Control of Stimuli Sensitivity in pH-Switchable LCST/UCST-Type Thermosensitive Dendrimers by Changing the Dendrimer Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
3. Results and Discussion
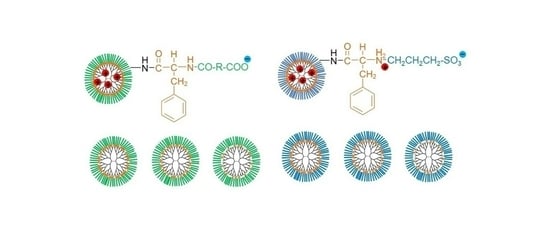
3.1. Synthesis of Various Anionic Terminal Dendrimers Bearing Phe
3.2. Thermosensitivity at Different pH Values
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Lee, V.E.; Liu, R.; Priestley, D.R. Responsive polymers as smart nanomaterials enable diverse applications. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent advances in dual temperature responsive block copolymers and their potential as biomedical applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Arotçaréna, M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the inside and the outside of aggregates of water-soluble block copolymers with double thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef]
- Costa, R.O.R.; Freitas, R.F.S. Phase behavior of poly(N-isopropylacrylamide) in binary aqueous solutions. Polymer 2002, 43, 5879–5885. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Amemori, S.; Kokado, K.; Sada, K. Fundamental molecular design for precise control of thermoresponsiveness of organic polymers by using ternary systems. J. Am. Chem. Soc. 2012, 134, 8344–8347. [Google Scholar] [CrossRef]
- Plamper, F.A.; Ballauff, M.A.; Müller, H.E. Tuning the thermoresponsiveness of weak polyelectrolytes by pH and light: Lower and upper critical-solution temperature of poly(N,N-dimethylaminoethyl methacrylate). J. Am. Chem. Soc. 2007, 129, 14538–14539. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch it inside-out: “Schizophrenic” behavior of all thermoresponsive UCST–LCST diblock copolymers. Langmuir 2019, 35, 9660−9676. [Google Scholar] [CrossRef]
- Vishnevetskaya, N.S.; Hildebrand, V.; Nizardo, N.M.; Ko, C.-H.; Di, Z.; Radulescu, A.; Barnsley, L.C.; Müller-Buschbaum, P.; Laschewsky, A.; Papadakis, C.M. All-in-one “schizophrenic” self-assembly of orthogonally tuned thermoresponsive diblock copolymers. Langmuir 2019, 35, 6441–6452. [Google Scholar] [CrossRef]
- Cao, H.; Guo, F.; Chen, Z.; Kong, X.Z. Preparation of thermoresponsive polymer nanogels of oligo(ethylene glycol) diacrylate-methacrylic acid and their property characterization. Nanoscale Res. Lett. 2018, 13, 209. [Google Scholar] [CrossRef] [Green Version]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The role of branch cell symmetry and other critical nanoscale design parameters in the determination of dendrimer encapsulation properties. Biomolecules 2020, 10, 642. [Google Scholar] [CrossRef]
- Kojima, C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010, 7, 307–319. [Google Scholar] [CrossRef]
- Haba, Y.; Harada, A.; Takagishi, T.; Kono, K. Rendering poly(amidoamine) or poly(propylenimine) dendrimers temperature sensitive. J. Am. Chem. Soc. 2004, 126, 12760–12761. [Google Scholar] [CrossRef]
- Haba, Y.; Kojima, C.; Harada, A.; Kono, K. Comparison of thermosensitive properties of poly(amidoamine) dendrimers with peripheral N-isopropylamide groups and linear polymers with the same groups. Angew. Chem. Int. Ed. 2007, 46, 234–237. [Google Scholar] [CrossRef]
- Li, X.; Haba, Y.; Ochi, K.; Yuba, E.; Harada, A.; Kono, K. PAMAM dendrimers with an oxyethylene unit-enriched surface as biocompatible temperature-sensitive dendrimers. Bioconjug. Chem. 2013, 24, 282–290. [Google Scholar] [CrossRef]
- Kojima, C.; Irie, K.; Tada, T.; Tanaka, N. Temperature-sensitive elastin-mimetic dendrimers: Effect of peptide length and dendrimer generation to temperature sensitivity. Biopolymers 2014, 101, 603–612. [Google Scholar] [CrossRef]
- Tamaki, M.; Fukushima, D.; Kojima, C. Dual pH-sensitive and UCST-type thermosensitive dendrimers: Phenylalanine-modified polyamidoamine dendrimers with carboxyl termini. RSC Adv. 2018, 8, 28147–28151. [Google Scholar] [CrossRef] [Green Version]
- Tono, Y.; Kojima, C.; Haba, Y.; Takahashi, T.; Harada, A.; Yagi, S.; Kono, K. Thermosensitive properties of poly(amidoamine) dendrimers with peripheral phenylalanine residues. Langmuir 2006, 22, 4920–4922. [Google Scholar] [CrossRef]
- Tamaki, M.; Kojima, C. pH-Switchable LCST/UCST-type thermosensitive behaviors of phenylalanine-modified zwitterionic dendrimers. RSC Adv. 2020, 10, 10452–10460. [Google Scholar] [CrossRef] [Green Version]
- Shiba, H.; Nishio, M.; Sawada, M.; Tamaki, M.; Michigami, M.; Nakai, S.; Nakase, I.; Fujii, I.; Matsumoto, A.; Kojima, C. Carboxy-terminal dendrimers with phenylalanine for pH-sensitive delivery system into immune cells including T cells. J. Mater. Chem. B 2022, 10, 2463–2470. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Williams, R. pKa Data Compiled by R. Williams. Available online: https://organicchemistrydata.org/hansreich/resources/pka/pka_data/pka-compilation-williams.pdf (accessed on 7 April 2022).
