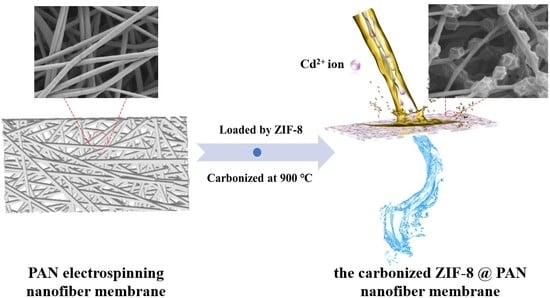
Preparation of the Carbonized Zif−8@PAN Nanofiber Membrane for Cadmium Ion Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
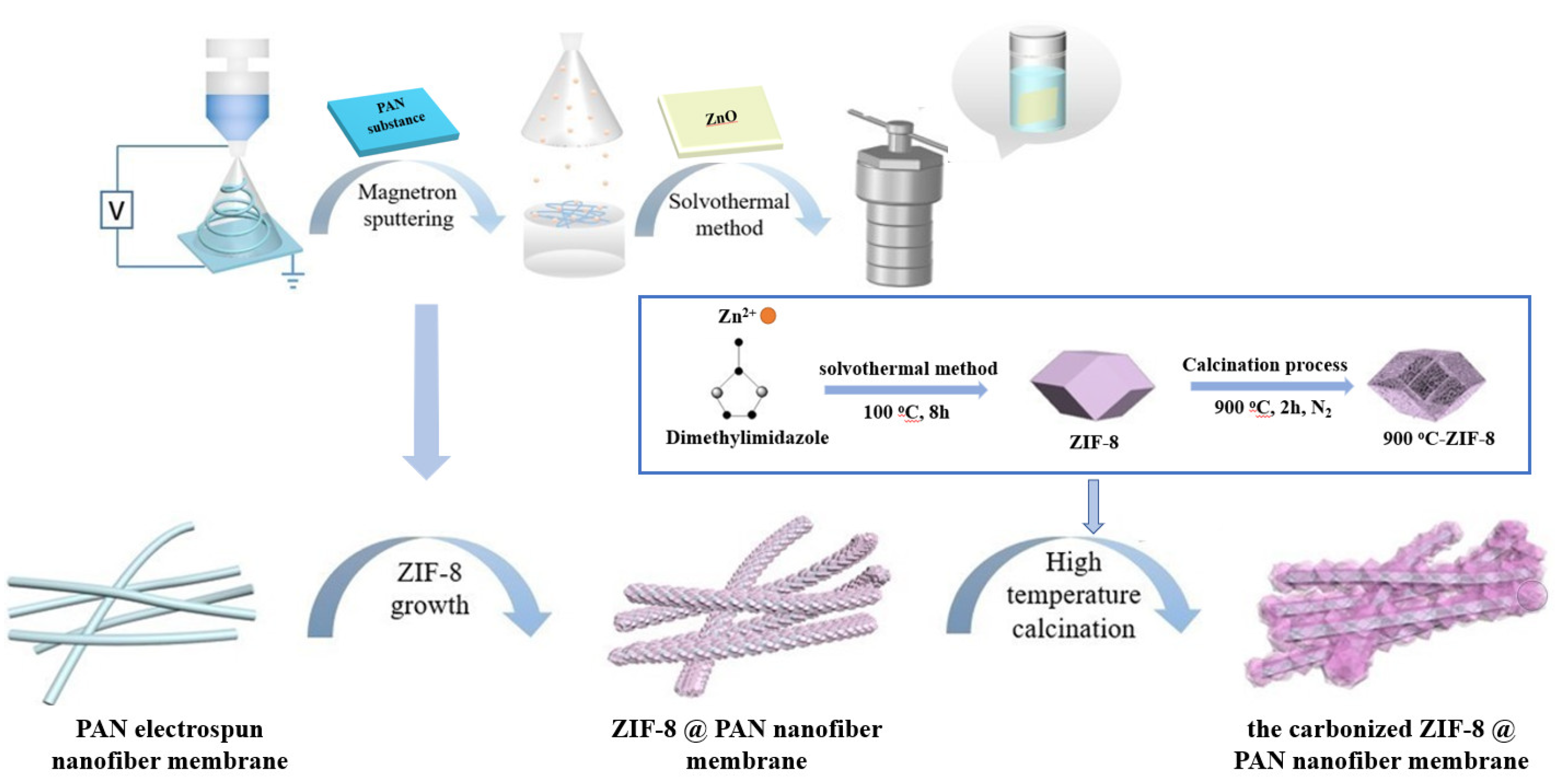
2.2. Preparation of ZnO@PAN Nanofiber Membrane
2.3. Preparation of ZIF−8@PAN Nanofiber Membrane
2.4. Preparation of the Carbonized ZIF−8@PAN Nanofiber Membrane
2.5. Characterization
2.6. Adsorption Performance
3. Results and Discussion
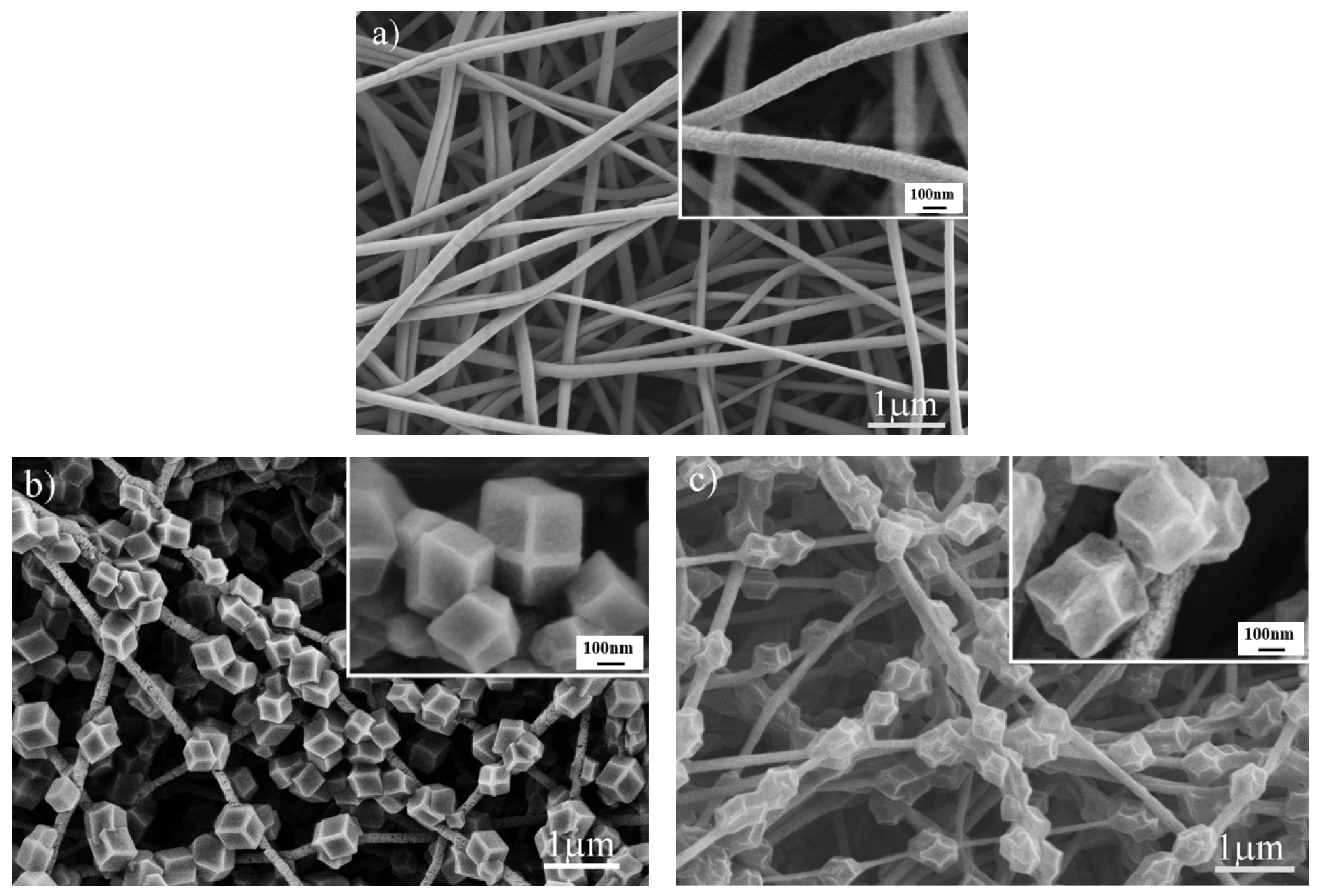
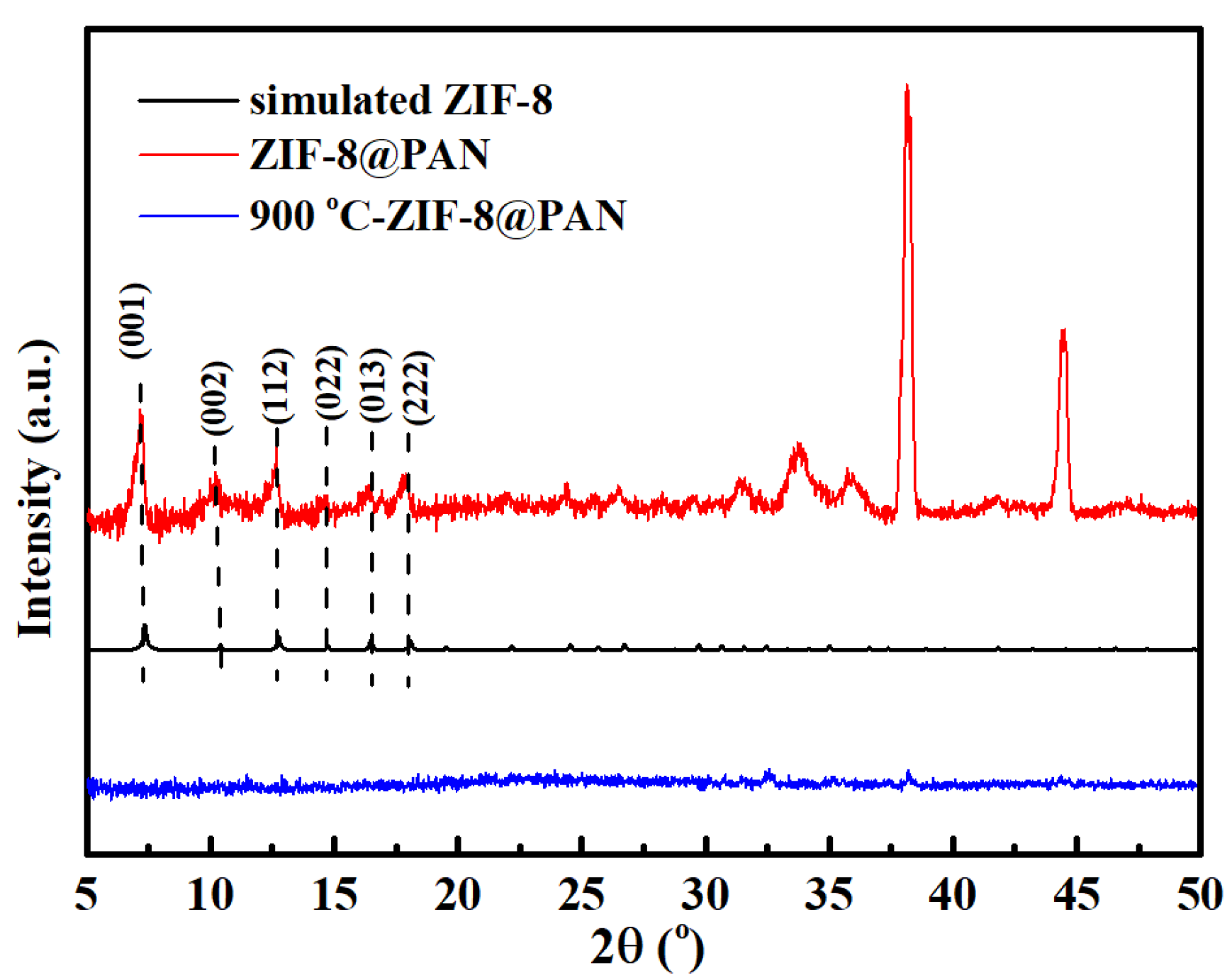
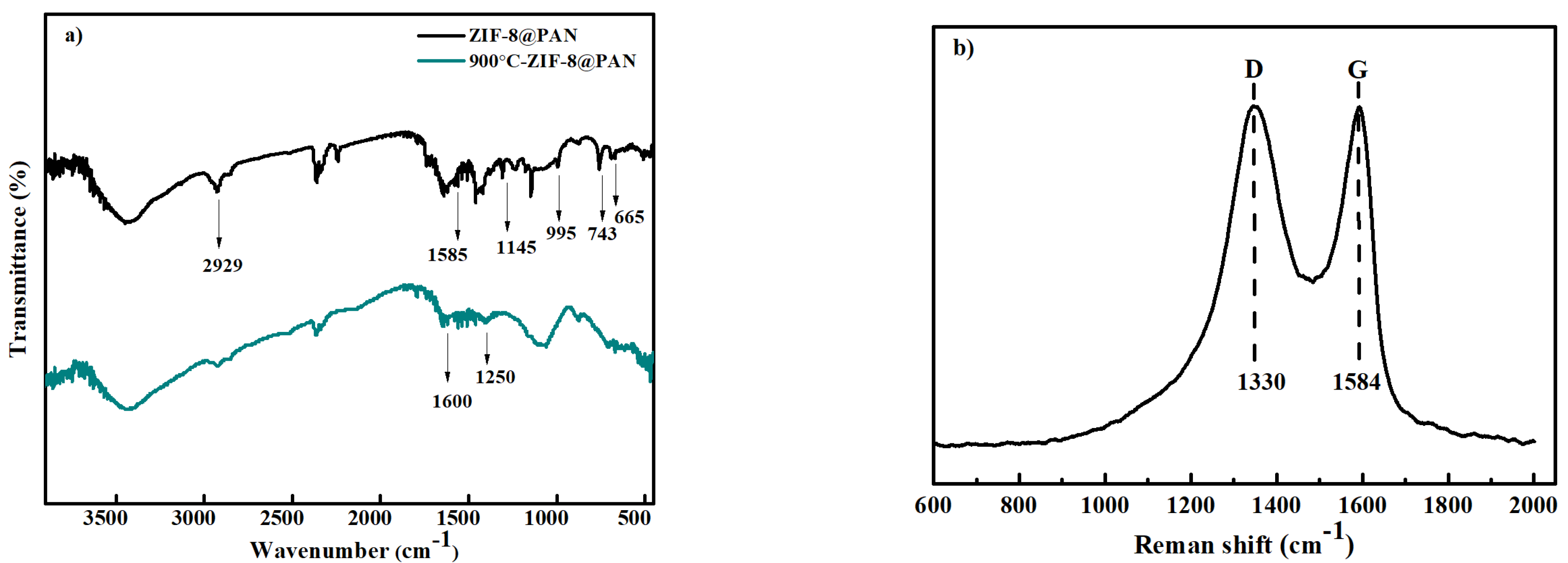
3.1. Characterization of Adsorbents
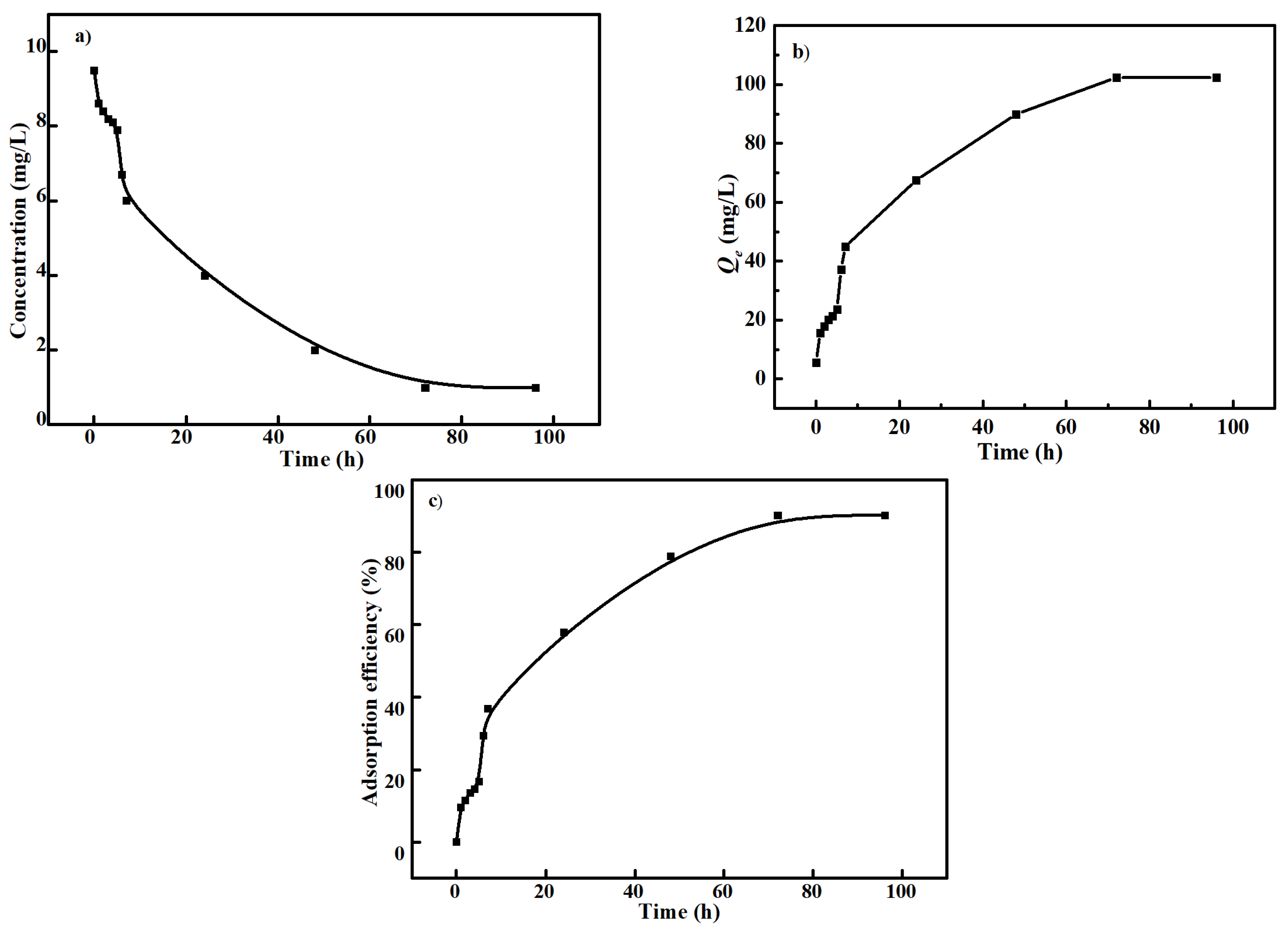
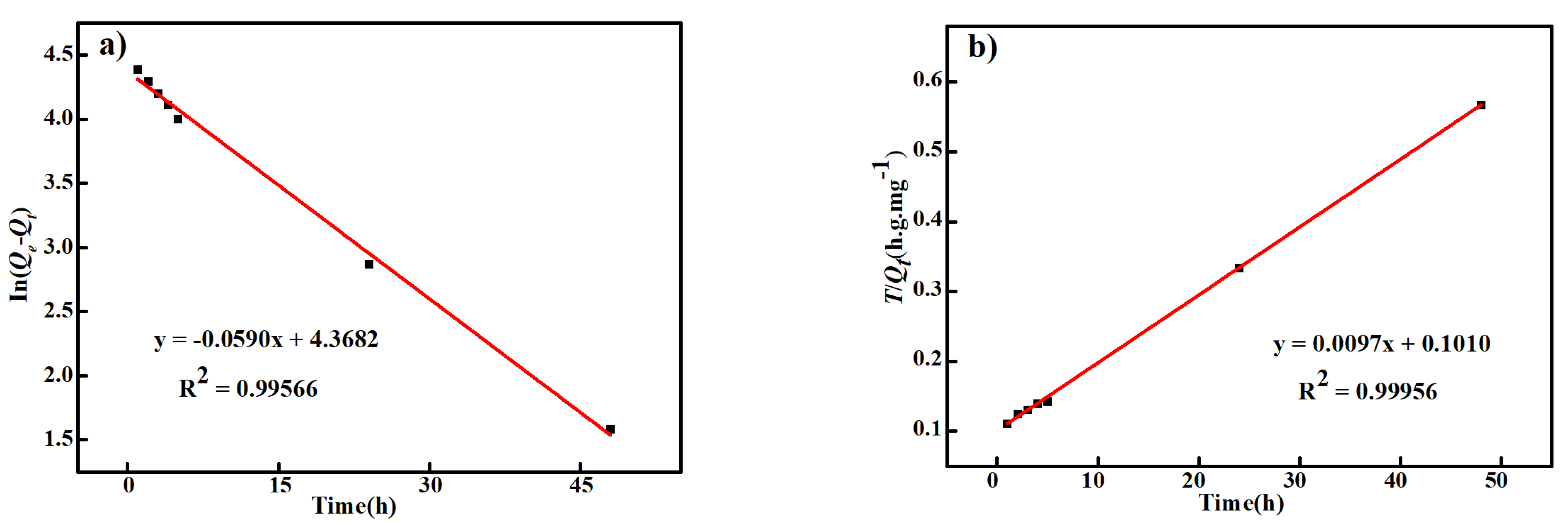
3.2. Cd2+ Adsorption Performance
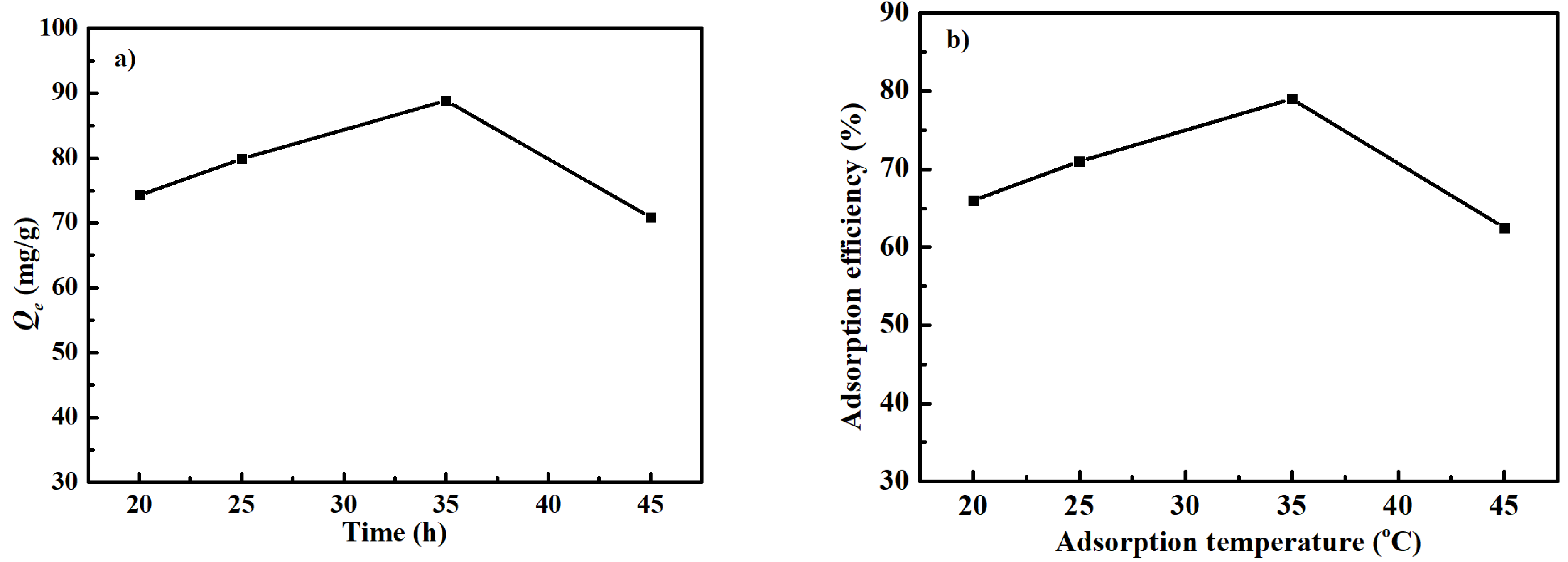
3.3. Effect of Temperature
3.4. Adsorption Thermodynamics Analysis
3.5. Effect of pH
3.6. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Miwornunyuie, N.; Chen, L.; Huang, J.Y.; Amaniampong, P.S.; Koomson, D.A.; Ewusi-Mensah, D.; Xue, W.C.; Li, G.; Lu, H. Sustainable Application of ZIF−8 for Heavy-Metal Removal in Aqueous Solutions. Sustainability 2021, 13, 984. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, H.W.; Wang, X.X.; Yu, S.J.; Chen, Z.S.; Zhang, P.; Chen, L.; Song, G.; Alharbi, N.S.; Rabah, S.O. Zeolitic imidazolate framework-based nanomaterials for the capture of heavy metal ions and radionuclides: A review. Chem. Eng. J. 2021, 406, 127139–127158. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bhattacharjee, S.; Kumar, V.; Singh, A.K. A study on the interdependence of heavy metals while contributing to groundwater pollution index. Environ. Sci. Pollut. R. 2021, 28, 25798–25807. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Niu, Z.G. Evaluation model of major heavy metals pollution factors in coastal waters and sediments. Desalin. Water. Treat. 2019, 149, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Song, X.H.; Zhao, T.T.; Xiao, Y.J.; Wang, Y.R.; Chen, X. A phosphorylethanolamine-functionalized super-hydrophilic 3D graphene-based foam filter for water purification. J. Hazard. Mater. 2018, 343, 298–303. [Google Scholar] [CrossRef]
- Xu, Z.S.; Huang, W.Y.; Xie, H.J.; Feng, X.Q.; Wang, S.F.; Song, H.N.; Xiong, J.H.; Mailhot, G. Co-adsorption and interaction mechanism of cadmium and sulfamethazine onto activated carbon surface. Colloid. Surf. A 2021, 619, 126540. [Google Scholar] [CrossRef]
- Sun, J.T.; Li, M.F.; Zhang, Z.H.; Guo, J.J. Unravelling the adsorption disparity mechanism of heavy-metal ions on the biomass-derived hierarchically porous carbon. Appl. Surf. Sci. 2019, 471, 615–620. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Modified-MOF-808-loaded polyacrylonitrile membrane for highly efficient, simultaneous emulsion separation and heavy metal ion removal. ACS. Appl. Mater. Inter. 2020, 12, 39227–39235. [Google Scholar] [CrossRef]
