-tricalcium phosphate scaffolds with smaller pore sizes improve in vivo bone regeneration and biomechanical properties in a critical-sized calvarial defect rat model. Adv. Healthc. Mater. 2018, 7, 1800441. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Nazhat, S.N.; Sheikh Md Fadzullah, S.H.; Maquet, V.; Boccaccini, A.R. Mechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle-filled composites for tissue engineering scaffolds. Compos. Sci. Technol. 2007, 67, 1139–1147. [Google Scholar] [CrossRef]
- Goshulak, P.; Samiezadeh, S.; Aziz, M.S.R.; Bougherara, H.; Zdero, R.; Schemitsch, E.H. The biomechanical effect of anteversion and modular neck offset on stress shielding for short-stem versus conventional long-stem hip implants. Med. Eng. Phys. 2016, 38, 232–240. [Google Scholar] [CrossRef]
- Chanlalit, C.; Shukla, D.R.; Fitzsimmons, J.S.; An, K.-N.; O’Driscoll, S.W. Stress shielding around radial head prostheses. J. Hand. Surg. Am. 2012, 37, 2118–2125. [Google Scholar] [CrossRef]
- Sumner, D.R. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef]
- Bragdon, C.R.; Burke, D.; Lowenstein, J.D.; O’Connor, D.O.; Ramamurti, B.; Jasty, M.; Harris, W.H. Differences in stiffness of the interface between a cementless porous implant and cancellous bone in vivo in dogs due to varying amounts of implant motion. J. Arthroplast. 1996, 11, 945–951. [Google Scholar] [CrossRef]
- Maniatopoulos, C.; Pilliar, R.M.; Smith, D.C. Threaded versus porous-surfaced designs for implant stabilization in bone-endodontic implant model. J. Biomed. Mater. Res. 1986, 20, 1309–1333. [Google Scholar] [CrossRef]
- Cheng, L.; Suresh, K.S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; Camci-Unal, G.; et al. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. Int. J. Nanomed. 2021, 16, 4289–4319. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lossdörfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L.; Schwartz, Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur. Cell Mater. 2003, 6, 22–27. [Google Scholar] [CrossRef]
- Masuda, T.; Yliheikkilä, P.K.; Felton, D.A.; Cooper, L.F. Generalizations regarding the process and phenomenon of osseointegration. Part, I. In vivo studies. Int. J. Oral Maxillofac. Implant. 1998, 13, 17–29. [Google Scholar]
- Chen, H.; Wang, C.; Zhu, X.; Zhang, K.; Fan, Y.; Zhang, X. Fabrication of porous titanium scaffolds by stack sintering of microporous titanium spheres produced with centrifugal granulation technology. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 182–188. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005, 1, 211–222. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Cerardi, A.; Caneri, M.; Meneghello, R.; Concheri, G.; Ricotta, M. Mechanical characterization of polyamide cellular structures fabricated using selective laser sintering technologies. Mater. Des. 2013, 46, 910–915. [Google Scholar] [CrossRef]
- Mukherjee, K.; Gupta, S. Bone ingrowth around porous-coated acetabular implant: A three-dimensional finite element study using mechanoregulatory algorithm. Biomech. Model. Mechanobiol. 2016, 15, 389–403. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. ISO: Geneva, Switzerland, 2018. Available online: http://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/06/89/68936.html (accessed on 18 February 2022).
- Daigle, B.; Torsekar, M. The EU Medical Device Regulation and the US Medical Device Industry. J. Int. Econ. 2019, 1. Available online: https://heinonline.org/HOL/LandingPage?handle=hein.journals/jice2019&div=10&id=&page= (accessed on 1 January 2022).
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Regulation and Approval of Medical Devices: 1976–2020. JAMA 2021, 326, 420–432. [Google Scholar] [CrossRef]
- FDA. Biological Responses to Metal Implants; FDA: Silver Spring, MD, USA, 2011.
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts. What they can offer and what they cannot. J. Bone Jt. Surg. Ser. B 2007, 89, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef] [Green Version]
- Rotatori, D.; Lee, E.J.; Sleeva, S. The evolution of the workforce during the fourth industrial revolution. Hum. Resour. Dev. Int. 2021, 24, 92–103. [Google Scholar] [CrossRef]
- Kumar, L.; Haleem, A.; Tanveer, Q.; Javaid, M.; Shuaib, M.; Kumar, V. Rapid manufacturing: Classification and recent development. Int. J. Adv. Eng. Res. Sci. 2017, 4, 29–40. [Google Scholar] [CrossRef]
- Akilbekova, D.; Mektepbayeva, D. Patient specific in situ 3D printing. In 3D Printing in Medicine; Woodhead Publishing: Cambridge, UK, 2017; pp. 91–113. [Google Scholar]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.-J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf.-Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Hofland, E.C.; Baran, I.; Wismeijer, D.A. Correlation of Process Parameters with Mechanical Properties of Laser Sintered PA12 Parts. Adv. Mater. Sci. Eng. 2017, 2017, 4953173. [Google Scholar] [CrossRef] [Green Version]
- Tolochko, N.K.; Khlopkov, Y.V.; Mozzharov, S.E.; Ignatiev, M.B.; Laoui, T.; Titov, V.I. Absorptance of powder materials suitable for laser sintering. Rapid Prototyp. J. 2000, 39, 88. [Google Scholar] [CrossRef]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 39, 88. [Google Scholar] [CrossRef] [Green Version]
- Józwik, J.; Ostrowski, D.; Milczarczyk, R.; Krolczyk, G.M. Analysis of relation between the 3D printer laser beam power and the surface morphology properties in Ti-6Al-4V titanium alloy parts. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 215. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Gong, H.; Pal, D.; Rafi, K.; Starr, T.; Stucker, B. Influences of Energy Density on Porosity and Microstructure of Selective Laser Melted 17-4PH Stainless Steel. In Proceedings of the 2013 Solid Freeform Fabrication Symposium, Austin, TX, USA, 12–14 August 2013; Available online: http://utw10945.utweb.utexas.edu/Manuscripts/2013/2013-37-Gu.pdf (accessed on 27 April 2021).
