Polymers in Technologies of Additive and Inkjet Printing of Dosage Formulations
Abstract
:1. Introduction
2. The Methods for Manufacturing Personalized Dosage Formulations
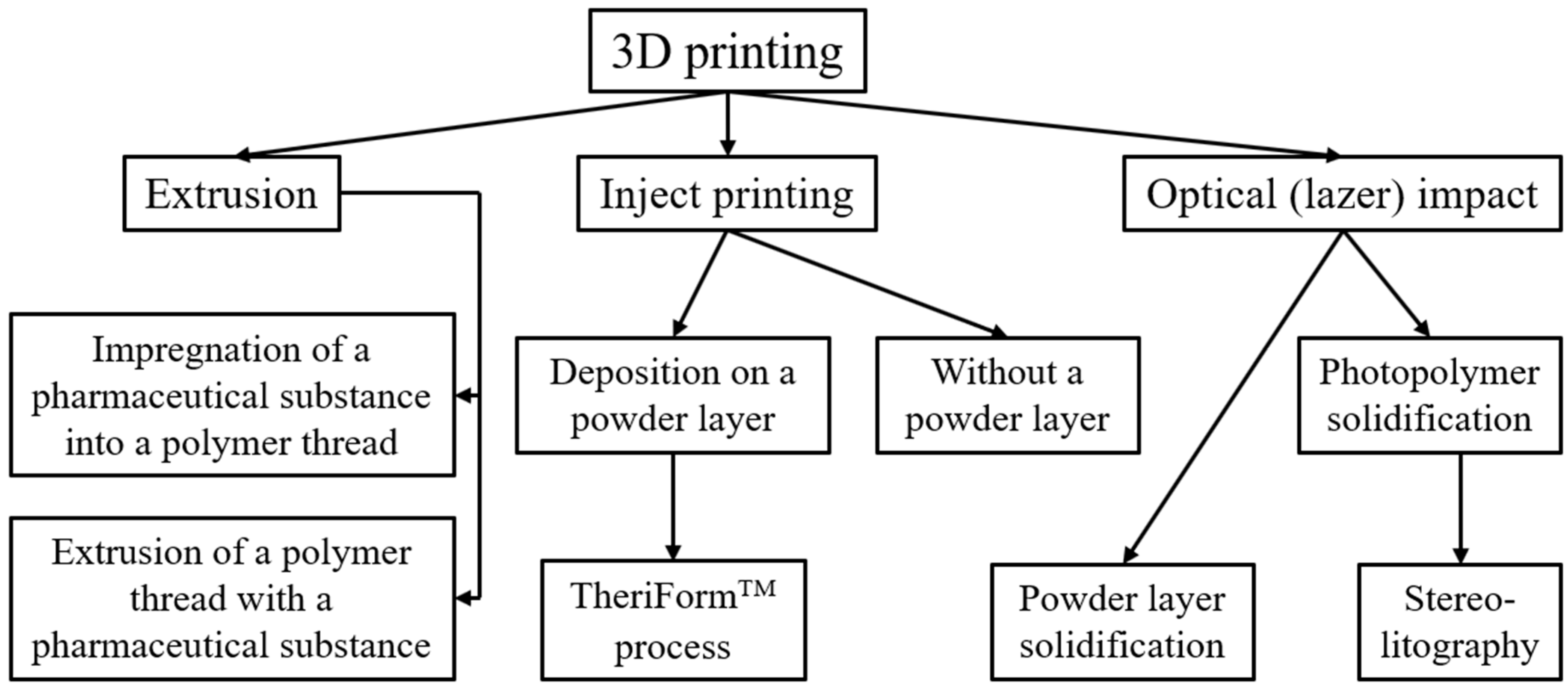
2.1. Obtaining DF with 3D Printing
2.2. Preparation of Dosage Formulations Using 2D Inkjet Printing
2.3. The Main Ways of 4-D Modification of 3D and 2D Printing
3. Polymers for Additive, Inkjet, and 4D Printing
4. Directions of Issue Development
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dedov, I.I.; Tyulpakov, A.N.; Chekhonin, V.P.; Baklaushev, V.P.; Archakov, A.I.; Moshkovsky, S.A. Personalized medicine: Current state and prospects. Bull. Russ. Acad. Med. Sci. 2012, 67, 4–12. [Google Scholar]
- Blynskaya, E.V.; Tishkov, S.V.; Alekseev, K.V. Three-dimensional printing technologies for the creation of solid dosage forms. Devel. Registr. Med. 2018, 3, 20–29. [Google Scholar]
- Yunus, Y.; Mahadzir, N.A.; Mohamed Ansari, M.N.; Tg Abd Aziz, T.H.; Mohd Afdzaluddin, A.; Anwar, H.; Wang, M.; Ismail, A.G. Review of the Common Deposition Methods of Thin-Film Pentacene, Its Derivatives, and Their Performance. Polymers 2022, 14, 1112. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, K.V.; Blynskaya, E.V.; Tishkov, S.V.; Alekseev, V.K.; Ivanov, A.A.; Minaev, S.V.; Kondakov, S.E.; Ikhalainen, E.S. Technology two-dimensional printing of dosage forms in the system of personalized medicine. Biopharm. J. 2020, 12, 14–21. [Google Scholar]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet printing as a novel medicine formulation technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef]
- Vakili, H.; Kolakovic, R.; Genina, N.; Marmion, M.; Salo, H.; Ihalainen, P.; Peltonen, J.; Sandler, N. Hyperspectral imaging in quality control of inkjet printed personalised dosage forms. Int. J. Pharm. 2015, 483, 244–249. [Google Scholar] [CrossRef]
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yuan, J.; Cheng, Q.; Wei, P.; Cheng, G.J.; Chang, C. Additive printing of recyclable anti-counterfeiting patterns with sol–gel cellulose nanocrystal inks. Nanoscale 2021, 13, 11808–11816. [Google Scholar] [CrossRef]
- Behroozfar, A.; Bhuiyan, M.E.H.; Daryadel, S.; Edwards, D.; Rodriguez, B.J.; Minary-Jolandan, M. Additive printing of pure nanocrystalline nickel thin films using room environment electroplating. Nanotechnology 2019, 31, 055301. [Google Scholar] [CrossRef]
- Pacewicz, K.; Sobotka, A.; Gołek, Ł. Characteristic of materials for the 3D printed building constructions by additive printing. MATEC. EDP Sci. 2018, 222, 01013. [Google Scholar] [CrossRef]
- Pizarro, F.; Salazar, R.; Rajo-Iglesias, E.; Rodriguez, M.; Fingerhuth, S.; Hermosilla, G. Parametric study of 3D additive printing parameters using conductive filaments on microwave topologies. IEEE Access 2019, 7, 106814–106823. [Google Scholar] [CrossRef]
- Cosker, M.; Lizzi, L.; Ferrero, F.; Staraj, R.; Ribero, J.M. Realization of 3-D flexible antennas using liquid metal and additive printing technologies. IEEE Antennas Wirel. Propag. Lett. 2016, 16, 971–974. [Google Scholar] [CrossRef]
