Unfilled Natural Rubber Compounds Containing Bio-Oil Cured with Different Curing Systems: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NR Compounds
2.3. Testing and Characterizations
2.3.1. Swelling Test
2.3.2. Cure Behavior
2.3.3. Crosslink Density
2.3.4. Tensile Properties
2.3.5. Hardness
2.3.6. Compression Set
3. Results and Discussion
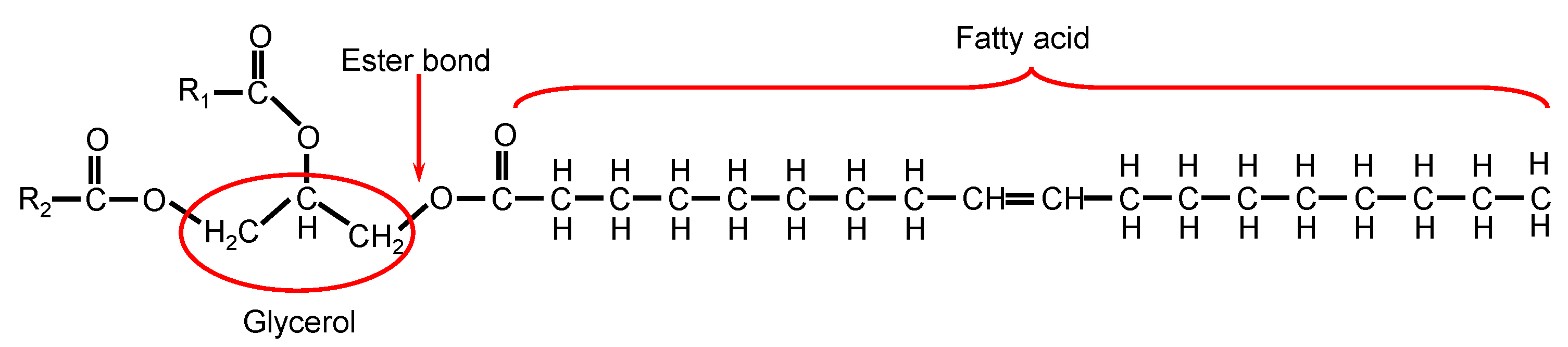
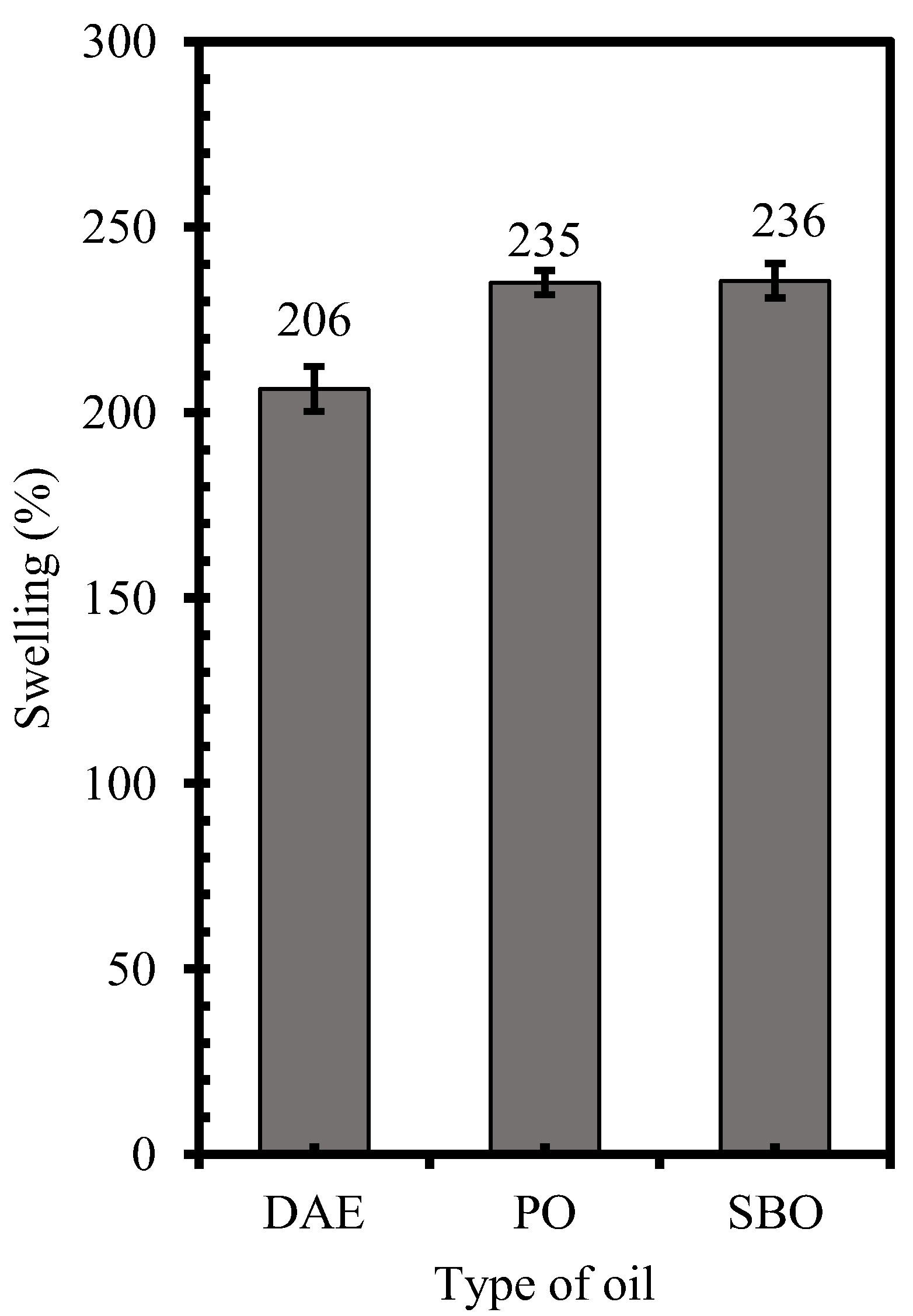
3.1. Solubility Aspects
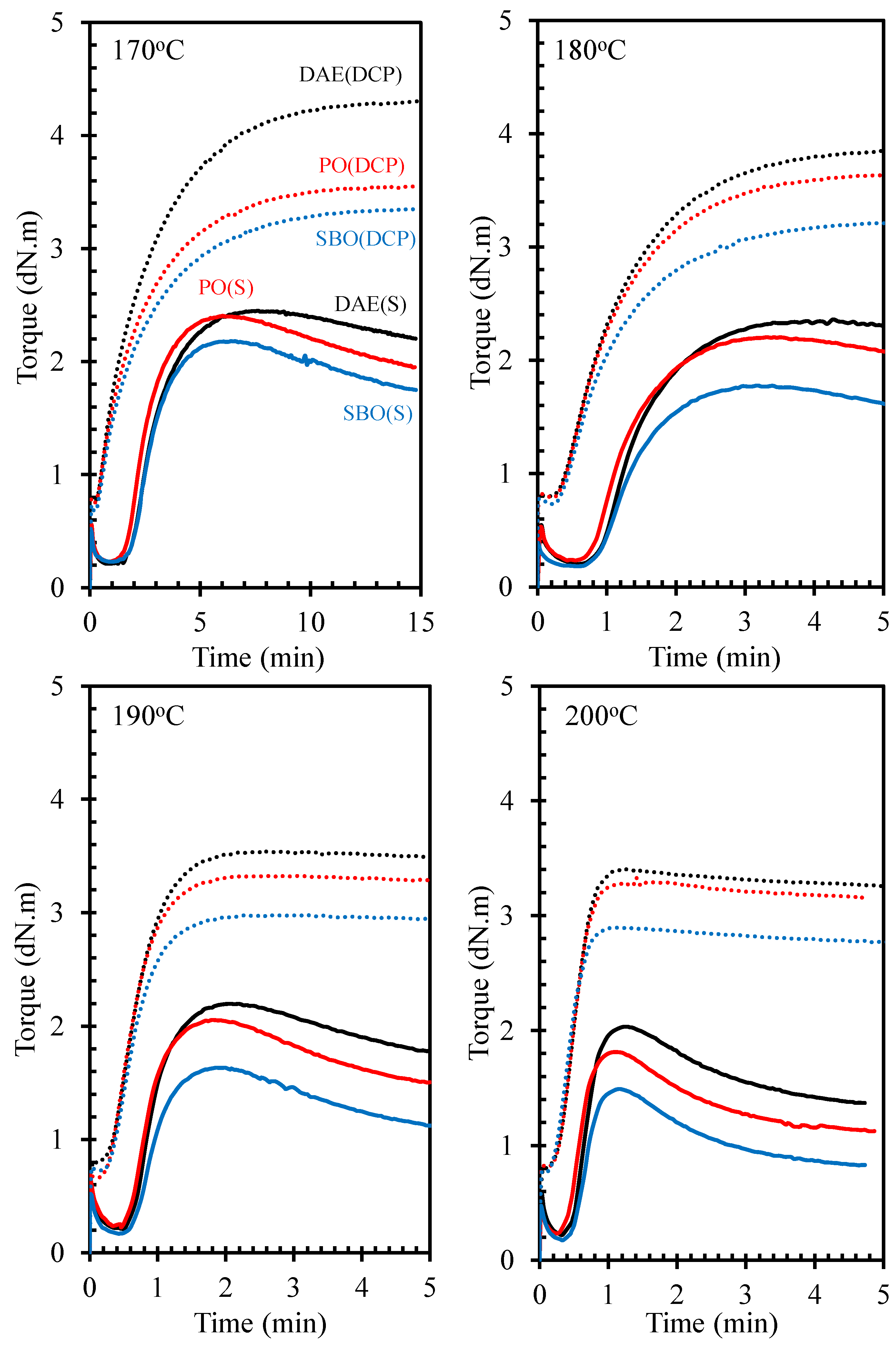
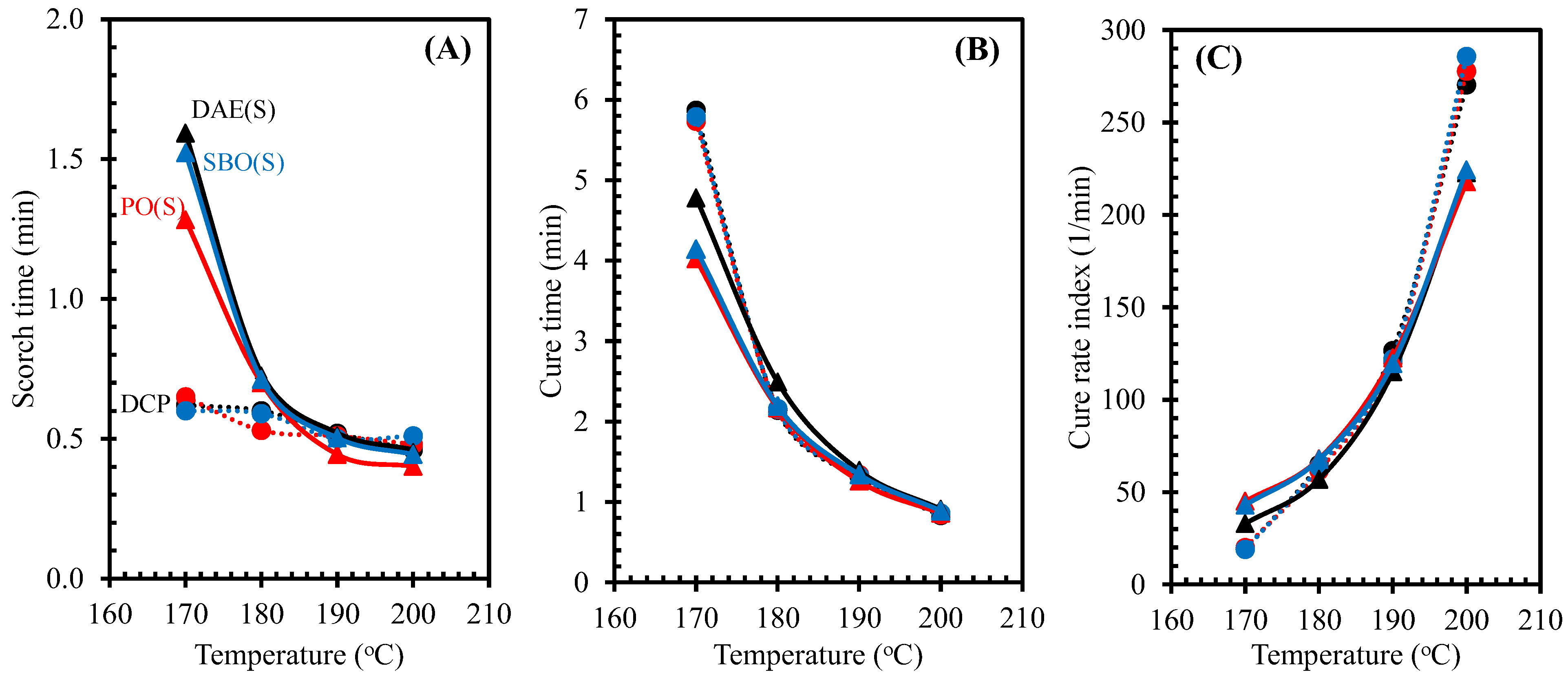
3.2. Cure Characteristics
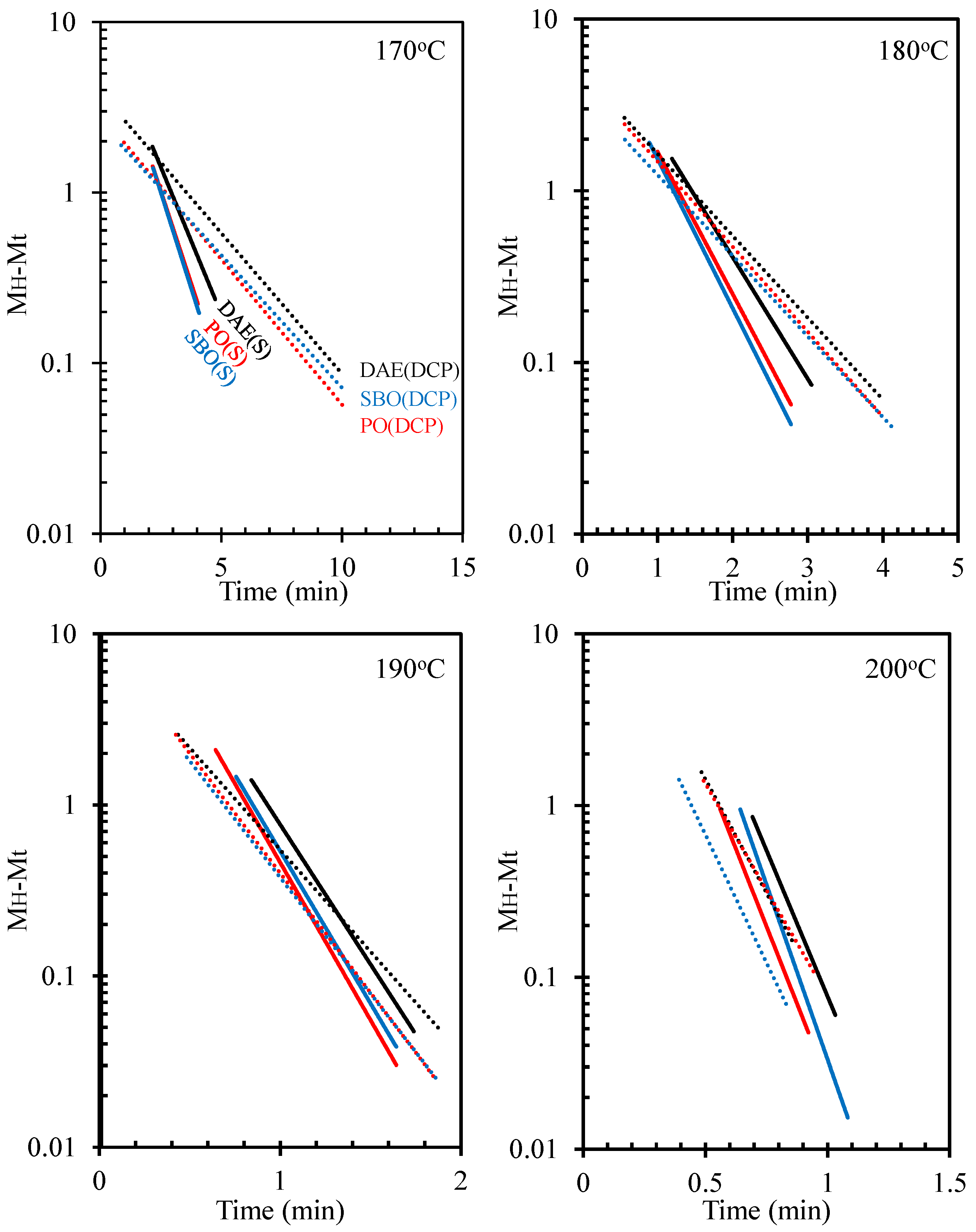
3.3. Cure Kinetics
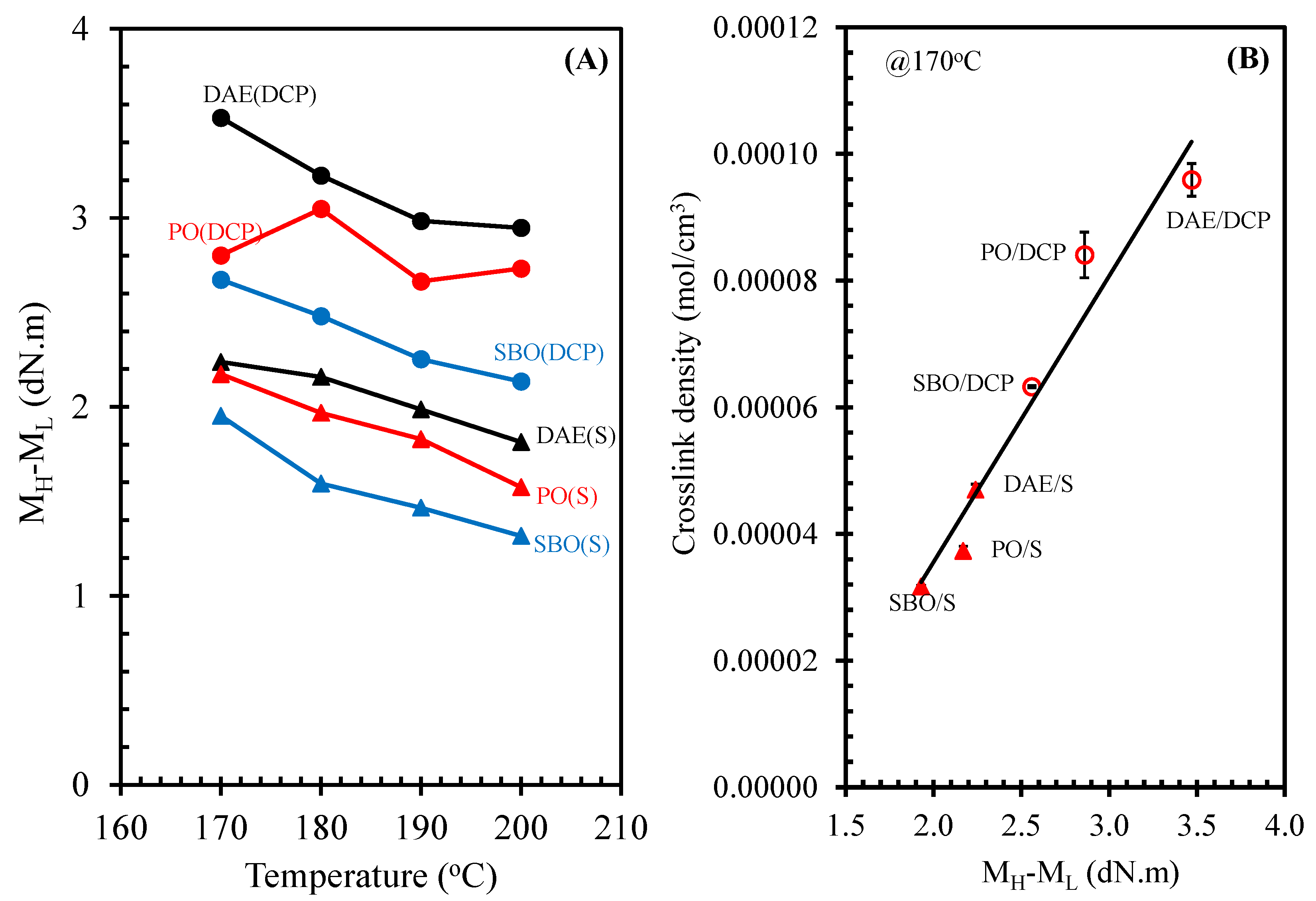
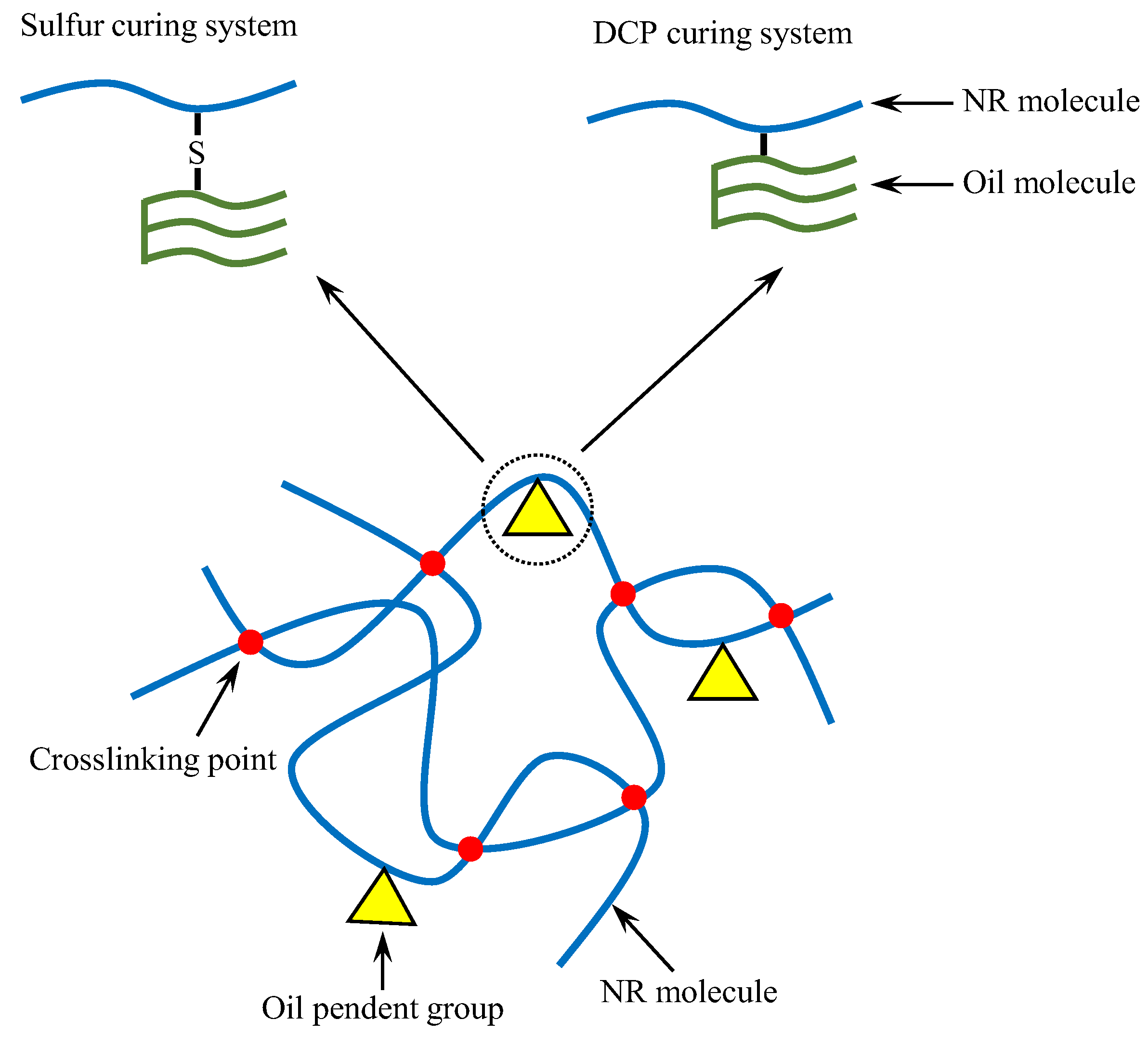
3.4. Crosslink Density
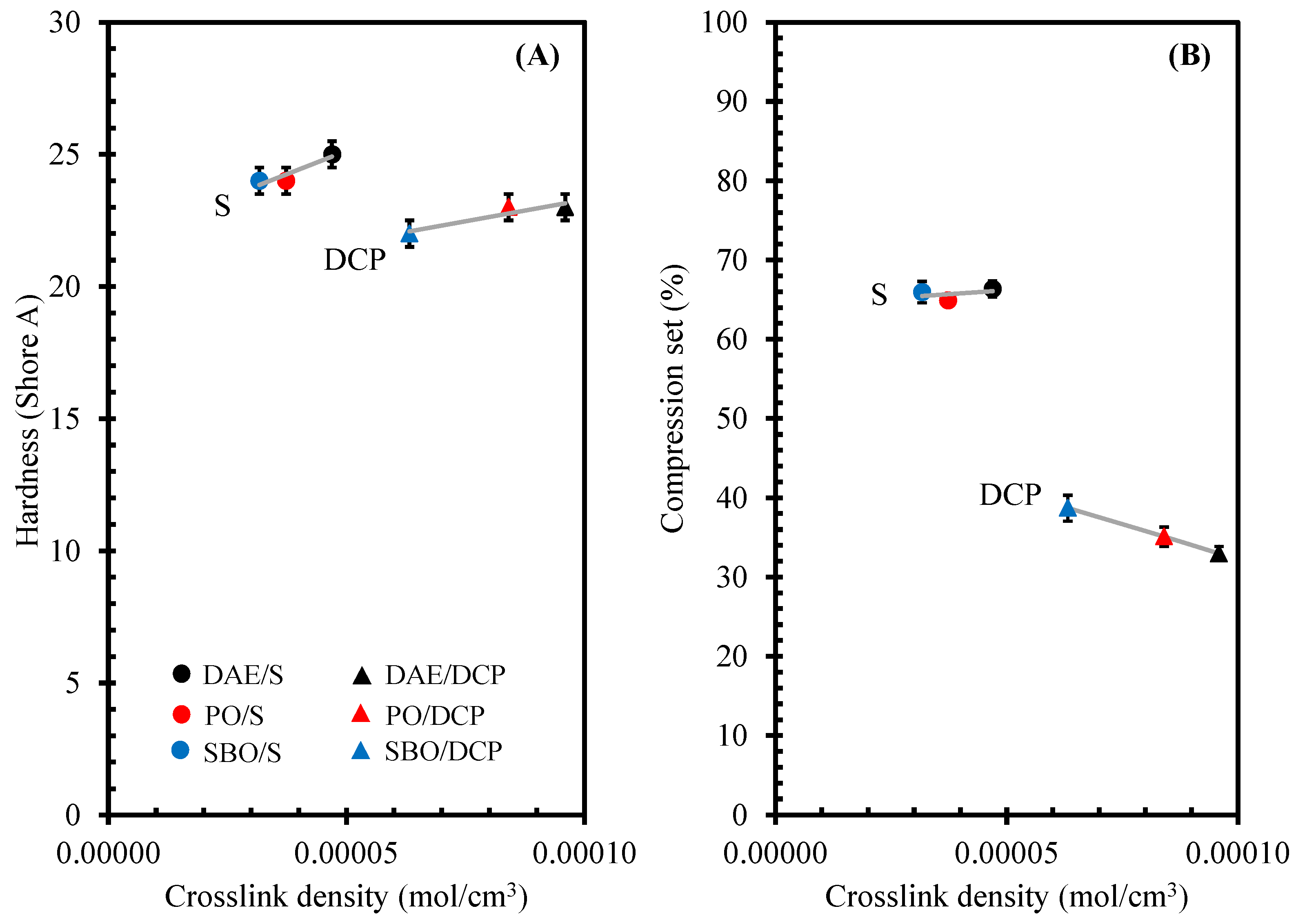
3.5. Tensile Properties, Hardness, and Compression Set of NR Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baranwal, C.K.; Stephens, L.H. Basic Elastomer Technol; Rubber Division: Akron, OH, USA, 2001; pp. 165–207. [Google Scholar]