- Ho, H.C.H.; Cheung, W.L.; Gibson, I. Morphology and Properties of Selective Laser Sintered Bisphenol A Polycarbonate. Ind. Eng. Chem. Res. 2003, 42, 1850–1862. [Google Scholar] [CrossRef]
- Tan, K.H.; Chua, C.K.; Leong, K.F.; Cheah, C.M.; Cheang, P.; Abu Bakar, M.S.; Cha, S.W. Scaffold development using selective laser sintering of polyetheretherketone–hydroxyapatite biocomposite blends. Biomaterials 2003, 24, 3115–3123. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Materials perspective of polymers for additive manufacturing with selective laser sintering. J. Mater. Res. 2014, 29, 1824–1832. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.C.; Vail, N.K.; Barlow, J.W.; Beaman, J.J.; Bourell, D.L.; Marcus, H.L. Selective laser sintering of polymer-coated silicon carbide powders. Ind. Eng. Chem. Res. 1995, 34, 1641–1651. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Vandenbroucke, B.; Van Vaerenbergh, J.; Naert, I. Digital manufacturing of biocompatible metal frameworks for complex dental prostheses by means of SLS/SLM. In Proceedings of the 2nd Internet Conference on Advanced Research in Virtual and Rapid Prototyping, Leiria, Portugal, 28 September–1 October 2005; pp. 139–145. [Google Scholar]
- Chalancon, A.; Bourell, D. Measured energy densities for polyamide 12 and comparison of values calculated for laser sintering. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 8–10 August 2016; pp. 2217–2223. [Google Scholar]
- Scipioni Bertoli, U.; Wolfer, A.J.; Matthews, M.J.; Delplanque, J.-P.R.; Schoenung, J.M. On the limitations of Volumetric Energy Density as a design parameter for Selective Laser Melting. Mater. Des. 2017, 113, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Sampson, R.; Lancaster, R.; Sutcliffe, M.; Carswell, D.; Hauser, C.; Barras, J. An improved methodology of melt pool monitoring of direct energy deposition processes. Opt. Laser Technol. 2020, 127, 106194. [Google Scholar] [CrossRef]
- Thanki, A.; Goossens, L.; Mertens, R.; Probst, G. Study of keyhole-porosities in selective laser melting using X-ray computed tomography. In Proceedings of the 2019 iCT, Padova, Italy, 13–15 February 2019; Available online: https://lirias.kuleuven.be/retrieve/530678 (accessed on 30 September 2021).
- Yadav, P.; Rigo, O.; Arvieu, C.; Le Guen, E.; Lacoste, E. In Situ Monitoring Systems of The SLM Process: On the Need to Develop Machine Learning Models for Data Processing. Crystals 2020, 10, 524. [Google Scholar] [CrossRef]
- Alkahari, M.R.; Furumoto, T.; Ueda, T.; Hosokawa, A.; Tanaka, R.; Abdul Aziz, M.S. Thermal conductivity of metal powder and consolidated material fabricated via selective Laser Melting. Key Eng. Mater. 2012, 523, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Mertens, R.; Vrancken, B.; Holmstock, N.; Kinds, Y.; Kruth, J.-P.; Van Humbeeck, J. Influence of Powder Bed Preheating on Microstructure and Mechanical Properties of H13 Tool Steel SLM Parts. Phys. Procedia 2016, 83, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.; Spontak, R.J.; Koch, C.C.; Saw, C.K.; Balik, C.M. Structural changes in poly(ethylene terephthalate) induced by mechanical milling. Polymer 2000, 41, 7147–7157. [Google Scholar] [CrossRef]
- Jonna, S.; Lyons, J. Processing and properties of cryogenically milled post-consumer mixed plastic waste. Polym. Test. 2005, 24, 428–434. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Zhao, Z.; Wang, M.; Zhang, J. Study in performance and morphology of polyamide 12 produced by selective laser sintering technology. Rapid Prototyp. J. 2018, 24, 813–820. [Google Scholar] [CrossRef]
- Duan, B.; Cheung, W.L.; Wang, M. Optimized fabrication of Ca–P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 15001. [Google Scholar] [CrossRef]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, X.; Zhan, Q.; Wang, K. Microstructure evolution and mechanical properties of in-situ Ti6Al4V–TiB composites manufactured by selective laser melting. Compos. Part B 2021, 207, 108567. [Google Scholar] [CrossRef]
- Gu, D.; Zhang, H.; Dai, D.; Xia, M.; Hong, C.; Poprawe, R. Laser additive manufacturing of nano-TiC reinforced Ni-based nanocomposites with tailored microstructure and performance. Compos. Part B 2019, 163, 585–597. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Sun, H.; Huang, S.; Zeng, F. Effect of the properties of the polymer materials on the quality of selective laser sintering parts. Proc. Inst. Mech. Eng. Part L J. Mat. Des. Appl. 2004, 218, 247–252. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Polymer powders for selective laser sintering (SLS). In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2015. [Google Scholar] [CrossRef] [Green Version]
