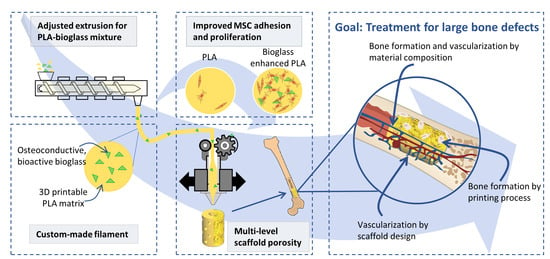
3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filament Fabrication
2.2. Sample Fabrication
2.3. Filament Diameter
2.4. Imaging
2.5. Image Analysis
2.6. Assessment of Mechanical Properties
2.7. Calcium Release
2.8. Ethics
2.9. Establishment and Characterization of the MSC Pool
2.10. Adhesion of MSCs
2.11. Statistical Analysis
3. Results
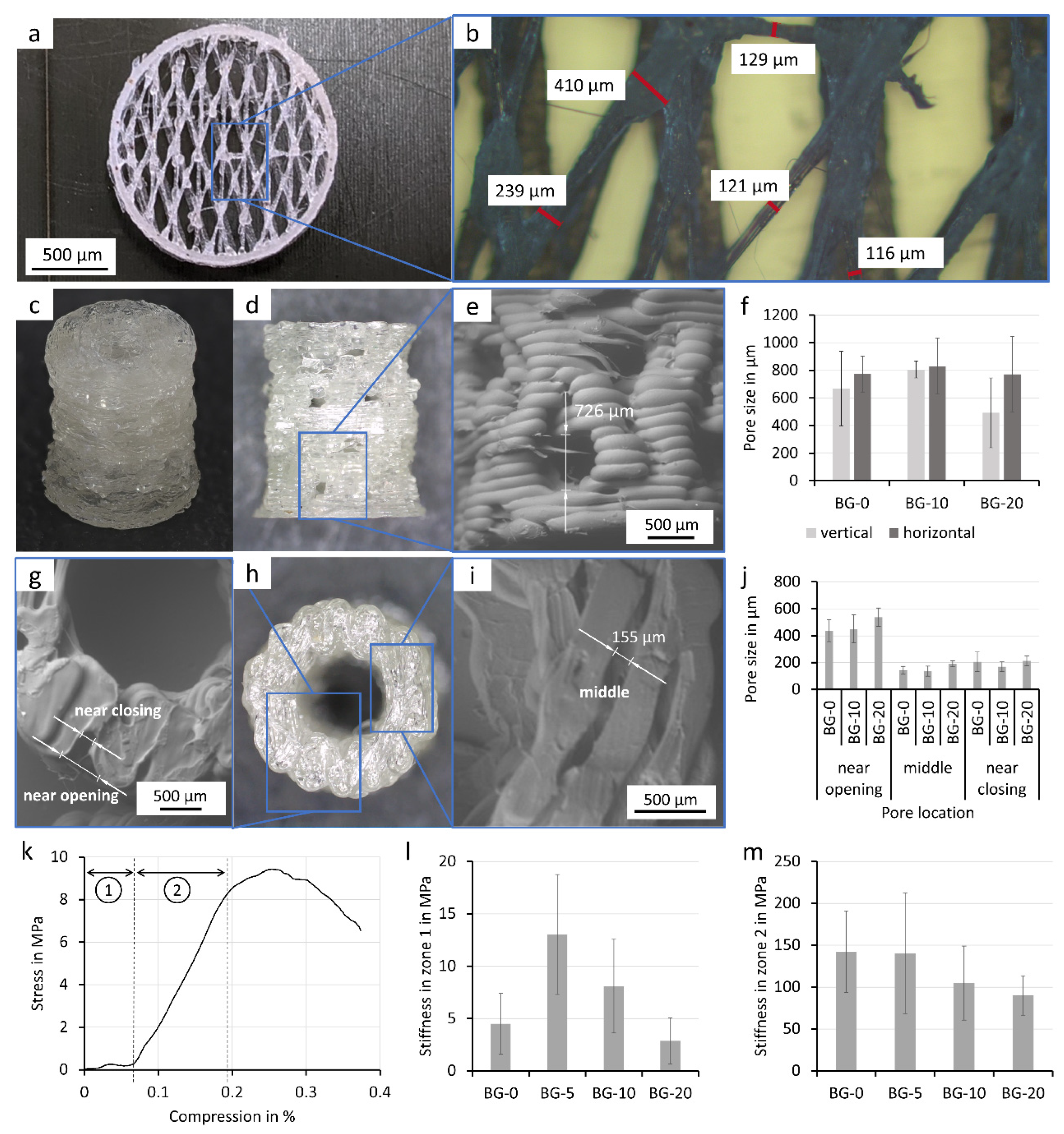
3.1. Filament Extrusion
3.2. Composite Filament Characterization
3.3. Processing of Composite Filament
3.4. FFF Printed Porous Scaffolds
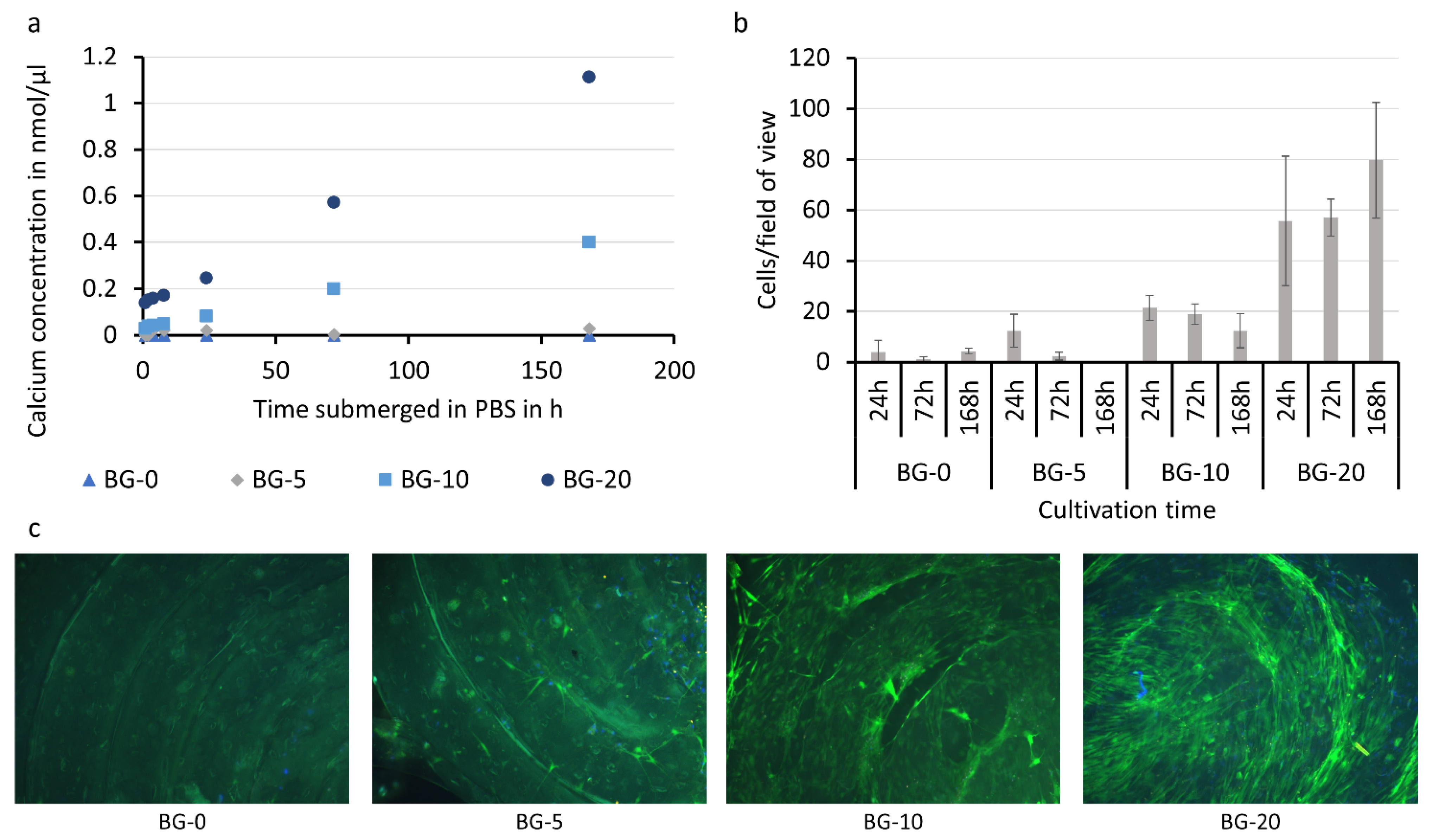
3.5. Bio-Chemical Characterization of PLA-Bioglass Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L.V.H. Autogenous Iliac Crest Bone Graft: Complications and Functional Assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components—Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef]
- Murphy, C.; Kolan, K.C.R.; Long, M.; Li, W.; Leu, M.C.; Semon, J.A.; Day, D.E. 3D Printing of a Polymer Bioactive Glass Composite for Bone Repair. In Proceedings of the 27th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 8–10 August 2016. [Google Scholar]
- Distler, T.; Fournier, N.; Grünewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef]
- Massera, J.; Hupa, L. Influence of SrO substitution for CaO on the properties of bioactive glass S53PJ. Mater. Sci. Mater. Med. 2013, 25, 657–668. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2009, 93A, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Leppäranta, O.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Hupa, M.; Ylänen, H.; Salonen, J.I.; Viljanen, M.K.; Eerola, E. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J. Mater. Sci. Mater. Med. 2007, 19, 547–551. [Google Scholar] [CrossRef]
- Xiao, W.; Zaeem, M.A.; Li, G.; Bal, B.S.; Rahaman, M.N. Tough and strong porous bioactive glass-PLA composites for structural bone repair. J. Mater. Sci. 2017, 52, 9039–9054. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Bortolin, M.; Toscano, M.; Mattina, R.; Romanò, C.L. Antimicrobial activity and resistance selection of different bioglass S53P4 formulations against multidrug resistant strains. Future Microbiol. 2015, 10, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.-H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017, 317, 119–131. [Google Scholar] [CrossRef]