- Trabelsi, S.; Albouy, P.-A.; Rault, J. Stress-induced crystallization around a crack tip in natural rubber. Macromolecules 2002, 35, 10054–10061. [Google Scholar] [CrossRef]
- Le Cam, J.-B.; Toussaint, E. The mechanism of fatigue crack growth in rubbers under severe loading: The effect of stress-induced crystallization. Macromolecules 2010, 43, 4708–4714. [Google Scholar] [CrossRef] [Green Version]
- Brüning, K.; Schneider, K.; Roth, S.V.; Heinrich, G. Strain-induced crystallization around a crack tip in natural rubber under dynamic load. Polymer 2013, 54, 6200–6205. [Google Scholar] [CrossRef]
- Beurrot-Borgarino, S.; Huneau, B.; Verron, E.; Rublon, P. Strain-induced crystallization of carbon black-filled natural rubber during fatigue measured by in situ synchrotron X-ray diffraction. Int. J. Fatigue 2013, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Saintier, N.; Cailletaud, G.; Piques, R. Cyclic loadings and crystallization of natural rubber: An explanation of fatigue crack propagation reinforcement under a positive loading ratio. Mater. Sci. Eng. A 2011, 528, 1078–1086. [Google Scholar] [CrossRef]
- Moneypenny, H.G.; Menting, K.H.; Gragg, F.M. Rubber compounding chemistry and applications. In General Compounding; Rodgers, B., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; Volume 1, pp. 334–363. [Google Scholar]
- Morris, G. Developments in rubber technology-1 Improving product performance. In Plasticizers; Whelan, A., Lee, K.S., Eds.; Applied Science Publishers Ltd.: Essex, UK, 1979; Volume 1, pp. 207–226. [Google Scholar]
- The Swedish national chemicals inspectorate. KEMI Reports, Rep. 5/03; Swedish National Chemicals Inspectorate: Sundbyberg, Sweden, 1993. [Google Scholar]
- Directive 2005/69/EC. Off. J. Eur. Union 2005, 51, L323.
- Commission regulation (EC) no 552/2009. Off. J. Eur. Union 2009, 7, L164.
- Petchkaew, A.; Sahakaro, K.; Noordermeer, J.W.M. Petroleum-based safe process oils in NR, SBR and their blends: Study on unfilled compounds. Part I. Oil characteristics and solubility aspects. KGK Kautsch. Gummi Kunstst. 2013, 66, 43–47. [Google Scholar]