- Wudy, K.; Drummer, D. Aging effects of polyamide 12 in selective laser sintering: Molecular weight distribution and thermal properties. Addit. Manuf. 2019, 25, 1–9. [Google Scholar] [CrossRef]
- Drummer, D.; Harder, R.G.; Witt, G.; Wegner, A.; Wudy, K.; Drexler, M. Long-term Properties of Laser Sintered Parts of Polyamide 12-Influence of Storage Time and Temperature on the Aging Behavior. Int. J. Recent Contrib. Eng. Sci. IT 2015, 3, 20–27. [Google Scholar] [CrossRef]
- Benedetti, L.; Brulé, B.; Decreamer, N.; Evans, K.E.; Ghita, O. Shrinkage behaviour of semi-crystalline polymers in laser sintering: PEKK and PA12. Mater. Des. 2019, 181, 107906. [Google Scholar] [CrossRef]
- Winnacker, M. Polyamides and their functionalization: Recent concepts for their applications as biomaterials. Biomater. Sci. 2017, 5, 1230–1235. [Google Scholar] [CrossRef]
- Xiang, C.; Etrick, N.R.; Frey, M.W.; Norris, E.J.; Coats, J.R. Structure and Properties of Polyamide Fabrics with Insect-Repellent Functionality by Electrospinning and Oxygen Plasma-Treated Surface Coating. Polymers 2020, 12, 2196. [Google Scholar] [CrossRef]
- Niu, X.; Qin, M.; Xu, M.; Zhao, L.; Wei, Y.; Hu, Y.; Lian, X.; Liang, Z.; Chen, S.; Chen, W.; et al. Coated electrospun polyamide-6/chitosan scaffold with hydroxyapatite for bone tissue engineering. Biomed. Mater. 2021, 16, 25014. [Google Scholar] [CrossRef]
- McKeen, L.W. Introduction to Plastics and Polymers. In Film Properties of Plastics and Elastomers, 4th ed.; McKeen, L.W., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 1–24. [Google Scholar]
- Wudy, K.; Drummer, D.; Kühnlein, F.; Drexler, M. Influence of degradation behavior of polyamide 12 powders in laser sintering process on produced parts. AIP Conf. Proc. 2014, 1593, 691–695. [Google Scholar]
- Goodridge, R.D.; Tuck, C.J.; Hague, R.J.M. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Bai, J.; Goodridge, R.D.; Hague, R.J.M.; Song, M. Improving the mechanical properties of laser-sintered polyamide 12 through incorporation of carbon nanotubes. Polym. Eng. Sci. 2013, 53, 1937–1946. [Google Scholar] [CrossRef]
- Mazur, M.; Leary, M.; McMillan, M.; Sun, S.; Shidid, D.; Brandt, M. Laser Additive Manufacturing: Materials, Design, Technologies, and Applications; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Griessbach, S.; Lach, R.; Grellmann, W. Structure–property correlations of laser sintered nylon 12 for dynamic dye testing of plastic parts. Polym. Test. 2010, 29, 1026–1030. [Google Scholar] [CrossRef]
- Bourell, D.L.; Watt, T.J.; Leigh, D.K.; Fulcher, B. Performance Limitations in Polymer Laser Sintering. Phys. Procedia 2014, 56, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.-R.D.; Hornung, K.-H.D.; Feldmann, R.D.; Smigerski, H.-J.D. Verfahren Zur Herstellung von Pulverfoermigen Beschichtungsmitteln Auf Der Basis von Polyamiden MIT Mindestens 10 Ali-Phatisch Gebundenen Kohlenstoffatomen Pro Carbonamidgruppe. European Patent AT:1820T, 15 December 1982. Available online: https://patents.google.com/patent/AT1820T/en-20US4325121.pdf (accessed on 8 May 2021).
- Senff, H.; Gaboriau, C. Method for Preparing Polyamide Powder by Anionic Polymerisation. U.S. Patent 20100113661A1, 6 May 2010. Available online: https://patentimages.storage.googleapis.com/ff/1b/bf/1d071c259b1681/US20100113661A1.pdf (accessed on 9 May 2021).
- De Campos Vidal, B. Using the FT-IR linear dichroism method for molecular order determination of tendon collagen bundles and nylon 6. Acta Histochem. 2013, 115, 686–691. [Google Scholar] [CrossRef]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Das, S.; Hollister, S.J.; Flanagan, C.; Adewunmi, A.; Bark, K.; Chen, C.; Ramaswamy, K.; Rose, D.; Widjaja, E. Freeform fabrication of Nylon-6 tissue engineering scaffolds. Rapid Prototyp. J. 2003, 9, 43–49. [Google Scholar] [CrossRef]
- Das, S.; Hollister, S.J.; Flanagan, C.; Adewunmi, A.; Bark, K.; Chen, C.; Ramaswamy, K.; Rose, D.; Widjaja, E. Computational Design, Freeform Fabrication and Testing of Nylon-6 Tissue Engineering Scaffolds. MRS Online Proc. Libr. 2002, 758, 57. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lian, T.; Zhang, B.; Du, Y.; Du, K.; Xiang, N.; Jung, D.W.; Wang, G.; Osaka, A. Design and Mechanical Compatibility of Nylon Bionic Cancellous Bone Fabricated by Selective Laser Sintering. Materials 2021, 14, 1965. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Nuyken, O.; Pask, S.D. Ring-Opening Polymerization—An Introductory Review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef] [Green Version]
- Chuenjitkuntaworn, B.; Osathanon, T.; Nowwarote, N.; Supaphol, P.; Pavasant, P. The efficacy of polycaprolactone/hydroxyapatite scaffold in combination with mesenchymal stem cells for bone tissue engineering. J. Biomed. Mater. Res. A 2016, 104, 264–271. [Google Scholar] [CrossRef]