- Zhang, M.; Lan, G.H.; Qiu, H.Y.; Zhang, T.L.; Li, W.J.; Hu, X.Q. Preparation of ion exchange resin using soluble starch and acrylamide by graft polymerization and hydrolysis. Environ. Sci. Pollut. R. 2019, 26, 3803–3813. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Kim, S.G.; Bae, H.S.; Ryu, H.I.; Chung, H.S.; Seo, Y.S.; Cho, M.; Shea, P.J.; Jang, J.S. Simultaneous and synergistic effect of heavy metal adsorption on the enhanced photocatalytic performance of a visible-light-driven RS-TONR/TNT composite. Environ. Res. 2020, 180, 108651. [Google Scholar] [CrossRef]
- Zheng, X.D.; Li, A.; Hua, J.; Zhang, Y.Z.; Li, Z.Y. Crown Ether Grafted Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Membrane for Highly Selective Adsorption and Separation of Lithium Ion. Nanomaterials 2021, 11, 2668. [Google Scholar] [CrossRef]
- Salleh, N.A.B.; Afifi, A.M.; Mohamad, E.N. Studies on properties and adsorption ability of bilayer chitosan/PVA/PVDF electrospun nanofibrous. Desalination Water Treat. 2020, 206, 177–188. [Google Scholar] [CrossRef]
- Liu, S.; Mukai, Y. Selective adsorption and separation of proteins by ligand-modified nanofiber fabric. Polymers 2021, 13, 2313. [Google Scholar] [CrossRef]
- Ren, G.F.; Qu, Z.G.; Wang, X.L.; Zhang, J.F. Liquid water transport and mechanical performance of electrospun gas diffusion layers. Int. J. Green Energy 2022, 19, 210–218. [Google Scholar] [CrossRef]