- Nejad, Z.M.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Amoli, M.S. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef]
- Adriana Martel Estrada, S.; Olivas Armendáriz, I.; Torres García, A.; Francisco Hernández Paz, J.; Alejandra Rodríguez González, C. Evaluation of in Vitro Bioactivity of 45S5 Bioactive Glass/Poly Lactic Acid Scaffolds Produced by 3D Printing. Int. J. Compos. Mater. 2017, 7, 144–149. [Google Scholar] [CrossRef]
- Björkenheim, R.; Strömberg, G.; Pajarinen, J.; Ainola, M.; Uppstu, P.; Hupa, L.; Böhling, T.O.; Lindfors, N.C. Polymer-coated bioactive glass S53P4 increases VEGF and TNF expression in an induced membrane model in vivo. J. Mater. Sci. 2017, 52, 9055–9065. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, E.; Björkenheim, R.; Strömberg, G.; Ainola, M.; Uppstu, P.; Aalto-Setälä, L.; Leino, V.-M.; Hupa, L.; Pajarinen, J.; Lindfors, N.C. S53P4 bioactive glass scaffolds induce BMP expression and integrative bone formation in a critical-sized diaphysis defect treated with a single-staged induced membrane technique. Acta Biomater. 2021, 126, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, J.L.; Wang, J.; Mikos, A.G.; Zhang, S. Hierarchically designed bone scaffolds: From internal cues to external stimuli. Biomaterials 2019, 218, 119334. [Google Scholar] [CrossRef] [PubMed]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of Bone Marrow Mononuclear Cells on Biomaterials for Bone Tissue Engineering In Vitro. BioMed Res. Int. 2015, 2015, 762407. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Siebert, H.; Spiehl, D.; Nienhaus, V.; Krauße, M.; Dörsam, E. Prozessentwicklung Zur Herstellung Feiner Gitterstrukturen per Schmelzschichtung Development of a Process for the Production of Fine Grid Structures by the Use of Fused Filament Fabrication Kurzfassung Short Abstract. Rapid. Tech Fachkongress 2018, 16, 148–161. [Google Scholar]
- Söhling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef]