- Alekseev, K.V.; Blynskaya, E.V.; Tishkov, S.V.; Alekseev, V.K.; Ivanov, A.A.; Minaev, S.V.; Kondakov, S.E.; Ikhalainen, E.S. Features two-dimensional printing of dosage forms in pharmaceutical technology. J. Pharm. Qual. Assur. Issues 2020, 2, 28–39. [Google Scholar]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet printing for pharmaceutical applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Ivanova, O.; Williams, C.; Campbell, T. Additive manufacturing (AM) and nanotechnology: Promises and challenges. Rapid Pro. J. 2013, 19, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef]
- Alekseev, K.V.; Blynskaya, E.V.; Tishkov, S.V.; Ivanov, A.A.; Alekseev, V.K. Three-dimensional additive printing in the technology of dosage forms. J. Pharm. Qual Assur. Issues 2020, 27, 4–17. [Google Scholar]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 549, 283–292. [Google Scholar] [CrossRef]
- Dimitrov, D.; Schreve, K.; De Beer, N. Advances in three dimensional printing–state of the art and future perspectives. Rapid Pro. J. 2006, 12, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of fused deposition modeling process parameters: A review of current research and future prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Hoque, M.E.; Chuan, Y.L.; Pashby, I. Extrusion based rapid prototyping technique: An advanced platform for tissue engineering scaffold fabrication. Biopolymers 2012, 97, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q.; Snyder, J.; Wang, C.; Timmer, M.; Hammer, J.; Guceri, S.; Sun, W. Fabrication of three-dimensional scaffolds using precision extrusion deposition with an assisted cooling device. Biofabrication 2011, 3, 34–109. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Salmela, I.; Fallarero, A.; Rosling, A.; Khajeheian, M.; Kolakovic, R.; Genina, N.; Nyman, J.; Vuorela, P. Towards fabrication of 3D printed medical devices to prevent biofilm formation. Int. J. Pharm. 2014, 459, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Utela, B.; Storti, D.; Anderson, R.; Ganter, M.A. Review of process development steps for new material systems in three dimensional printing (3DP). J. Manuf. Processes 2008, 10, 96–104. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Palo, M. Design and Development of Personalized Dosage Forms by Printing Technology; Painosalama Oy: Turku, Finland, 2017; pp. 1–64. [Google Scholar]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Thabet, Y.; Breitkreutz, J. Printing pharmaceuticals by inkjet technology: Proof of concept for stand-alone and continuous in-line printing on orodispersible films. J. Manuf. Processes 2018, 35, 205–215. [Google Scholar] [CrossRef]
- Genina, N.; Janben, E.M.; Breitenbach, A.; Breitkreutz, J.; Sandler, N. Evaluation of different substrates for inkjet printing of rasagiline mesylate. Eur. J. Pharm. Biopharm. 2013, 85, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Nuzzo, R.G.; Mahadevan, L.; Gladman, A.S.; Matsumoto, E.A. Biomimetic 4D printing. Nat. Mat. 2016, 15, 413–418. [Google Scholar]
