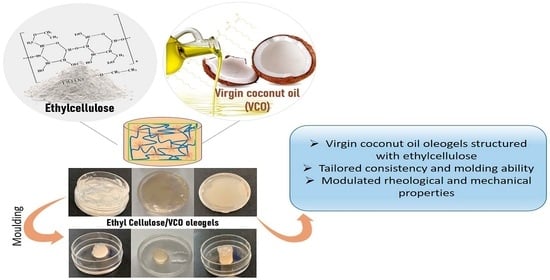
Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethylcellulose/Virgin Coconut Oil Oleogels Formation
2.3. Rheology
2.4. Oil Binding Capability
2.5. Free Fatty Acids Determination
2.6. Fourier Transform Infrared Spectroscopy
2.7. Oil Migration Ability and Stability Tests
Oil Migration Ability
2.8. Stability Assays
2.9. Antioxidant Activity
2.10. Differential Scanning Calorimetry
2.11. Statistical Analysis
3. Results and Discussion
3.1. Rheology
3.2. Oil Binding Capability
3.3. Structural Features
3.4. Oil Migration
3.5. Antioxidant Activity
3.6. Stability Behavior
3.7. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bascuas, S.; Salvador, A.; Hernando, I.; Quiles, A. Designing Hydrocolloid-Based Oleogels with High Physical, Chemical, and Structural Stability. Front. Sustain. Food Syst. 2020, 4, 111. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Ag, M. Development, Characterization, and Utilization of Food-Grade Polymer Oleogels. Annu. Rev. Food Sci. Technol. 2016, 7, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Amin, K.A.M.; Razali, M.H. Mechanical and Antibacterial Activities Study of Gellan Gum/Virgin Coconut Oil Film Embedded Norfloxacin. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 440, p. 012001. [Google Scholar] [CrossRef]
- Singh, A.; Auzanneau, F.I.; Rogers, M.A. Advances in edible oleogel technologies–A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Bascuas, S.; Morell, P.; Hernando, I.; Quiles, A. Recent trends in oil structuring using hydrocolloids. Food Hydrocoll. 2021, 118, 106612. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-Based Systems for the Delivery of Bioactive Compounds in Foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for development of health-promoting food products. Food Sci. Hum. Wellness 2020, 9, 31–39. [Google Scholar] [CrossRef]
- Rogers, M.A.; Wright, A.J.; Marangoni, A.G. Oil organogels: The fat of the future? Soft Matter 2009, 5, 1594–1596. [Google Scholar] [CrossRef]
- Giacintucci, V.; Di Mattia, C.D.; Sacchetti, G.; Flamminii, F.; Gravelle, A.J.; Baylis, B.; Dutcher, J.R.; Marangoni, A.G.; Pittia, P. Ethylcellulose oleogels with extra virgin olive oil: The role of oil minor components on microstructure and mechanical strength. Food Hydrocoll. 2018, 84, 508–514. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Barbut, S.; Marangoni, A.G. Fractionation of ethylcellulose oleogels during setting. Food Funct. 2013, 4, 153–161. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Gravelle, A.J.; Barbut, S.; Marangoni, A.G. Temperature effects on the gelation of ethylcellulose oleogels. Food Hydrocoll. 2015, 46, 76–83. [Google Scholar] [CrossRef]
- Roopan, S.M. An Overview of Phytoconstituents, Biotechnological Applications, and Nutritive Aspects of Coconut (Cocos nucifera). Appl. Biochem. Biotechnol. 2016, 179, 1309–1324. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Amin, I. Virgin coconut oil: Emerging functional food oil. Trends Food Sci. Technol. 2009, 20, 481–487. [Google Scholar] [CrossRef]
- Khor, Y.P.; Koh, S.P.; Long, K.; Long, S.; Ahmad, S.Z.; Tan, C.P. A comparative study of the physicochemical properties of a virgin coconut oil emulsion and commercial food supplement emulsions. Molecules 2014, 19, 9187–9202. [Google Scholar] [CrossRef] [Green Version]
- Dayrit, F. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Intahphuak, S.; Khonsung, P.; Panthong, A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm. Biol. 2010, 48, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Pandiselvam, R.; Ramarathinam, M.; Beegum, S.; Mathew, A. Virgin Coconut Oil infused healthy cosmetics. Indian Coconut J. 2019, 45, 30–32. [Google Scholar]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different Types of Gel Carriers as Metronidazole Delivery Systems to the Oral Mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Gomes, J.M.; Vilas-Boas, Â.; Pirraco, R.P.; Reis, R.L. Approach on chitosan/virgin coconut oil-based emulsion matrices as a platform to design superabsorbent materials. Carbohydr. Polym. 