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Sources 2022, 525, 231121. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, W.K.; Qu, Z.G.; Ren, G.F.; Wang, H.C. Surface roughness dominated wettability of carbon fiber in gas diffusion layer materials revealed by molecular dynamics simulations. Int. J. Hydrogen Energy 2021, 46, 26489–26498. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, B.; Qu, Z.G.; Zhang, J.F.; Jin, Z.G. Effects of graphite microstructure evolution on the anisotropic thermal conductivity of expanded graphite/paraffin phase change materials and their thermal energy storage performance. Int. J. Heat Mass Tran. 2020, 155, 119853. [Google Scholar] [CrossRef]
- Wang, X.L.; Qu, Z.G.; Ren, G.F.; Feng, C.; Cheng, F. Prolonged yield platform in bioinspired three dimensional carbon materials derived from crack deflection. Mater. Lett. 2020, 270, 127759. [Google Scholar] [CrossRef]
- Orleans-Boham, H.; Elessawy, N.A.; El-Shazly, A.; Elkady, M.F. Production and characterization of nano-polyaniline and carbonized polyaniline for potential application as energy storage devices. Mater. Today 2020, 33, 1909–1912. [Google Scholar] [CrossRef]
- Abedi, M.; Chenar, M.P.; Sadeghi, M. Surface modification of PAN hollow fiber membrane by chemical reaction. Fiber. Polym. 2015, 16, 788–793. [Google Scholar] [CrossRef]
- Almasian, A.; Giahi, M.; Fard, G.C.; Dehdast, S.; Maleknia, L. Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: Filtration performance, antifouling and regeneration behavior. Chem. Eng. J. 2018, 351, 1166–1178. [Google Scholar] [CrossRef]
