Study on Microwave Curing of Unsaturated Polyester Resin and Its Composites Containing Calcium Carbonate
Abstract
:1. Introduction
2. Experimental
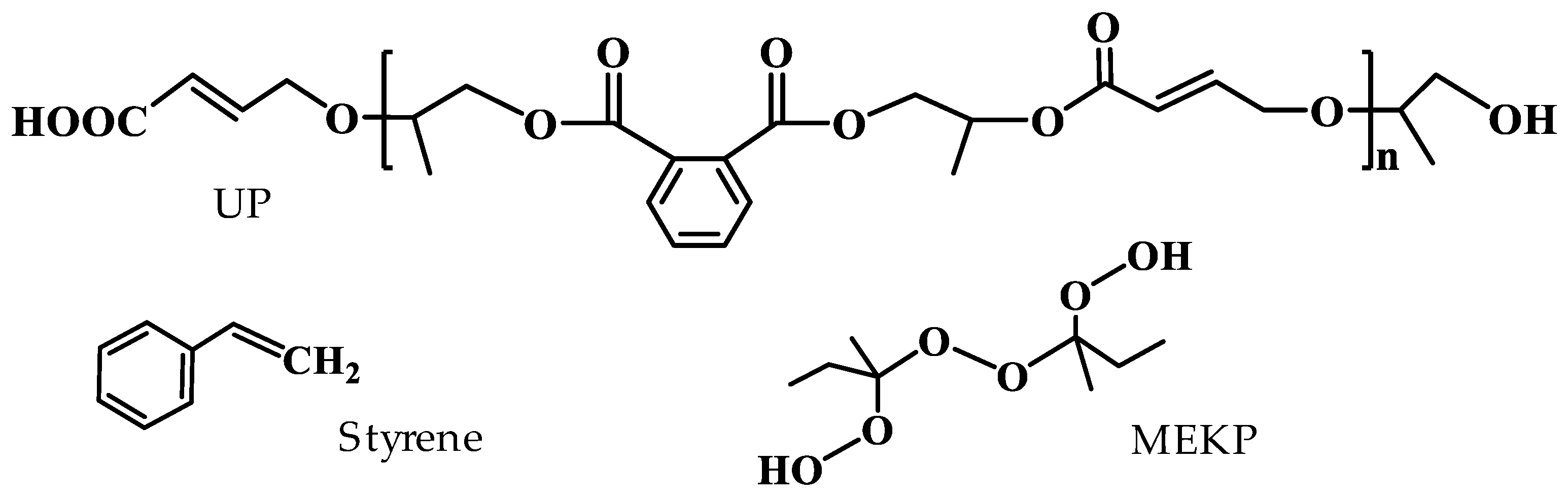
2.1. Materials
2.2. Curing of UPR Composites
2.2.1. Sample Preparation