- Park, P.I.P.; Jonnalagadda, S. Predictors of glass transition in the biodegradable poly-lactide and poly-lactide-co-glycolide polymers. J. Appl. Polym. Sci. 2006, 100, 1983–1987. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. The Relationship between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef]
- Ziemath, E.C. Degradation of the surface of a metasilicate glass due to atmosphere moisture. Quim. Nova 1998, 21, 356–360. [Google Scholar] [CrossRef]
- Larrañaga, A.; Ramos, D.; Amestoy, H.; Zuza, E.; Sarasua, J.-R. Coating of bioactive glass particles with mussel-inspired polydopamine as a strategy to improve the thermal stability of poly(l-lactide)/bioactive glass composites. RSC Adv. 2015, 5, 65618–65626. [Google Scholar] [CrossRef] [Green Version]
- Caraseva, I.; Muñoz, J.; Sarasua, J.R.; Zuza, E. Effect of the Zeta-Potential of Oxide Particles in the Thermal Stability and Hydrolytic Degradation of Polylactides. In Proceedings of the 31st Conference of the European Society for Biomaterials, Porto, Portugal, 5–9 September 2021; pp. 927–928. [Google Scholar]
- Vanaei, H.R.; Khelladi, S.; Deligant, M.; Shirinbayan, M.; Tcharkhtchi, A. Numerical Prediction for Temperature Profile of Parts Manufactured using Fused Filament Fabrication. J. Manuf. Process. 2022, 76, 548–558. [Google Scholar] [CrossRef]
- Dressaire, E.; Sauret, A. Clogging of microfluidic systems. Soft Matter 2016, 13, 37–48. [Google Scholar] [CrossRef]
- Hafez, A.; Liu, Q.; Finkbeiner, T.; Alouhali, R.A.; Moellendick, T.E.; Santamarina, J.C. The effect of particle shape on discharge and clogging. Sci. Rep. 2021, 11, 3309. [Google Scholar] [CrossRef]
- Beran, T.; Mulholland, T.; Henning, F.; Rudolph, N.; Osswald, T.A. Nozzle clogging factors during fused filament fabrication of spherical particle filled polymers. Addit. Manuf. 2018, 23, 206–214. [Google Scholar] [CrossRef]