- Bahl, S.; Dolma, J.; Jyot Singh, J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2021, 39, 31–34. [Google Scholar] [CrossRef]
- BaoLin, G.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar]
- Górecka, Ż.; Idaszek, J.; Kołbuk, D.; Choińska, E.; Chlanda, A.; Święszkowski, W. The effect of diameter of fibre on formation of hydrogen bonds and mechanical properties of 3D-printed PCL. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111072. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Sailema-Palate, G.P.; Vidaurre, A.; Campillo-Fernández, A.J.; Castilla-Cortázar, I. A comparative study on Poly(ε-caprolactone) film degradation at extreme pH values. Polym. Degrad. Stab. 2016, 130, 118–125. [Google Scholar] [CrossRef]
- Aoyama, T.; Uto, K.; Shimizu, H.; Ebara, M.; Kitagawa, T.; Tachibana, H.; Suzuki, K.; Kodaira, T. Development of a new poly-ε-caprolactone with low melting point for creating a thermoset mask used in radiation therapy. Sci. Rep. 2021, 11, 20409. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D printed PCL/β-TCP cross-scale scaffold with high-precision fiber for providing cell growth and forming bones in the pores. Mater. Sci. Eng. C 2021, 127, 112197. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, e2001232. [Google Scholar] [CrossRef]
- Kinstlinger, I.S.; Bastian, A.; Paulsen, S.J.; Hwang, D.H.; Ta, A.H.; Yalacki, D.R.; Schmidt, T.; Miller, J.S. Open-Source Selective Laser Sintering (OpenSLS) of Nylon and Biocompatible Polycaprolactone. PLoS ONE 2016, 11, e0147399. [Google Scholar] [CrossRef] [Green Version]
- Mazzoli, A.; Ferretti, C.; Gigante, A.; Salvolini, E.; Mattioli-Belmonte, M. Selective laser sintering manufacturing of polycaprolactone bone scaffolds for applications in bone tissue engineering. Rapid Prototyp. J. 2015, 21, 386–392. [Google Scholar] [CrossRef]
- Yamada, H.; Evans, F.G. Strength of Biological Materials; Williams & Wilkins: Baltimore, MD, USA, 1970. [Google Scholar]
- Gu, X.; Zha, Y.; Li, Y.; Chen, J.; Liu, S.; Du, Y.; Zhang, S.; Wang, J. Integrated polycaprolactone microsphere-based scaffolds with biomimetic hierarchy and tunable vascularization for osteochondral repair. Acta Biomater. 2022, 141, 190–197. [Google Scholar] [CrossRef]
- Liang, X.; Duan, P.; Gao, J.; Guo, R.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp Composite Scaffold for Osteochondral Tissue Engineering and Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef]
- Demirors, M. The History of Polyethylene. In 100+ Years of Plastics Leo Baekeland and Beyond; Strom, T.E., Rasmussen, S.C., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 115–145. [Google Scholar]
- González-Aguilar, G.A.; Cruz, R.; Baez, R.; Wang, C.Y. Storage quality of bell peppers pretreated with hot water and polyethylene packaging. J. Food Qual. 1999, 22, 287–299. [Google Scholar] [CrossRef]
- Hamadouche, M.; Biau, D.J.; Huten, D.; Musset, T.; Gaucher, F. The use of a cemented dual mobility socket to treat recurrent dislocation. Clin. Orthop. Relat. Res. 2010, 468, 3248–3254. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Pascu, M. Practical Guide to Polyethylene; Rapra Technology Limited: Shropshire, UK, 2005; pp. 4–7. [Google Scholar]
- Sharma, R.K. Use of HDPE implants in facial skeletal augmentation: Should we rush for it? Indian J. Plast. Surg. 2010, 43, 40–41. [Google Scholar] [CrossRef] [Green Version]
- Hindy, P.; Hong, J.; Lam-Tsai, Y.; Gress, F. A comprehensive review of esophageal stents. Gastroenterol. Hepatol. 2012, 8, 526–534. [Google Scholar]
- Langlois, J.; Hamadouche, M. Recent update on crosslinked polyethylene in total hip arthroplasty. SICOT J. 2020, 6, 13. [Google Scholar] [CrossRef]
- Wegner, A. New Polymer Materials for the Laser Sintering Process: Polypropylene and Others. Phys. Procedia 2016, 83, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Amorim, F.L.; Lohrengel, A.; Neubert, V.; Higa, C.F.; Czelusniak, T. Selective laser sintering of Mo-CuNi composite to be used as EDM electrode. Rapid Prototyp. J. 2014, 20, 59–68. [Google Scholar] [CrossRef]
- Khalil, Y.; Kowalski, A.; Hopkinson, N. Influence of energy density on flexural properties of laser-sintered UHMWPE. Addit. Manuf. 2016, 10, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Ferrage, L.; Bertrand, G.; Lenormand, P.; Grossin, D.; Ben-Nissan, B. A review of the additive manufacturing (3DP) of bioceramics: Alumina, zirconia (PSZ) and hydroxyapatite. J. Aust. Ceram. Soc. 2017, 53, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Salmoria, G.V.; Ahrens, C.H.; Klauss, P.; Paggi, R.A.; Oliveira, R.G.; Lago, A. Rapid manufacturing of polyethylene parts with controlled pore size gradients using selective laser sintering. Mater. Res. 2007, 10, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Paxton, N.C.; Dinoro, J.; Ren, J.; Ross, M.T.; Daley, R.; Zhou, R.; Bazaka, K.; Thompson, R.G.; Yue, Z.; Beirne, S.; et al. Additive manufacturing enables personalised porous high-density polyethylene surgical implant manufacturing with improved tissue and vascular ingrowth. Appl. Mater. Today 2021, 22, 100965. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Vaish, A.; Vaishya, R. Three-Dimensional-Printed Polyether Ether Ketone Implants for Orthopedics. Indian J. Orthop. 2019, 53, 377–379. [Google Scholar] [CrossRef]