2020, 249, 116839. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Mohamad, S.F.; Ibrahim, M.A.; Mat Amin, K.A. Evaluation of Gellan Gum Film Containing Virgin Coconut Oil for Transparent Dressing Materials. Adv. Biomater. 2014, 2014, 351248. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wan, Y.P.E.; Marangoni, A.G.; Henry, C.J. Effects of liquid oil vs. oleogel co-ingested with a carbohydrate-rich meal on human blood triglycerides, glucose, insulin and appetite. Food Funct. 2017, 8, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.Y.; Peh, E.; Lau, E.; Marangoni, A.G.; Henry, C.J. Physical Form of Dietary Fat Alters Postprandial Substrate Utilization and Glycemic Response in Healthy Chinese Men. J. Nutr. 2017, 147, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, P.; Tabibiazar, M.; Roufegarinejad, L.; Babazadeh, A. Development of behenic acid-ethyl cellulose oleogel stabilized Pickering emulsions as low calorie fat replacer. Int. J. Biol. Macromol. 2020, 150, 974–981. [Google Scholar] [CrossRef]
- Dawson, R.; Elliot, D.; Elliot, W.; Jones, K. Data for Biochemical Research. In Biochemical Education; Wood, E.J., Ed.; Oxford Science Publisher: Oxford, UK, 1987; Volume 15, p. 97. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Laredo, T.; Barbut, S.; Marangoni, A.G. Molecular interactions of polymer oleogelation. Soft Matter. 2011, 7, 2734–2743. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Barbut, S.; Marangoni, A.G. Ethylcellulose oleogels: Manufacturing considerations and effects of oil oxidation. Food Res. Int. 2012, 48, 578–583. [Google Scholar] [CrossRef]
- Ye, X.; Li, P.; Lo, Y.M.; Fu, H.; Cao, Y. Development of Novel Shortenings Structured by Ethylcellulose Oleogels. J. Food Sci. 2019, 84, 1456–1464. [Google Scholar] [CrossRef]
- Yılmaz, E.; Uslu, E.K.; Toksöz, B. Structure, Rheological and Sensory Properties of Some Animal Wax Based Oleogels. J. Oleo Sci. 2020, 69, 1317–1329. [Google Scholar] [CrossRef]
- Patel, A.R.; Babaahmadi, M.; Lesaffer, A.; Dewettinck, K. Rheological Profiling of Organogels Prepared at Critical Gelling Concentrations of Natural Waxes in a Triacylglycerol Solvent. J. Agric. Food Chem. 2015, 63, 4862–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Hernández, A.K.; Pérez-Martínez, J.D.; Gallegos-Infante, J.A.; Toro-Vazquez, J.F.; Ornelas-Paz, J.J. Rheological properties of ethyl cellulose-monoglyceride-candelilla wax oleogel vis-a-vis edible shortenings. Carbohydr. Polym. 2021, 252, 117171. [Google Scholar] [CrossRef] [PubMed]
- Kwon, U.H.; Chang, Y.H. Rheological and Physicochemical Properties of Oleogel with Esterified Rice Flour and Its Suitability as a Fat Replacer. Foods 2022, 11, 242. [Google Scholar] [CrossRef]
- Luo, S.Z.; Hu, X.F.; Jia, Y.J.; Pan, L.H.; Zheng, Z.; Zhao, Y.Y.; Mu, D.D.; Zhong, X.Y.; Jiang, S.T. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: Preparation, characterization and potential application. Food Hydrocoll. 2019, 95, 76–87. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef]
- Abdollahi, M.; Goli, S.A.H.; Soltanizadeh, N. Physicochemical Properties of Foam-Templated Oleogel Based on Gelatin and Xanthan Gum. Eur. J. Lipid Sci Technol. 2020, 122, 1900196. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Muste, S. Application of Analytical Methods for the Comprehensive Analysis of Oleogels-A Review. Polymers 2021, 13, 1934. [Google Scholar] [CrossRef]
- Szymańska, I.; Żbikowska, A.; Kowalska, M. Physical stability of model emulsions based on ethyl cellulose oleogels. Int. Agrophys. 2020, 34, 289–300. [Google Scholar] [CrossRef]
- Haj Eisa, A.; Laufer, S.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. Stabilization of Ethyl-Cellulose Oleogel Network Using Lauric Acid. Eur. J. Lipid Sci. Technol. 2020, 122, 1900044. [Google Scholar] [CrossRef]
- Trivedi, M.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.; Jana, S. Characterization of Physicochemical and Thermal Properties of Biofield Treated Ethyl Cellulose and Methyl Cellulose. Int. J. Biomed. Mater. Res. 2015, 3, 83–91. [Google Scholar]