- Campbell, T.A.; Tibbits, S.; Garrett, B. The Next Wave: 4D Printing; Atlantic: Washington, DC, USA, 2014; pp. 1–16. [Google Scholar]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekkanen, A.M.; Mondschein, R.J.; Williams, C.B.; Long, T.E. 3D printing polymers with supramolecular functionality for biological applications. Biomacromolecules 2017, 18, 2669–2687. [Google Scholar] [CrossRef]
- Louzao, I.; Koch, B.; Taresco, V.; Ruiz-Cantu, L.; Irvine, D.J.; Roberts, C.J.; Tuck, C.; Cameron, A.; Hague, R.; Wildman, R.; et al. Identification of novel “Inks” for 3D printing using high-throughput screening: Bioresorbable photocurable polymers for controlled drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 6841–6848. [Google Scholar] [CrossRef]
- Nadgorny, M.; Ameli, A. Functional polymers and nanocomposites for 3D printing of smart structures and devices. ACS Appl. Mater. Interfaces 2018, 10, 17489–17507. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.R.; Goyanes, A.; Basi, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf. Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Lee, K.J.; Kang, A.; Delfino, J.J.; West, T.G.; Chetty, D.; Monkhouse, D.C.; Yoo, J. Evaluation of critical formulation factors in the development of a rapidly dispersing captopril oral dosage form. Drug Dev. Ind. Pharm. 2003, 29, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Wang, T.; Guo, Y.; Derby, B. Ink-jet printing of zirconia: Coffee staining and line stability. J. Am. Ceram. Soc. 2011, 94, 3787–3792. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Raijada, D.; Genina, N.; Fors, D.; Wisaeus, E.; Peltonen, J.; Rantanen, J.; Sandler, N. A step toward development of printable dosage forms for poorly soluble drugs. J. Pharm. Sci. 2013, 102, 3694–3704. [Google Scholar] [CrossRef]
- Planchette, C.; Pichler, H.; Wimmer-Teubenbacher, M.; Gruber, M.; Gruber-Wölfler, H.; Mohr, S.; Tetyczkad, C.; Hsiaoa, W.-K.; Paudela, A.; Roblegg, E.; et al. Printing medicines as orodispersible dosage forms: Effect of substrate on the printed micro-structure. Int. J. Pharm. 2016, 509, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Combining inkjet printing and amorphous nanonization to prepare personalized dosage forms of poorly-soluble drugs. Eur. J. Pharm. Biopharm. 2015, 96, 314–321. [Google Scholar] [CrossRef]
- Buanz, A.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Buanz, A.B.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, M.O.; Gaisford, S. Ink-jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 494, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Vakili, H.; Nyman, J.O.; Genina, N.; Preis, M.; Sandler, N. Application of a colorimetric technique in quality control for printed pediatric orodispersible drug delivery systems containing propranolol hydrochloride. Int. J. Pharm. 2016, 511, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Toth, S.J.; Simpson, G.J.; Taylor, L.S.; Harris, M.T. Effect of substrates on naproxen-polyvinylpyrrolidone solid dispersions formed via the drop printing technique. J. Pharm. Sci. 2013, 102, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, L.; Giridhar, A.; Taylor, L.S.; Harris, M.T.; Reklaitis, G.V. Dropwise additive manufacturing of pharmaceutical products for solvent-based dosage forms. J. Pharm. Sci. 2014, 103, 496–506. [Google Scholar] [CrossRef]