- Melander, W.; Horváth, C. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins: An interpretation of the lyotropic series. Arch. Biochem. Biophys. 1977, 183, 200–215. [Google Scholar] [CrossRef]





| Dendrimer 1 | Phe | Suc/CHex/SO3Na | Thermo- Sensitivity | |
|---|---|---|---|---|
| In Feed | Bound | Bound | ||
| PAMAM-Phe-Suc 2 | 96 | 56 | 57 | LCST/UCST |
| PAMAM-Phe64-CHex | 96 | 64 | 62 | UCST |
| PAMAM-Phe46-CHex | 62 | 46 | ~64 | UCST |
| PAMAM-Phe35-CHex | 46 | 35 | ~64 | UCST |
| PAMAM-Phe27-CHex | 38 | 27 | 62 | LCST/UCST |
| PAMAM-Phe16-CHex | 27 | 16 | ~64 | LCST/UCST |
| PAMAM-Phe57-SO3Na64 2 | 96 | 57 | 64 | LCST/UCST |
| PAMAM-Phe37-SO3Na68 | 49 | 37 | 68 | LCST/UCST |
| PAMAM-Phe27-SO3Na61 | 38 | 27 | 61 | LCST/UCST |
| PAMAM-Phe16-SO3Na62 | 27 | 16 | 62 | UCST |
| Dendrimer | Temperature (°C) | |||
|---|---|---|---|---|
| pH 7 | pH 5 | pH 4.5 | pH 4 | |
| PAMAM-Phe64-CHex | 60 (U) | Turbid | Turbid | Slightly |
| turbid | ||||
| PAMAM-Phe46-CHex | 46 (U) | >80 (U) | Turbid | Slightly |
| turbid | ||||
| PAMAM-Phe35-CHex | <20 (U) | >80 (U) | Turbid | Slightly |
| turbid | ||||
| PAMAM-Phe27-CHex | Clear | Turbid | 32 (L) | Clear |
| PAMAM-Phe16-CHex | Clear | >80 (U) | 68 (L) | Clear |
| Dendrimer | Temperature (°C) | ||||
|---|---|---|---|---|---|
| pH 6.5 | pH 6 | pH 5.5 | pH 5 | pH 4 | |
| PAMAM-Phe57-SO3Na64 | 36 (U) | 68 (U) | 24 (L)/ | 50 (L) | Clear |
| 68 (U) | |||||
| PAMAM-Phe37-SO3Na68 | Clear | 38 (U) | 62 (U) | 25 (L)/ | Clear |
| 76 (U) | |||||
| PAMAM-Phe27-SO3Na61 | Clear | 37 (U) | 55 (U) | 21 (L)/ | Clear |
| >80 (U) | |||||
| PAMAM-Phe16-SO3Na62 | Clear | Clear | 38 (U) | 44 (U) | Clear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, C.; Fu, Y.; Tamaki, M. Control of Stimuli Sensitivity in pH-Switchable LCST/UCST-Type Thermosensitive Dendrimers by Changing the Dendrimer Structure. Polymers 2022, 14, 2426. https://doi.org/10.3390/polym14122426
Kojima C, Fu Y, Tamaki M. Control of Stimuli Sensitivity in pH-Switchable LCST/UCST-Type Thermosensitive Dendrimers by Changing the Dendrimer Structure. Polymers. 2022; 14(12):2426. https://doi.org/10.3390/polym14122426
Chicago/Turabian StyleKojima, Chie, Yunshen Fu, and Mamiko Tamaki. 2022. "Control of Stimuli Sensitivity in pH-Switchable LCST/UCST-Type Thermosensitive Dendrimers by Changing the Dendrimer Structure" Polymers 14, no. 12: 2426. https://doi.org/10.3390/polym14122426
APA StyleKojima, C., Fu, Y., & Tamaki, M. (2022). Control of Stimuli Sensitivity in pH-Switchable LCST/UCST-Type Thermosensitive Dendrimers by Changing the Dendrimer Structure. Polymers, 14(12), 2426. https://doi.org/10.3390/polym14122426