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. Application of FTIR Spectroscopy for the Determination of Virgin Coconut Oil in Binary Mixtures with Olive Oil and Palm Oil. J. Am. Oil Chem. Soc. 2010, 87, 601–606. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. The gelation of oil using ethyl cellulose. Carbohydr. Polym. 2015, 117, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Illam, S.P.; Raghavamenon, A.C. Health impacts of different edible oils prepared from coconut (Cocos nucifera): A comprehensive review. Trends Food Sci. Technol. 2018, 80, 1–7. [Google Scholar] [CrossRef]
- Ng, Y.J.; Tham, P.E.; Khoo, K.S.; Cheng, C.K.; Chew, K.W.; Show, P.L. A comprehensive review on the techniques for coconut oil extraction and its application. Bioprocess Biosyst. Eng. 2021, 44, 1807–1818. [Google Scholar] [CrossRef]
- Marina, A.M.; Man, Y.B.; Nazimah, S.A.; Amin, I. Antioxidant capacity and phenolic acids of virgin coconut oil. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 2), 114–123. [Google Scholar] [CrossRef]
- Elçin, A.E. In Vitro and In Vivo Degradation of Oxidized Acetyl- and Ethyl-Cellulose Sponges. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 407–418. [Google Scholar] [CrossRef]
- Lai, H.L.; Pitt, K.; Craig, D.Q.M. Characterisation of the thermal properties of ethylcellulose using differential scanning and quasi-isothermal calorimetric approaches. Int. J. Pharm. 2010, 386, 178–184. [Google Scholar] [CrossRef]
- Dubernet, C.; Rouland, J.; Benoit, J. Comparative study of two ethylcellulose forms (raw material and microspheres) carried out through thermal analysis. Int. J. Pharm. 1990, 64, 99–107. [Google Scholar]
- Marikkar, J.M.N. Differential Scanning Calorimetric Analysis of Virgin Coconut Oil, Palm Olein, and their Adulterated Blends. CORD 2019, 35, 34–42. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Sajeevkumar, V.A.; Sharma, G.K. Melting, crystallization and storage stability of virgin coconut oil and its blends by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). J. Food Sci. Technol. 2017, 54, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Identification | Ethylcellulose Content (%) | Virgin Coconut Oil Content (%) | Molding Temperature |
|---|---|---|---|
| EC/VCO 5/95_RT | 5 | 95 | RT |
| EC/VCO 5/95_37 °C | 5 | 95 | 37 °C |
| EC/VCO 10/90_RT | 10 | 90 | RT |
| EC/VCO 10/90_37 °C | 10 | 90 | 37 °C |
| EC/VCO 15/85_RT | 15 | 85 | RT |
| EC/VCO 15/85_37 °C | 15 | 85 | 37 °C |
| Composition | G′ (kPa) | G″ (kPa) | tanδ |
|---|---|---|---|
| EC/VCO 5/95_RT | 1.13 ± 0.12 | 7.46 ± 0.68 | 6.60 |
| EC/VCO 5/95_37 °C | 0.83 ± 0.05 | 0.59 ± 0.30 | 0.71 |
| EC/VCO 10/90_RT | 127.0 ± 22.62 | 19.55 ± 5.26 | 0.15 |
| EC/VCO 10/90_37 °C | 199.20 ± 92.35 | 33.03 ± 13.17 | 0.17 |
| EC/VCO 15/85_RT | 106.45 ± 0.35 | 16.37 ± 0.60 | 0.15 |
| EC/VCO 15/85_37 °C | 279.65 ± 41.79 | 50.90 ± 8.62 | 0.18 |
| Sample | Weight Loss (%) | |
|---|---|---|
| PBS | pH = 5 | |
| EC/VCO 5/95_RT | 73.9 ± 14.8 | 61.1 ± 6.4 |
| EC/VCO 5/95_37 °C | * | * |
| EC/VCO 10/90_RT | 23.9 ± 2.9 | 18.1 ± 2.9 |
| EC/VCO 10/90_37 °C | 13.20 ± 4.5 | 21.06 ± 5.9 |
| EC/VCO 15/85_RT | 16.20 ± 6.2 | 11.12 ± 1.9 |
| EC/VCO 15/85_37 °C | 23.66 ± 1.8 | 19.18 ± 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Lobo, F.C.M.; Gomes, J.M.; Reis, R.L. Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil. Polymers 2022, 14, 2473. https://doi.org/10.3390/polym14122473
Silva SS, Rodrigues LC, Fernandes EM, Lobo FCM, Gomes JM, Reis RL. Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil. Polymers. 2022; 14(12):2473. https://doi.org/10.3390/polym14122473
Chicago/Turabian StyleSilva, Simone S., Luísa C. Rodrigues, Emanuel M. Fernandes, Flávia C. M. Lobo, Joana M. Gomes, and Rui L. Reis. 2022. "Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil" Polymers 14, no. 12: 2473. https://doi.org/10.3390/polym14122473
APA StyleSilva, S. S., Rodrigues, L. C., Fernandes, E. M., Lobo, F. C. M., Gomes, J. M., & Reis, R. L. (2022). Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil. Polymers, 14(12), 2473. https://doi.org/10.3390/polym14122473