- Elele, E.; Shen, Y.; Susarla, R.; Khusid, B.; Keyvan, G.; Michniak-Kohn, B. Electrodeless electrohydrodynamic drop-on-demand encapsulation of drugs into porous polymer films for fabrication of personalized dosage units. J. Pharm. Sci. 2012, 101, 2523–2533. [Google Scholar] [CrossRef]
- Janßen, E.M.; Schliephacke, R.; Breitenbach, A.; Breitkreutz, J. Drug-printing by flexographic printing technology—A new manufacturing process for orodispersible films. Int. J. Pharm. 2013, 441, 818–825. [Google Scholar] [CrossRef]
- Zimmer, Ł.; Kasperek, R.; Poleszak, E. Modern polymers in matrix tablets technology. Polim. W Med. 2014, 44, 189–196. [Google Scholar] [PubMed]
- Song, T.H.; Jang, J.; Choi, Y.J.; Shim, J.H.; Cho, D.W. 3D-printed drug/cell carrier enabling effective release of cyclosporin A for xenogeneic cell-based therapy. Cell Transplant. 2015, 24, 2513–2525. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Meng, F.; Johnson, B.N.; Kong, Y.L.; Tian, L.; Yeh, Y.W.; Masters, N.; Singamaneni, S.; McAlpine, M.C. 3D printed programmable release capsules. Nano Lett. 2015, 15, 5321–5329. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’brien, J.; Zhang, L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef]
- Jain, V.; Haider, N.; Jain, K. 3D printing in personalized drug delivery. Curr. Pharm. Des. 2018, 24, 5062–5071. [Google Scholar] [CrossRef]
- Joshi, M. Role of eudragit in targeted drug delivery. Int. J. Curr. Pharm. Res. 2013, 5, 58–62. [Google Scholar]
- Breger, J.C.; Yoon, C.; Xiao, R.; Wang, M.O.; Fisher, J.P.; Nguyen, T.D.; Gracias, D.H. Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl. Mater. Interfaces 2015, 7, 3398–3405. [Google Scholar] [CrossRef]
- Zarek, M.; Mansour, N.; Shapira, S.; Cohn, D. 4D printing of shape memory-based personalized endoluminal medical devices. Macromol. Rapid Commun. 2017, 38, 1600628. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Fortin, D.; Xia, H.; Zhao, Y. Poly (vinyl alcohol)–poly (ethylene glycol) double-network hydrogel: A general approach to shape memory and self-healing functionalities. Langmuir 2015, 31, 11709–11716. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Raviv, D.; Zhao, W.; McKnelly, C.; Papadopoulou, A.; Kadambi, A.; Shi, B.; Hirsch, S.; Dikovsky, D.; Zyracki, M.; Olguin, C.; et al. Active printed materials for complex self-evolving deformations. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Clay, N.E.; Sen, S.; Melhem, M.; Qin, E.; Kong, H.; Bashir, R. 3D printing enables separation of orthogonal functions within a hydrogel particle. Biomed. Microdevices 2016, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.; Xu, T.; Miszuk, J.M.; Picciani, P.H.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef]
- Solanki, A.B.; Parikh, J.R.; Parikh, R.H. Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level Box-Behnken design. Aaps Pharmscitech. 2007, 8, 43–49. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNT s for bone cell growth stimulation. J. Biomed. Mater. Res. Part B 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Li, S.; Hu, S.G.; Chang, W.C.; Jeng, U.S.; Hsu, S.H. Synthesis of thermoresponsive amphiphilic polyurethane gel as a new cell printing material near body temperature. ACS Appl. Mater. Interfaces 2015, 7, 27613–27623. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef]