- Alharbi, H.E.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Karim, M.R. Electrospun Bilayer PAN/Chitosan Nanofiber Membranes Incorporated with Metal Oxide Nanoparticles for Heavy Metal Ion Adsorption. Coatings 2020, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.W.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.H.; Zhou, M.; Xiao, L.H.; Liu, S.Y.; Zhang, M.M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef]
- Devaraj, M.; Sasikumar, Y.; Rajendran, S.; Ponce, L.C. Review-Metal Organic Framework Based Nanomaterials for Electrochemical Sensing of Toxic Heavy Metal Ions: Progress and Their Prospects. J. Electrochem. Soc. 2021, 168, 037513. [Google Scholar] [CrossRef]
- Wen, J.; Hu, X.H. Metal selectivity and effects of co-existing ions on the removal of Cd, Cu, Ni, and Cr by ZIF−8-EGCG nanoparticles. J. Colloid. Interf. Sci. 2021, 589, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight studies on Metal-Organic Framework nanofibrous membrane adsorption and activation for heavy metal ions removal from aqueous solution. Acs. Appl. Mater. Inter. 2018, 10, 18619–18629. [Google Scholar] [CrossRef] [PubMed]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, Y.H.; Sun, Z.J.; Yang, C.H.; Tang, D.Y. Effective strategy to fabricate ZIF−8@ZIF−8/polyacrylonitrile nanofibers with high loading efficiency and improved removing of Cr(VI). Colloids Surf. A 2020, 603, 125292. [Google Scholar] [CrossRef]