2.2.2. Thermal Curing
2.2.3. Microwave Curing
2.3. Characterizations
3. Results and Discussion
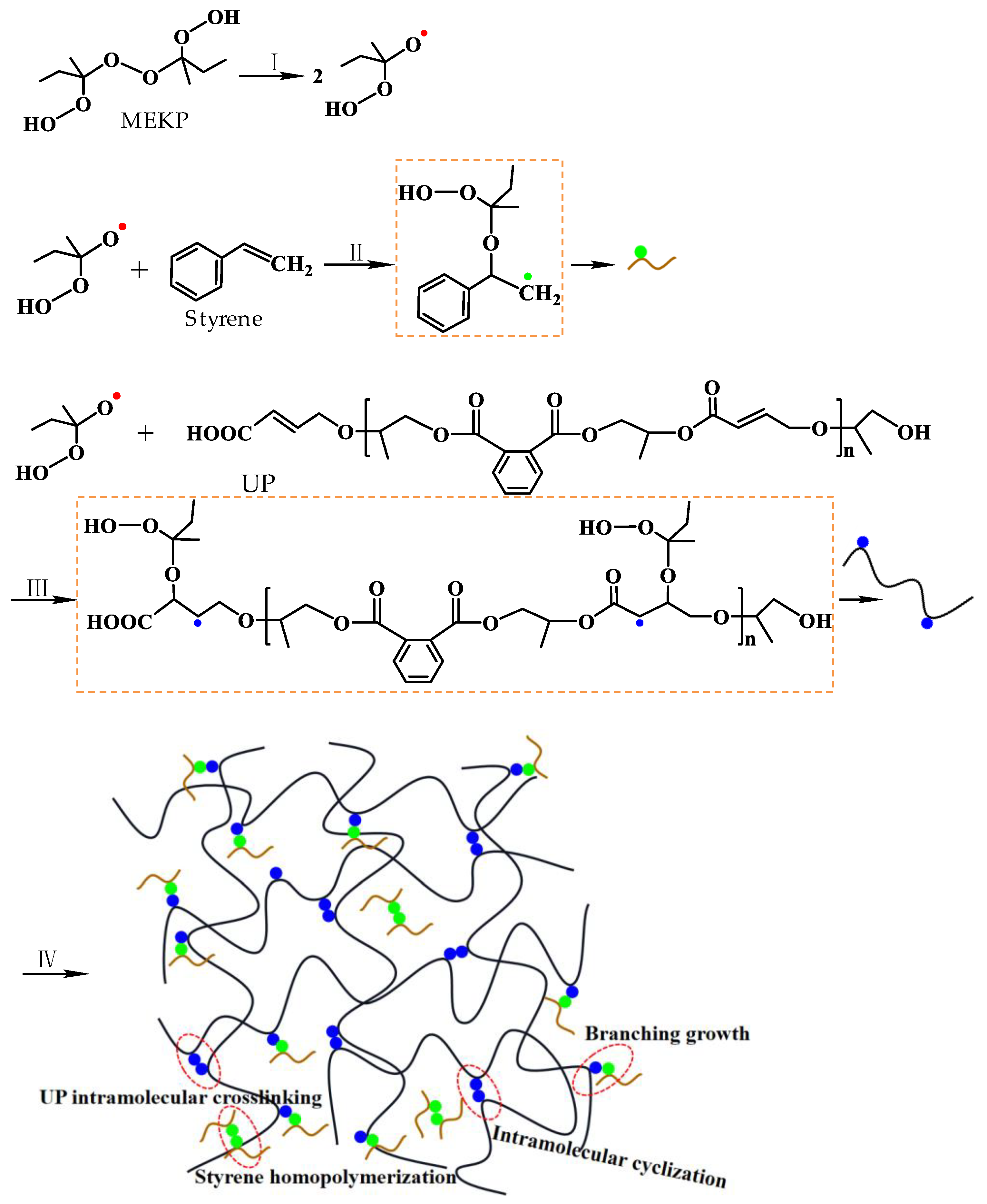