- Abbadessa, A.; Blokzijl, M.M.; Mouser, V.H.M.; Marica, P.; Malda, J.; Hennink, W.E.; Vermonden, T. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Fulbandhe, V.M.; Jobanputra, C.R.; Wadher, K.J.; Umekar, M.J.; Bhoyar, G.S. Evaluation of release retarding property of gum damar and gum copal in combination with hydroxypropyl methylcellulose. Indian J. Pharm. Sci. 2012, 74, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Larush, L.; Kaner, I.; Fluksman, A.; Tamsut, A.; Pawar, A.A.; Lesnovski, P.; Benny, O.; Magdassi, S. 3D printing of responsive hydrogels for drug-delivery systems. J. 3d Print. Med. 2017, 1, 219–229. [Google Scholar] [CrossRef]
- Palo, M.; Kogermann, K.; Laidmäe, I.; Meos, A.; Preis, M.; Heinamaki, J.; Sandler, N. Development of oromucosal dosage forms by combining electrospinning and inkjet printing. Mol. Pharm. 2017, 14, 808–820. [Google Scholar] [CrossRef]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Unger, K.; Salzmann, P.; Masciullo, C.; Cecchini, M.; Koller, G.; Coclite, A.M. Novel Light-Responsive Biocompatible Hydrogels Produced by Initiated Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2017, 9, 17408–17416. [Google Scholar] [CrossRef]
- Zhang, K.; Geissler, A.; Standhardt, M.; Mehlhase, S.; Gallei, M.; Chen, L.; Marie Thiele, C. Moisture-responsive films of cellulose stearoyl esters showing reversible shape transitions. Sci. Rep. 2015, 5, 11011. [Google Scholar] [CrossRef]
- Jamal, M.; Kadam, S.S.; Xiao, R.; Jivan, F.; Onn, T.M.; Fernandes, R.; Nguyen, T.D.; Gracias, D.H. Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers. Adv. Healthc. Mater. 2013, 2, 1142–1150. [Google Scholar] [CrossRef]
- Preis, M.; Rosenholm, J.M. Printable nanomedicines: The future of customized drug delivery? Ther. Deliv. 2017, 8, 721–723. [Google Scholar] [CrossRef] [Green Version]

| Technology | Source Material | Polymer(s) Exploited | Mode and Resolution | References |
|---|---|---|---|---|
| 3D | ||||
| Fused deposition modeling FDM | Filament | Thermoplastic polymers, such as polycarbonate, ABS, PLA and nylon | Extrusion and deposition, 50–200 (Rapide Lite 500) | [23,24,25] |
| Stereolithography SLA | Liquid photo polymer | Photopolymer (epoxy or acrylate based resin) | Laser scanning and UV induced curing, 10 (DWSLAB XFAB) | [44] |
| Selective laser sintering SLS | Powder | Polykaprolaktam, polyamides, etc. | Laser scanning and heat induced sintering, 80 (Spo230 HS) | [45,46] |
| Inkjetprinting | Powder | Any powder Es, as well as polymers that correct the rheological characteristics of the liquid | Drop-on-demand binder printing, 100–250 (Plan B, Ytec3D) | [47] |
| Pressure assisted microsyringes (PAM) | Liquid polymer | Polymer with effective viscosity to form a suspension, with optimum shear and compression yield strength to avoid nozzle blockage, e.g., HPMC, carbomers, etc. | The piston of the pouring machine creates a pressure of ~3–5 bar and squeezes out the polymer; (3D printer (Fab@Home) resolution 25 µm) | [27] |