- Zhang, H. Fire-Safe Polymers and Polymer Composites; Office of Aviation Research, Federal Aviation Administration: Washington, DC, USA, 2004. [Google Scholar]
- Berretta, S.; Evans, K.E.; Ghita, O. Processability of PEEK, a new polymer for High Temperature Laser Sintering (HT-LS). Eur. Polym. J. 2015, 68, 243–266. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, A.; Skornyakov, I.; Shishkovsky, I. The Setup Design for Selective Laser Sintering of High-Temperature Polymer Materials with the Alignment Control System of Layer Deposition. Machines 2018, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Yazdani, B.; Chen, B.; Benedetti, L.; Davies, R.; Ghita, O.; Zhu, Y. A new method to prepare composite powders customized for high temperature laser sintering. Compos. Sci. Technol. 2018, 167, 243–250. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.; Ghita, O. Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Chen, P.; Cai, H.; Li, Z.; Li, M.; Wu, H.; Su, J.; Wen, S.; Zhou, Y.; Liu, J.; Wang, C.; et al. Crystallization kinetics of polyetheretherketone during high temperature-selective laser sintering. Addit. Manuf. 2020, 36, 101615. [Google Scholar] [CrossRef]
- Shackleford, A.S.D.; Williams, R.J.; Brown, R.; Wingham, J.R.; Majewski, C. Degradation of Laser Sintered polyamide 12 parts due to accelerated exposure to ultraviolet radiation. Addit. Manuf. 2021, 46, 102132. [Google Scholar] [CrossRef]
- Akande, S.O.; Dalgarno, K.W.; Munguia, J.; Pallari, J. Assessment of tests for use in process and quality control systems for selective laser sintering of polyamide powders. J. Mater. Process. Technol. 2016, 229, 549–561. [Google Scholar] [CrossRef]
- Roskies, M.; Jordan, J.O.; Fang, D.; Abdallah, M.-N.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J. Biomater. Appl. 2016, 31, 132–139. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.D.; De, A.; Zhang, W. Additive manufacturing of metallic components--process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Zadpoor, A.A. Mechanical performance of additively manufactured meta-biomaterials. Acta Biomater. 2019, 85, 41–59. [Google Scholar] [CrossRef]
- Du Plessis, A.; Broeckhoven, C.; Yadroitsava, I.; Yadroitsev, I.; Hands, C.H.; Kunju, R.; Bhate, D. Beautiful and Functional: A Review of Biomimetic Design in Additive Manufacturing. Addit. Manuf. 2019, 27, 408–427. [Google Scholar] [CrossRef]
- Harrysson, O.L.A.; Cansizoglu, O.; Marcellin-Little, D.J.; Cormier, D.R.; West, H.A. Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mater. Sci. Eng. C 2008, 28, 366–373. [Google Scholar] [CrossRef]
- Bragdon, C.R.; Jasty, M.; Greene, M.; Rubash, H.E.; Harris, W.H. Biologic fixation of total hip implants. Insights gained from a series of canine studies. J. Bone Jt. Surg. 2004, 86 (Suppl. 2), 105–117. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tian, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. J. Mater. Sci. Technol. 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.H.; Zadpoor, A.A. Rationally designed meta-implants: A combination of auxetic and conventional meta-biomaterials. Mater. Horiz. 2018, 5, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Jones, J.R.; Hench, L.L. Regeneration of trabecular bone using porous ceramics. Curr. Opin. Solid State Mater. Sci. 2003, 7, 301–307. [Google Scholar] [CrossRef]
- Zhu, T.; Ren, H.; Li, A.; Liu, B.; Cui, C.; Dong, Y.; Tian, Y.; Qiu, D. Novel bioactive glass based injectable bone cement with improved osteoinductivity and its in vivo evaluation. Sci. Rep. 2017, 7, 3622. [Google Scholar] [CrossRef] [PubMed]
- Zimmer Biomet. Biologics U.S. Product Portfolio; Zimmer Biomet: Warsaw, IN, USA, 2020; Available online: https://www.zimmerbiomet.com/content/dam/zb-corporate/en/products/specialties/biologics/plasmax-plasma-concentration-system/BiologicsComprehensiveBrochureFinal2020.pdf (accessed on 29 January 2020).
- Synthes, C.M.F.; Norian CRS Rotary Mix. Injectable Calcium Phosphate Bone Cement. 2004. Available online: http://synthes.vo.llnwd.net/o16/Mobile/Synthes%20International/KYO/CMF/PDFs/036.000.650.pdf (accessed on 10 October 2021).