- Peng, L.C.; Zhang, X.L.; Sun, Y.X.; Xing, Y.; Li, C.J. Heavy metal elimination based on metal organic framework highly loaded on flexible nanofibers. Environ. Res. 2020, 188, 109742. [Google Scholar] [CrossRef]
- Zhang, T.H.; Li, P.Y.; Ding, S.P.; Wang, X.F. High-performance TFNC membrane with adsorption assisted for removal of Pb2+ and other contaminants. J. Hazard. Mater. 2022, 424, 127742. [Google Scholar] [CrossRef]
- Wang, C.L.; Yang, R.Q.; Wang, H.F. Synthesis of ZIF−8/fly ash composite for adsorption of Cu2+, Zn2+ and Ni2+ from aqueous solutions. Materials 2020, 13, 214. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.W.; Wang, B.X.; Zhu, L.Y.; Jiang, L. Properties of Cobalt- and Nickel-Doped ZIF−8 Framework Materials and Their Application in Heavy-Metal Removal from Wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef]
- Abbasi, Z.; Shamsaei, E.; Leong, S.K.; Ladewig, B.; Zhang, X.W.; Wang, H.T. Effect of carbonization temperature on adsorption property of ZIF−8 derived nanoporous carbon for water treatment. Micropor. Mesopor. Mat. 2016, 236, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, H.N.; Dowaidar, M.; Langel, U. Carbonized chitosan encapsulated hierarchical porous zeolitic imidazolate frameworks nanoparticles for gene delivery. Micropor. Mesopor. Mat. 2020, 302, 110200. [Google Scholar] [CrossRef]