- Petchkaew, A.; Sahakaro, K.; Noordermeer, J.W.M. Petroleum-based safe process oils in NR, SBR and their blends: Study on unfilled compounds. Part II. Properties. KGK Kautsch. Gummi Kunstst. 2013, 67, 21–27. [Google Scholar]
- Sahakaro, K.; Beraheng, A. Epoxidized natural oils as the alternative safe process oils in rubber compounds. Rubber Chem. Technol. 2011, 84, 200–214. [Google Scholar] [CrossRef]
- Boontawee, H.; Nakason, C.; Kaesaman, A.; Thitithammawong, A.; Chewchanwuttiwong, S. Benzyl esters of vegetable oils as processing oil in carbon black-filled SBR compounding: Chemical modification, characterization, and performance. Adv. Polym. Technol. 2017, 36, 320–330. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Boonkerd, K.; Nun-Anan, P.; Purbaya, M. Elucidation of the accelerated sulfur vulcanization of bio oil-extended natural rubber compounds. Polym. Adv. Technol. 2022, 33, 303–313. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Braton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Sovtić, N.; Predrag, K.S.; Bera, O.J.; Pavličević, J.M.; Govedarica, O.M.; Jovičić, M.C.; Govedarica, D.D. A review of environmentally friendly rubber production using different vegetable oils. Polym. Eng. Sci. 2020, 60, 1097–1117. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Batista, M.M.; Guirardello, R.; Krähenbühl, M.A. Determination of the hansen solubility parameters of vegetable oils, biodiesel, diesel, and biodiesel–diesel blends. J. Am. Oil Chem. Soc. 2015, 92, 95–109. [Google Scholar] [CrossRef]
- Cataldo, F.; da Ros, T. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- de la Peña-Gil, A.; Toro-Vazquez, J.F.; Rogers, M.A. Simplifying Hansen solubility parameters for complex edible fats and oils. Food Biophys. 2016, 11, 283–291. [Google Scholar] [CrossRef]
- Che Man, Y.B.; Moh, M.H.; van de Voort, F.R. Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 485–490. [Google Scholar] [CrossRef]
- Pietro, M.E.D.; Mannu, A.; Mele, A. NMR determination of free fatty acids in vegetable oils. Processes 2020, 8, 410–424. [Google Scholar] [CrossRef] [Green Version]
- Hayichelaeh, C.; Reuvekamp, L.A.E.M.; Dierkes, W.K.; Blume, A.; Noordermeer, J.W.M.; Sahakaro, K. Silica-reinforced natural rubber tire tread compounds containing bio-based process oils. I: Aspects of mixing sequence and epoxide content. Rubber Chem. Technol. 2020, 93, 360–377. [Google Scholar] [CrossRef]
- Siwarote, B.; Sae-Oui, P.; Wirasate, S.; Suchiva, K. Effects of bio-based oils on processing properties and cure characteristics of silica-filled natural rubber compounds. J. Rubber Res. 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Walsh, M.K. Immobilized enzyme technology for food applications. In Principles of Industrial Enzyme Technology; Woodhead Publishing Ltd., Abington Hall: Cambridge, UK, 2007. [Google Scholar]
- Clemente, T.E.; Cahoon, E.B. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, G.; Singh, R.P.; Nair, N.R.; Thomas, S. Development and characterization of novel EPDM/NR prophylactic waste composites. J. Mater. Sci. 2003, 38, 2469–2481. [Google Scholar] [CrossRef]
- Hassan, A.A.; Wang, S.; Anwar, F. Physiochemical characterization of soybean oil derived silanized factice and its interaction with styrene butadiene rubber/silica composite. Polym. Test. 2019, 78, 105933. [Google Scholar] [CrossRef]