- Vitoss|Stryker. 2020. Available online: https://www.stryker.com/us/en/spine/products/vitoss.html (accessed on 29 January 2020).
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Pryor, L.S.; Gage, E.; Langevin, C.-J.; Herrera, F.; Breithaupt, A.D.; Gordon, C.R.; Afifi, A.M.; Zins, J.E.; Meltzer, H.; Gosman, A.; et al. Review of bone substitutes. Craniomaxillofac. Trauma Reconstr. 2009, 2, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B Mater. Biol. Med. 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Zhuang, J.; Hu, H.; Peng, S.; Liu, D.; Liu, J. In vitro bioactivity and degradability of β-tricalcium phosphate porous scaffold fabricated via selective laser sintering. Biotechnol. Appl. Biochem. 2013, 60, 266–273. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Li, P.; Shuai, C.; Peng, S. Fabrication and Characterization of Porous 45S5 Glass Scaffolds via Direct Selective Laser Sintering. Mater. Manuf. Process. 2013, 28, 610–615. [Google Scholar] [CrossRef]
- Liu, F.H. Fabrication of Bioceramic Bone Scaffolds for Tissue Engineering. J. Mater. Eng. Perform. 2014, 23, 3762–3769. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E.; Tay, B.Y.; Zhao, Z.; Zhao, L.; Tian, Z.; Yang, S. Direct selective laser sintering and melting of ceramics: A review. Rapid Prototyp. J. 2017, 23, 611–623. [Google Scholar] [CrossRef]
- Seol, Y.J.; Park, D.Y.; Park, J.Y.; Kim, S.W.; Park, S.J.; Cho, D.-W. A new method of fabricating robust freeform 3D ceramic scaffolds for bone tissue regeneration. Biotechnol. Bioeng. 2013, 110, 1444–1455. [Google Scholar] [CrossRef]
- Grimal, Q.; Haupert, S.; Mitton, D.; Vastel, L.; Laugier, P. Assessment of cortical bone elasticity and strength: Mechanical testing and ultrasound provide complementary data. Med. Eng. Phys. 2009, 31, 1140–1147. [Google Scholar] [CrossRef]
- Lindner, M.; Hoeges, S.; Meiners, W.; Wissenbach, K.; Smeets, R.; Telle, R.; Poprawe, R.; Fischer, H. Manufacturing of individual biodegradable bone substitute implants using selective laser melting technique. J. Biomed. Mater. Res. A 2011, 97, 466–471. [Google Scholar] [CrossRef]
- Yeong, W.; Yap, C.Y.; Mapar, M.; Chua, C.K. State-of-the-art review on selective laser melting of ceramics. High Value Manuf. Adv. Res. Virtual Rapid Prototyp. 2013, 1, 65–70. [Google Scholar]
- Velez, M.; Kolan, K.C.R.; Leu, M.-C.; Hilmas, G.E.; Brown, R.F. Selective Laser Sintering Fabrication of 13–93 Bioactive Glass Bone Scaffolds. Biomater. Sci.—Process. Prop. Appl. 2011, 228, 185–193. [Google Scholar]
- Kolan, K.C.R.; Leu, M.C.; Hilmas, G.E.; Brown, R.F.; Velez, M. Fabrication of 13–93 bioactive glass scaffolds for bone tissue engineering using indirect selective laser sintering. Biofabrication 2011, 3, 25004. [Google Scholar] [CrossRef]
- Feng, P.; Gao, C.; Shuai, C.; Peng, S. Toughening and strengthening mechanisms of porous akermanite scaffolds reinforced with nano-titania. RSC Adv. 2015, 5, 3498–3507. [Google Scholar] [CrossRef]
- Lin, L.-L.; Shen, Y.-Y.; Zhang, J.-F.; Fang, M.-L. Microstructure and mechanical properties analysis of β-tricalcium phosphate/carbon nanotubes scaffold based on rapid prototyping. J. Shanghai Univ. 2009, 13, 349. [Google Scholar] [CrossRef]
- Feng, P.; Wei, P.; Li, P.; Gao, C.; Shuai, C.; Peng, S. Calcium silicate ceramic scaffolds toughened with hydroxyapatite whiskers for bone tissue engineering. Mater. Charact. 2014, 97, 47–56. [Google Scholar] [CrossRef]
- Feng, P.; Wu, P.; Gao, C.; Yang, Y.; Guo, W.; Yang, W.; Shuai, C. A Multimaterial Scaffold With Tunable Properties: Toward Bone Tissue Repair. Adv. Sci. 2018, 5, 1700817. [Google Scholar] [CrossRef]
- Savalani, M.M.; Hao, L.; Dickens, P.M.; Zhang, Y.; Tanner, K.E.; Harris, R.A. The effects and interactions of fabrication parameters on the properties of selective laser sintered hydroxyapatite polyamide composite biomaterials. Rapid Prototyp. J. 2012, 18, 16–27. [Google Scholar] [CrossRef]
- Hui, D.; Goodridge, R.D.; Scotchford, C.A.; Grant, D.M. Laser sintering of nano-hydroxyapatite coated polyamide 12 powders. Addit. Manuf. 2018, 22, 560–570. [Google Scholar] [CrossRef]
- Ramu, M.; Ananthasubramanian, M.; Kumaresan, T.; Gandhinathan, R.; Jothi, S. Optimization of the configuration of porous bone scaffolds made of Polyamide/Hydroxyapatite composites using Selective Laser Sintering for tissue engineering applications. Biomed. Mater. Eng. 2018, 29, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Das, S. Processing and properties of glass bead particulate-filled functionally graded Nylon-11 composites produced by selective laser sintering. Mater. Sci. Eng. A 2006, 437, 226–234. [Google Scholar] [CrossRef]