- Mohy-Eldin, M.S.; Elkady, M.F.; Abu-Saied, M.A.; Abdel Rahman, A.M.; Soliman, E.A.; Elzatahry, A.A.; Youssef, M.E. Removal of cadmium ions from synthetic aqueous solutions with a novel nanosulfonated poly(glycidyl methacrylate) cation exchanger: Kinetic and equilibrium studies. J. Appl. Polym. Sci. 2010, 118, 3111–3122. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Yu, J.D.; Jiang, C.Y.; Guan, Q.Q.; Ning, P.; Gu, J.J.; Chen, Q.L.; Zhang, J.M.; Miao, R.R. Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 2018, 195, 632–664. [Google Scholar] [CrossRef]
- Hsiao, Y.J.; Lin, L.Y. Efficient pore engineering in carbonized zeolitic imidazolate Framework-8 via chemical and physical methods as active materials for supercapacitors. J. Power Sources 2021, 486, 229370–229378. [Google Scholar] [CrossRef]
- Zheng, G.C.; Chen, Z.W.; Sentosun, K.; Pérez-Juste, I.; Bals, S.; Liz-Marzán, L.M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Hong, M. Shape control in ZIF−8 nanocrystals and metal nanoparticles@ZIF−8 heterostructures. Nanoscale 2017, 9, 16645–16651. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Q.; Liu, J.Y.; Zhu, L.X.; Hou, X.D.; Huang, L.; Lin, H.Q.; Cheng, J. Highly permeable mixed matrix materials comprising ZIF−8 nanoparticles in rubbery amorphous poly(ethylene oxide) for CO2 capture. Sep. Purif. Technol. 2018, 205, 58–65. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Ionic liquid-modified Pebax (R) 1657 membrane filled by ZIF−8 particles for separation of CO2 from CH4, N-2 and H-2. J. Membrane. Sci. 2017, 524, 652–662. [Google Scholar] [CrossRef]