| 3D printing by drop deposition (drop-on-drop) | Liquid polymer | Polymer system. PS must be soluble in a volatile solvent, using “ink” with an optimum viscosity between injector throughput and liquid leakage (PEG, HPMC, PLHA). | Drop-on-demand binder printing, 100–250 (Plan B, Ytec3D) | [48,49] |
| 2D | ||||
| Piezoelectric printing | Substrate | Substrate material: HPMC. Ink material: 40:60 (v/v) PEG 400 and ethanol; water; 5% (w/v) PEG 8000 in water | 25 µm | [50,51,52] |
| Thermal inkjet printing | Substrate | Substrate material: sodium PVA-CMC, HPMC. Ink material: 10:90 (v/v) glycerol and water; 30:70 (v/v) PPG and water; 10:20:70 (v/v/v) glycerol, methanol and water; DecoColour® yellow (Uchida of America Corp., Torrance, CA, USA) food solutions; 10% (v/v) food red solutions in 10:90 (v/v) mixture of glycerol and water | 9–10 µm | [33,53,54,55] |
| Drop deposition using a pump | Substrate | Substrate material: HPMC. Ink material: ethanol, FS/PVP complex | Wide range adjustable | [56,57] |
| Electrodynamic printing | Substrate | Substrate material: HPMC. Ink material: PEG 400; 2% (w/v) sodium lauryl sulfate in PEG 400 | 15–70 µm | [58] |
| Flexography | Substrate | Substrate material: HPMC. Ink material: PEG 400; 5:95 (w/w) HPC in ethanol; 5:95 (w/w) HPC in water | 30–75 µm | [50,59] |
| Polymer | Drug Delivery System | Printing Technology | References |
|---|---|---|---|
| Hydroxypropylmethylcellulose (HPMC) | Matrix tablets | 3D printing; by extrusion printing | [60] |
| Orally dispersible film | 2D printing (substrate material) | [33,52,57,58,59] | |
| Poly(lactic-co-glycolic acid) (PLGA) | Microsphere, capsules, tablets, nanospheres | 3D printing; by extrusion printing | [61,62,63,64] |
| Copolymers of methacrylic acid (Eudragit® RLPO, Eudragit® RL, Eudragit® E100) | Tablets (“rapid retard” systems, separable tablets, enteric dual pulsatile release, dual pulsatile release) | 3D printing, dropping powder: TheriForm™ process | [47,49] |
| PLGA (poly(lacto-co-glycolic acid)) and PLA (poly-L-lactide), PEG/HPMC | Matrix tablet | 3D printing by drop deposition (drop-on-drop) | [34,65] |
| HPMC, Methocel® K100M/Carbopol® 974P NF. | Matrix tablet | 3D printing (pressure-assisted microsyringes, PAM) | [27] |
| Polyvinyl alcohol, Eudragit® RL, RS | Matrix tablet, controlled release system | 3D printing (fused-deposition modeling, FDM) | [66] |
| pNIPAM-AAc | Nanoparticles of poly(N-isopropylacrylamide-coacrylic acid) (pNIPAM-AAc), polypropylene fumarate (PPF), iron oxide (Fe2O5) | 4D printing | [67] |
| Methacrylated polycaprolactone | Poly (e-caprolactone) (PCL) dimethyl acrylate, 2,4,6-trimethylbenzoyl-diphenylphosphine oxide (TPO) as a photoinitiator, vitamin E to prevent premature crosslinking, yellow 3GP toner | Stereolithography (Freeformpico 2 SLA digital laser printer) | [68] |
| PVA/PEG hydrogel | Polyvinyl alcohol (PVA)-polyethylene glycol (PEG) double sided hydrogel | 4D printing | [69] |