- Goodridge, R.D.; Shofner, M.L.; Hague, R.J.M.; McClelland, M.; Schlea, M.R.; Johnson, R.B.; Tuck, C.J. Processing of a Polyamide-12/carbon nanofibre composite by laser sintering. Polym. Test. 2011, 30, 94–100. [Google Scholar] [CrossRef]
- Bonfield, W.; Grynpas, M.D.; Tully, A.E.; Bowman, J.; Abram, J. Hydroxyapatite reinforced polyethylene--a mechanically compatible implant material for bone replacement. Biomaterials 1981, 2, 185–186. [Google Scholar] [CrossRef]
- Bonfield, W.; Doyle, C.; Tanner, K.E. In vivo evaluation of hydroxyapatite reinforced polyethylene composites. In Biological and Biomedical Performence of Biomaterials; Elsevier: Amsterdam, The Netherlands, 1986; pp. 153–159. [Google Scholar]
- Savalani, M.M.; Hao, L.; Harris, R.A. Evaluation of CO2 and Nd:YAG Lasers for the Selective Laser Sintering of HAPEX®. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2006, 220, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive manufacturing approaches for hydroxyapatite-reinforced composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int. J. Nanomed. 2013, 8, 4197–4213. [Google Scholar]
- Eshraghi, S.; Das, S. Micromechanical finite-element modeling and experimental characterization of the compressive mechanical properties of polycaprolactone-hydroxyapatite composite scaffolds prepared by selective laser sintering for bone tissue engineering. Acta Biomater. 2012, 8, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Doyle, H.; Lohfeld, S.; McHugh, P. Evaluating the effect of increasing ceramic content on the mechanical properties, material microstructure and degradation of selective laser sintered polycaprolactone/β-tricalcium phosphate materials. Med. Eng. Phys. 2015, 37, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Qian, G.; Zan, J.; Qi, F.; Zhao, Z.; Yang, W.; Peng, S.; Shuai, C. A co-dispersion nanosystem of graphene oxide @silicon-doped hydroxyapatite to improve scaffold properties. Mater. Des. 2021, 199, 109399. [Google Scholar] [CrossRef]
- Shuai, C.; Wu, P.; Zhong, Y.; Feng, P.; Gao, C.; Huang, W.; Zhou, Z.; Chen, L.; Shuai, C. Polyetheretherketone/poly (glycolic acid) blend scaffolds with biodegradable properties. J. Biomater. Sci. Polym. Ed. 2016, 27, 1434–1446. [Google Scholar] [CrossRef]
- Shuai, C.; Huang, W.; Feng, P.; Gao, C.; Shuai, X.; Xiao, T.; Deng, Y.; Peng, S.; Wu, P. Tailoring properties of porous Poly (vinylidene fluoride) scaffold through nano-sized 58s bioactive glass. J. Biomater. Sci. Polym. Ed. 2016, 27, 97–109. [Google Scholar] [CrossRef]
- Song, X.H.; Li, W.; Song, P.H.; Su, Q.Y.; Wei, Q.S.; Shi, Y.S.; Liu, K.; Liu, W.G. Selective laser sintering of aliphatic-polycarbonate/hydroxyapatite composite scaffolds for medical applications. Int. J. Adv. Manuf. Technol. 2015, 81, 15–25. [Google Scholar]
- Kuznetsova, D.S.; Timashev, P.S.; Dudenkova, V.V.; Meleshina, A.V.; Antonov, E.A.; Krotova, L.I.; Popov, V.K.; Bagratashvili, V.N.; Zagaynova, E.V. Comparative Analysis of Proliferation and Viability of Multipotent Mesenchymal Stromal Cells in 3D Scaffolds with Different Architectonics. Bull. Exp. Biol. Med. 2016, 160, 535–541. [Google Scholar] [CrossRef]
- Wiria, F.E.; Chua, C.K.; Leong, K.F.; Quah, Z.Y.; Chandrasekaran, M.; Lee, M.W. Improved biocomposite development of poly(vinyl alcohol) and hydroxyapatite for tissue engineering scaffold fabrication using selective laser sintering. J. Mater. Sci. Mater. Med. 2008, 19, 989–996. [Google Scholar] [CrossRef]
- Liao, H.T.; Lee, M.Y.; Tsai, W.W.; Wang, H.C.; Lu, W.C. Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type, I. J. Tissue 2016, 10, E337–E353. [Google Scholar] [CrossRef]
- Moiduddin, K.; Mian, S.H.; Alkhalefah, H.; Umer, U. Digital Design, Analysis and 3D Printing of Prosthesis Scaffolds for Mandibular Reconstruction. Metals 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Salmoria, G.V.; Klauss, P.; Zepon, K.M.; Kanis, L.A. The effects of laser energy density and particle size in the selective laser sintering of polycaprolactone/progesterone specimens: Morphology and drug release. Int. J. Adv. Manuf. Technol. 2013, 66, 1113–1118. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug. Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Salmoria, G.V.; Cardenuto, M.R.; Roesler, C.R.M.; Zepon, K.M.; Kanis, L.A. PCL/Ibuprofen Implants Fabricated by Selective Laser Sintering for Orbital Repair. Procedia CIRP 2016, 49, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Wu, F.; Gao, H.; Cui, K.; Xian, M.; Zhong, J.; Tian, Y.; Fan, S.; Wu, G. A Dexamethasone-Eluting Porous Scaffold for Bone Regeneration Fabricated by Selective Laser Sintering. ACS Appl. Bio Mater. 2020, 3, 8739–8747. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Jara, M.O.; Swinnea, S.; Pillai, A.R.; Maniruzzaman, M. Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions. Pharmaceutics 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Malczewski, P.; Pesta, E.; Łaszcz, M.; Mendyk, A.; Polak, S.; Dorożyński, P. Selective laser sintering (SLS) technique for pharmaceutical applications—Development of high dose controlled release printlets. Addit. Manuf. 2021, 38, 101761. [Google Scholar] [CrossRef]