- Zheng, F.C.; Yang, Y.; Chen, Q.W. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261–5271. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.N.; Zhang, Q.; Liu, Z.J.; Pan, K.F.; Dong, Y.H.; Li, Y.Y. Removal of Cu (II) by loofah fibers as a natural and low-cost adsorbent from aqueous solutions. J. Mol. Liq. 2014, 191, 73–78. [Google Scholar] [CrossRef]





| ΔHθ/(kJ·mol−1) | ΔSθ/(J·mol−1·K−1) | ΔGθ/(kJ·mol−1) | ||
|---|---|---|---|---|
| 293 K | 298 K | 308 K | ||
| 41.6 | 167.1 | −7.6 | −9.2 | −10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Feng, J.; Song, Y.; Xu, L.; Cui, X.; Yu, B. Preparation of the Carbonized Zif−8@PAN Nanofiber Membrane for Cadmium Ion Adsorption. Polymers 2022, 14, 2523. https://doi.org/10.3390/polym14132523
Sun H, Feng J, Song Y, Xu L, Cui X, Yu B. Preparation of the Carbonized Zif−8@PAN Nanofiber Membrane for Cadmium Ion Adsorption. Polymers. 2022; 14(13):2523. https://doi.org/10.3390/polym14132523
Chicago/Turabian StyleSun, Hui, Jiangli Feng, Yaoyao Song, Lei Xu, Xiaogang Cui, and Bin Yu. 2022. "Preparation of the Carbonized Zif−8@PAN Nanofiber Membrane for Cadmium Ion Adsorption" Polymers 14, no. 13: 2523. https://doi.org/10.3390/polym14132523
APA StyleSun, H., Feng, J., Song, Y., Xu, L., Cui, X., & Yu, B. (2022). Preparation of the Carbonized Zif−8@PAN Nanofiber Membrane for Cadmium Ion Adsorption. Polymers, 14(13), 2523. https://doi.org/10.3390/polym14132523