- Hoefling, A.; Lee, Y.J.; Theato, P. Sulfur-based polymer composites from vegetable oils and elemental sulfur: A sustainable active material for Li–S batteries. Macromol. Chem. Phys. 2017, 218, 1600303. [Google Scholar] [CrossRef]
- Syamin, Y.M.; Azemi, S.; Dzaraini, K. Evaluation of cooking oil as processing additive for natural rubber. ASEAN J. Sci. Technol. Dev. 2017, 34, 17–25. [Google Scholar] [CrossRef]
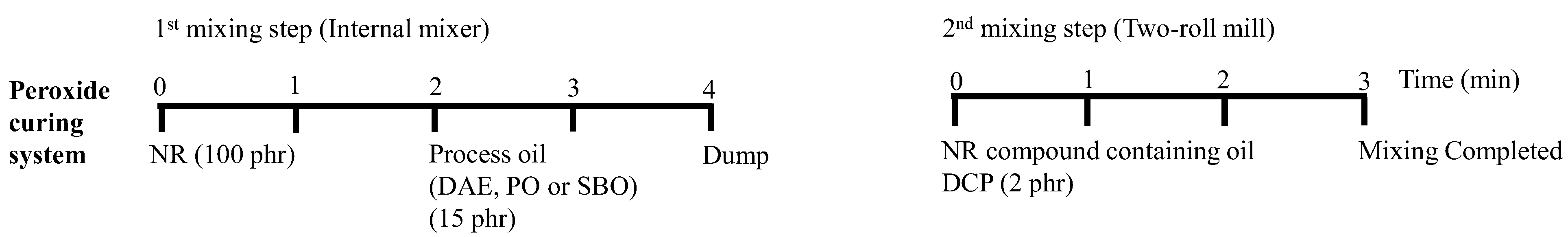



| Ingredient | Quantity (phr) |
|---|---|
| Peroxide | |
| NR | 100 |
| Processing oils * | 15 |
| DCP | 2 |
| Curing System | Type of Oil | Modulus at 300% Strain | Tensile Strength | Elongation at Break | |||
|---|---|---|---|---|---|---|---|
| Value (MPa) | Change (%) | Value (MPa) | Change (%) | Value (%) | Change (%) | ||
| DCP | DAE | 3.53 | 16.4 | 690 | |||
| PO | 2.91 | −18 | 14.2 | −13 | 740 | +7 | |
| SBO | 2.87 | −19 | 11.4 | −30 | 780 | +11 | |
| S | DAE | 2.94 | 28.5 | 830 | |||
| PO | 2.39 | −19 | 26.8 | −6 | 980 | +12 | |