| Acrylic acid copolymers | Epoxidized soybean oil acrylate contains three major fatty acid residues (stearic, oleic and linoleic acids) with pendant alkane groups that can freeze and improve shape hold at −18 °C. | Stereolithography (modified Solidoodle® 3D printer platform) | [70] |
| PEGDA/PHEMA | PEG-acrylate (PEGDA), iron(II, III) oxide (Fe5O4); 2-hydroxyethyl methacrylate (PHEMA) layer, micro and nanoparticles | 4D printing | [71] |
| Vinyl caprolactam/PE hydrogel | Vinyl caprolactam, polyethylene, epoxy diacrylate oligomer, Irgacure® 819 | StratasysConnex 500 multipurpose 3D printer | [72] |
| Polyethylene glycol based systems (PEG 400:ethanol, PEG 8000:water | Orally dispersible film | 2D piezoelectric printing (ink) | [50,52] |
| Polyethylene glycol 400 | 2D electrodynamic printing, flexography (ink) | [33,58] | |
| Poly (methacrylates) (Eudragit) | Nanocpasules, tablets | 3D printing; extrusion printing method; stereolithographic printing | [73] |
| Poly (ethylene glycol) diacrylate (PEGDA) | Hydrogel | 3D; 4D printing; stereolithography | [74] |
| Polyvinyl alcohol (PVA) | Tablets, capsule | 3D printing; by extrusion printing | [75] |
| Polylactic acid (PLA) | Nanofibres | 3D printing; by extrusion printing | [76] |
| Polyvinylpyrrolidone (PVP or Kollidon®) | Tablets; orally dispersible tablets (ODT) | 3D printing; by extrusion printing; 3D printing, dropping on TheriFlash™ powder | [77,78,79] |
| Poly (ε-caprolactone) (PCL) | Tablets, carbon nanotubes | 3D printing; extrusion printing method; laser sintering method | [80] |
| Polyurethane (PU) | Tablets, hydrogel | 3D printing; extrusion printing method; 3D printing (pressure-assisted microsyringes, PAM; (inner diameter, 260 µm, and outer diameter, 463.6 µm) | [81,82] |
| Pluronic | Hydrogel | 3D bioprinting with UV crosslinking | [83] |
| Poly(N-(2-hydroxypropyl) methacrylamide-mono/dilactate)-polyethylene glycol triblock copolymer (M15P10) | Thermosensitive hydrogel | 3D bioprinting; by extrusion printing | [84] |
| Ethylcellulose (EC) | Tablets | 3D printing; hot melt extrusion | [85] |
| Ethylene vinyl acetate | T-shaped intrauterine systems (IUS) and sub-cutaneous rods (SR) | 3D printing; by extrusion printing | [86] |
| Poly(methylmethacrylate) (PMMA) | Tablets | 3D printing by drip deposition | [78] |
| Methacrylic/cellulosic polymers | Tablets | 3D printing; hot melt extrusion | [34,87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blynskaya, E.V.; Tishkov, S.V.; Alekseev, K.V.; Vetcher, A.A.; Marakhova, A.I.; Rejepov, D.T. Polymers in Technologies of Additive and Inkjet Printing of Dosage Formulations. Polymers 2022, 14, 2543. https://doi.org/10.3390/polym14132543
Blynskaya EV, Tishkov SV, Alekseev KV, Vetcher AA, Marakhova AI, Rejepov DT. Polymers in Technologies of Additive and Inkjet Printing of Dosage Formulations. Polymers. 2022; 14(13):2543. https://doi.org/10.3390/polym14132543
Chicago/Turabian StyleBlynskaya, Evgenia V., Sergey V. Tishkov, Konstantin V. Alekseev, Alexandre A. Vetcher, Anna I. Marakhova, and Dovlet T. Rejepov. 2022. "Polymers in Technologies of Additive and Inkjet Printing of Dosage Formulations" Polymers 14, no. 13: 2543. https://doi.org/10.3390/polym14132543