- Hvid, I.; Christensen, P.; Søndergaard, J.; Christensen, P.B.; Larsen, C.G. Compressive Strength of Tibial Cancellous Bone: Instron® and Osteopenetrometer Measurements in an Autopsy Material. Acta Orthop. Scand. 1983, 54, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng. 2015, 137, 010802. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus–density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial Progenitor Cells and Mesenchymal Stem Cells Seeded onto β-TCP Granules Enhance Early Vascularization and Bone Healing in a Critical-Sized Bone Defect in Rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Viateau, V.; Guillemin, G.; Bousson, V.; Oudina, K.; Hannouche, D.; Sedel, L.; Logeart-Avramoglou, D.; Petite, H. Long-bone critical-size defects treated with tissue-engineered grafts: A study on sheep. J. Orthop. Res. 2007, 25, 741–749. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, W.; Sendi, P. Pathogenesis of implant-associated infection: The role of the host. Semin. Immunopathol. 2011, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Bone Defect Repair in Mice by Mesenchymal Stem Cells. In Animal Models for Stem Cell Therapy; Humana Press: New York, NY, USA, 2014; pp. 193–207. [Google Scholar]
- Gómez-Barrena, E.; Padilla-Eguiluz, N.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.-M.; Giordano, R.; et al. Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury 2020, 51, S63–S73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schätzlein, E.; Kicker, C.; Söhling, N.; Ritz, U.; Neijhoft, J.; Henrich, D.; Frank, J.; Marzi, I.; Blaeser, A. 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers 2022, 14, 2389. https://doi.org/10.3390/polym14122389
Schätzlein E, Kicker C, Söhling N, Ritz U, Neijhoft J, Henrich D, Frank J, Marzi I, Blaeser A. 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers. 2022; 14(12):2389. https://doi.org/10.3390/polym14122389
Chicago/Turabian StyleSchätzlein, Eva, Christoph Kicker, Nicolas Söhling, Ulrike Ritz, Jonas Neijhoft, Dirk Henrich, Johannes Frank, Ingo Marzi, and Andreas Blaeser. 2022. "3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering" Polymers 14, no. 12: 2389. https://doi.org/10.3390/polym14122389
APA StyleSchätzlein, E., Kicker, C., Söhling, N., Ritz, U., Neijhoft, J., Henrich, D., Frank, J., Marzi, I., & Blaeser, A. (2022). 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers, 14(12), 2389. https://doi.org/10.3390/polym14122389