| SBO | 2.23 | −24 | 21.8 | −24 | 990 | +19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayichelaeh, C.; Nun-Anan, P.; Purbaya, M.; Boonkerd, K. Unfilled Natural Rubber Compounds Containing Bio-Oil Cured with Different Curing Systems: A Comparative Study. Polymers 2022, 14, 2479. https://doi.org/10.3390/polym14122479
Hayichelaeh C, Nun-Anan P, Purbaya M, Boonkerd K. Unfilled Natural Rubber Compounds Containing Bio-Oil Cured with Different Curing Systems: A Comparative Study. Polymers. 2022; 14(12):2479. https://doi.org/10.3390/polym14122479
Chicago/Turabian StyleHayichelaeh, Chesidi, Phattarawadee Nun-Anan, Mili Purbaya, and Kanoktip Boonkerd. 2022. "Unfilled Natural Rubber Compounds Containing Bio-Oil Cured with Different Curing Systems: A Comparative Study" Polymers 14, no. 12: 2479. https://doi.org/10.3390/polym14122479
APA StyleHayichelaeh, C., Nun-Anan, P., Purbaya, M., & Boonkerd, K. (2022). Unfilled Natural Rubber Compounds Containing Bio-Oil Cured with Different Curing Systems: A Comparative Study. Polymers, 14(12), 2479. https://doi.org/10.3390/polym14122479