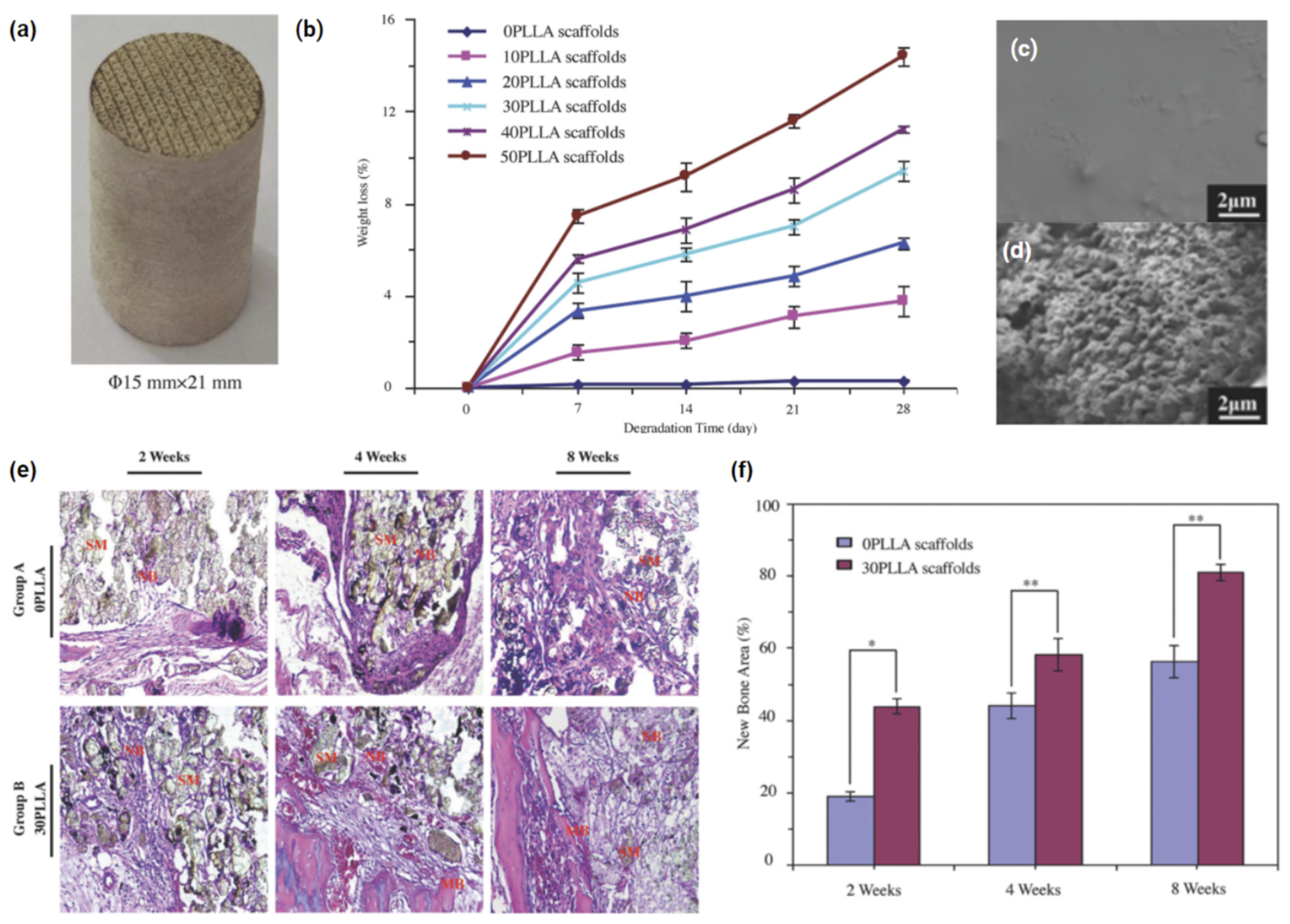
| Composite Formulation(s) | Print Specifications | Physical Attributes | Biological Response | Ref. |
|---|---|---|---|---|
| PCL/HA In wt% ratios of 100:0, 90:10, 80:20 and 70:30 | P = 1–1.2 W λ = 10.6 µm S = 152.4 µm T = N/A V = 914 mm/s Φ = 450 µm 50 °C bed temp | Increased HA concentration resulted in a higher E but a reduction in σUC | - | [196] |
| PCL/β-TCP In wt% ratios of 100:0, 90:10, 50:50, NB 50:50 utilised smaller PCL particles | P = 7 W λ = 10.6 µm S = N/A T = 0.11 mm V = N/A Φ = 410 µm 49 °C bed temp | Increasing β-TCP content was found to decrease the strength | In vivo bone formation significantly lower in PCL/TCP sintered composite compared to pure β-TCP | [197] |
| PLLA/GO@Si-HA | P = 3.5 W λ = N/A S = N/A T = N/A V = 180 mm/s | Compressive strength and modulus improved by 85% and 120% after incorporating GO@Si-HA, with a marginal improvement in hardness | 4 wk SBF: PLLA minimal, PLLA/GO minimal, PLLA/GO@Si-HA significantly improved appetite formation and MG-63 cell morphology and ALP activity after 7 days | [198] |
| PEEK PEEK/20%plyglycolicacid (PGA) PEEK/40%PGA | P = 100 W (max) λ = 10.6 µm S = 2.5 mm T = 0.1–0.2 mm V = 400 mm/min Φ = 800 µm | Increase in PGA concentration reduced compressive and tensile strength | PGA had no significant influence on MG-63 cell viability or morphology | [199] |
| Poly (vinylidene fluoride)/Bioactive glass 58s (PVDF/58s) | P = 100 W (max) λ = 10.6 µm S = 3 mm T = 0.1–0.2 mm V = 500 mm/min Φ = 800 µm | BG was found to be slightly exposed on the surface of scaffolds following EDS analysis | BG 58s addition improved osteoconductivity and osteoinductivity of scaffolds, following SBF and MG-63 cell seeding analysis | [200] |
| Aliphatic-polycarbonate/HA(a-PC/HA) a-PC a-PC/5 wt% HA a-PC/10 wt% HA a-PC/15 wt% HA | P = 11 W λ = 10.6 µm S = 0.15 mm T = 0.15 mm V = 2000 mm/s Φ = 200 µm 135 °C bed temp | Surface roughness and porosity (53 to 82%) increased with HA content, below 15 wt% ideal 6–7 times reduction in scaffold strength with HA compared to pure a-PC | Osteoconductivity unchanged by SLS processing | [201] |
| Poly[3,6-dimethyl-1,4-dioxane-2,5-dione]/HA | P = 10 W λ = 1.06 µm S = N/A T = N/A V = mm/s Φ = 125 µm | Young’s modulus increased from 6.4 to 8.4 GPa with HA addition | Sintered composite scaffolds improved ATSC attachment and viability, compared to foaming method and virgin polymer | [202] |
| PVA/HA 90:10 vol% 10–75 µm 50–100 µm | P = 10–20 W λ = 10.6 µm S = N/A T = N/A V = 1270–2540 mm/s & 2032 mm/s 65–75 °C bed temp & 80 °C bed temp for larger particles | Ball mixing was found to be best for homogenous blends of PVA and HA when compared to tumbler mixer. Larger particles also prevented clumping during layer deposition | - | [203] |
| PCL PCL/TCP PCL/TCP/collagen | P = 1 W (PCL) & 2 W (PCL/TCP) λ = N/A S = 0.2 mm T = N/A V = 500 mm/s 40 °C bed temp | Significant improvement of compressive modulus with addition of TCP, col no difference | Improved pASC attachment, viability and osteogenic differentiation (ALP and osteocalcin) with TCP and TCP/col addition, ALP activity highest at day 7 for all scaffolds (over 28 days). Woven bone and vasculature observed in vivo with composites, pure PCL was full of fibroblasts and granular tissue | [204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiNoro, J.N.; Paxton, N.C.; Skewes, J.; Yue, Z.; Lewis, P.M.; Thompson, R.G.; Beirne, S.; Woodruff, M.A.; Wallace, G.G. Laser Sintering Approaches for Bone Tissue Engineering. Polymers 2022, 14, 2336. https://doi.org/10.3390/polym14122336
DiNoro JN, Paxton NC, Skewes J, Yue Z, Lewis PM, Thompson RG, Beirne S, Woodruff MA, Wallace GG. Laser Sintering Approaches for Bone Tissue Engineering. Polymers. 2022; 14(12):2336. https://doi.org/10.3390/polym14122336
Chicago/Turabian StyleDiNoro, Jeremy N., Naomi C. Paxton, Jacob Skewes, Zhilian Yue, Philip M. Lewis, Robert G. Thompson, Stephen Beirne, Maria A. Woodruff, and Gordon G. Wallace. 2022. "Laser Sintering Approaches for Bone Tissue Engineering" Polymers 14, no. 12: 2336. https://doi.org/10.3390/polym14122336
APA StyleDiNoro, J. N., Paxton, N. C., Skewes, J., Yue, Z., Lewis, P. M., Thompson, R. G., Beirne, S., Woodruff, M. A., & Wallace, G. G. (2022). Laser Sintering Approaches for Bone Tissue Engineering. Polymers, 14(12), 2336. https://doi.org/10.3390/polym14122336