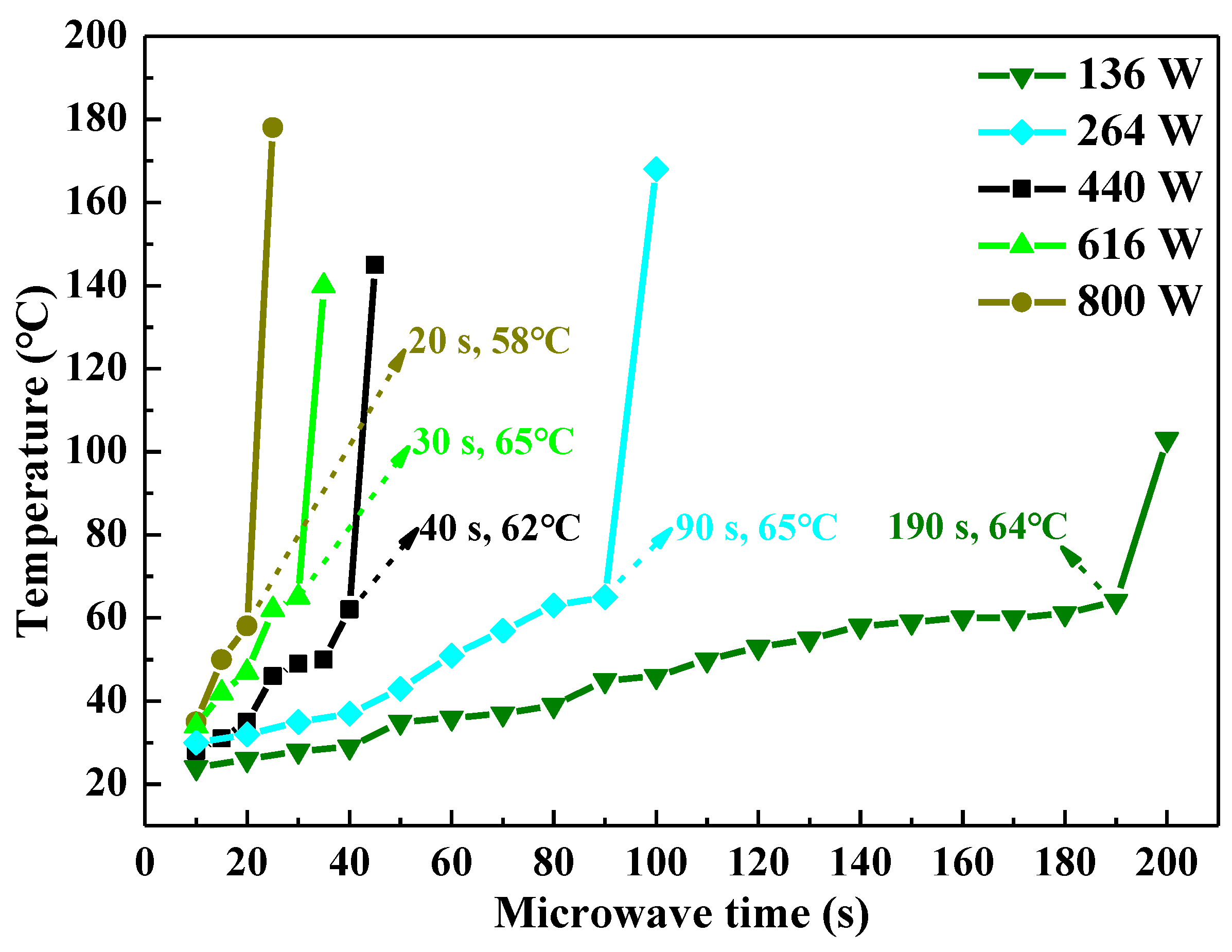
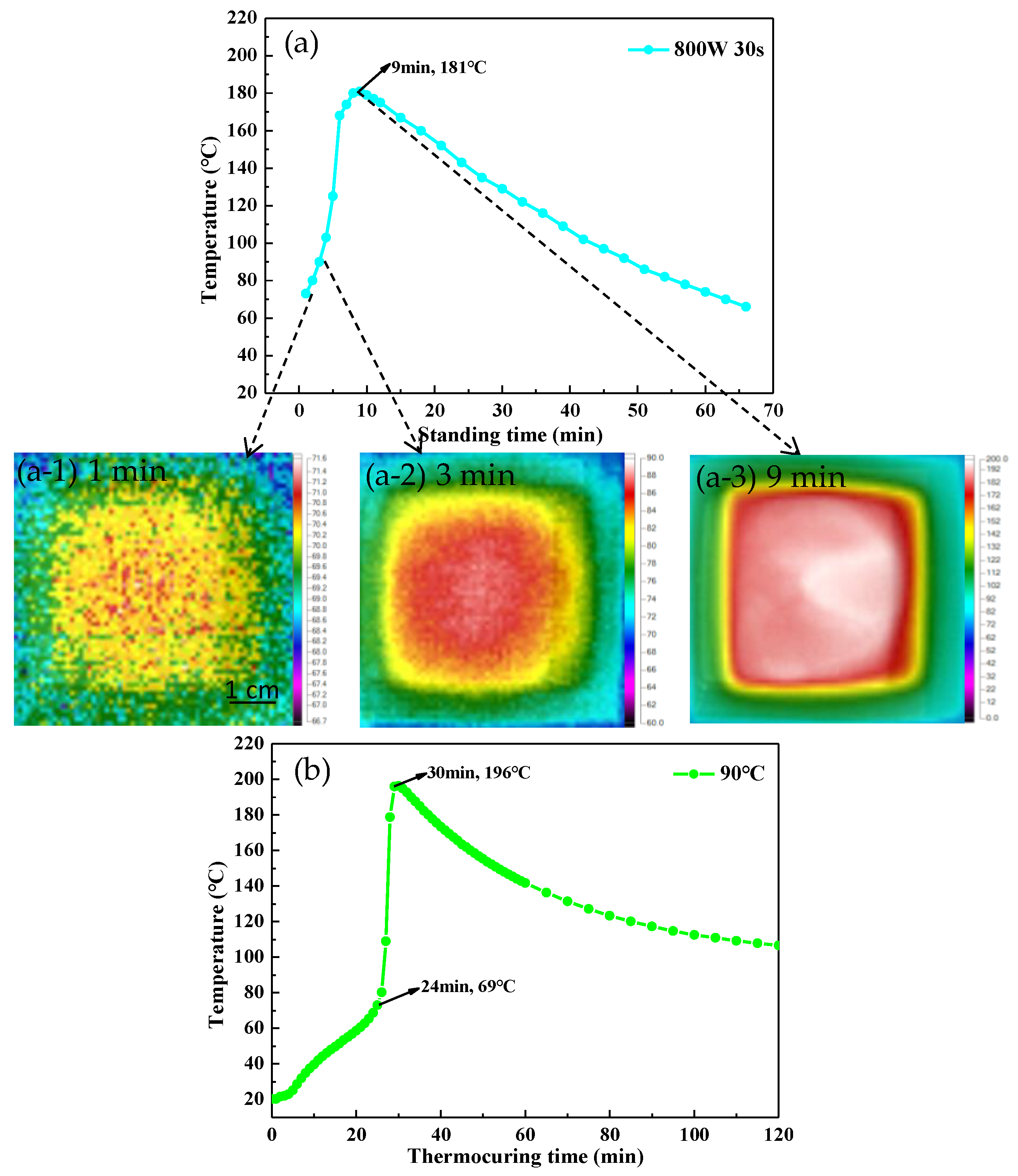
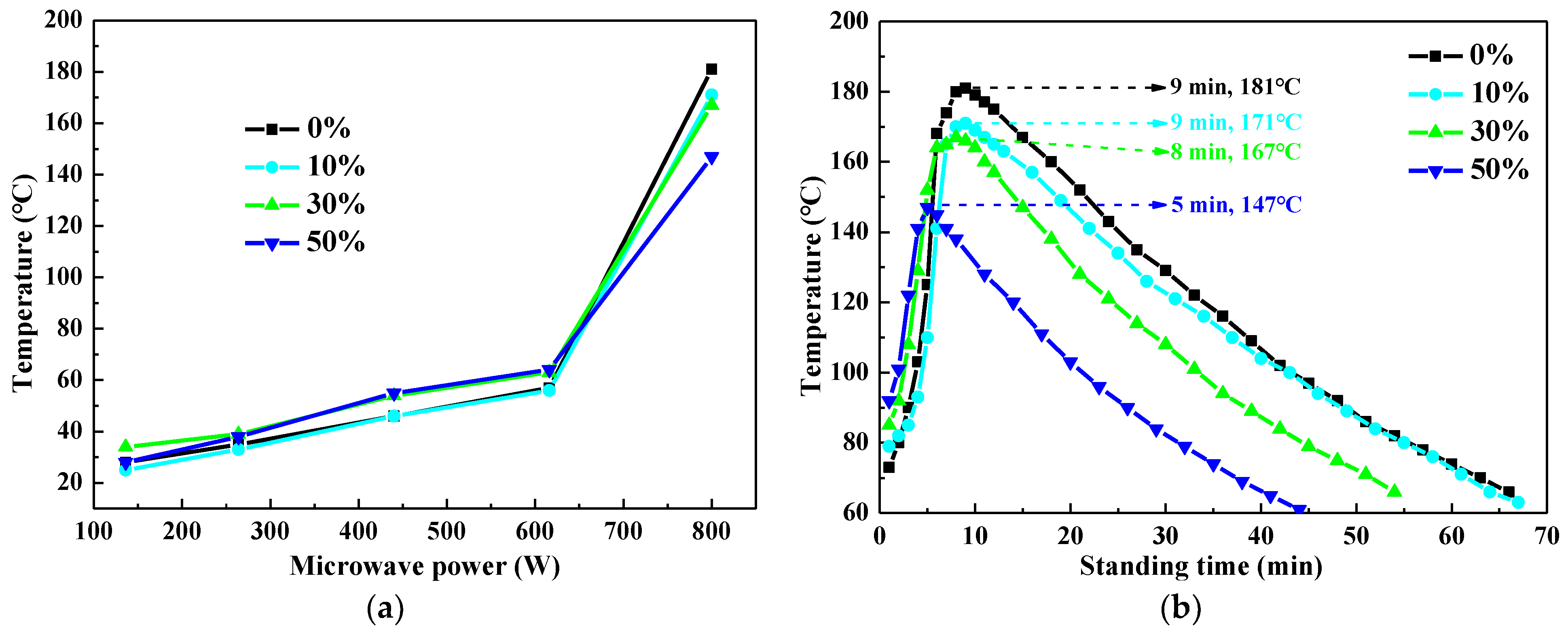
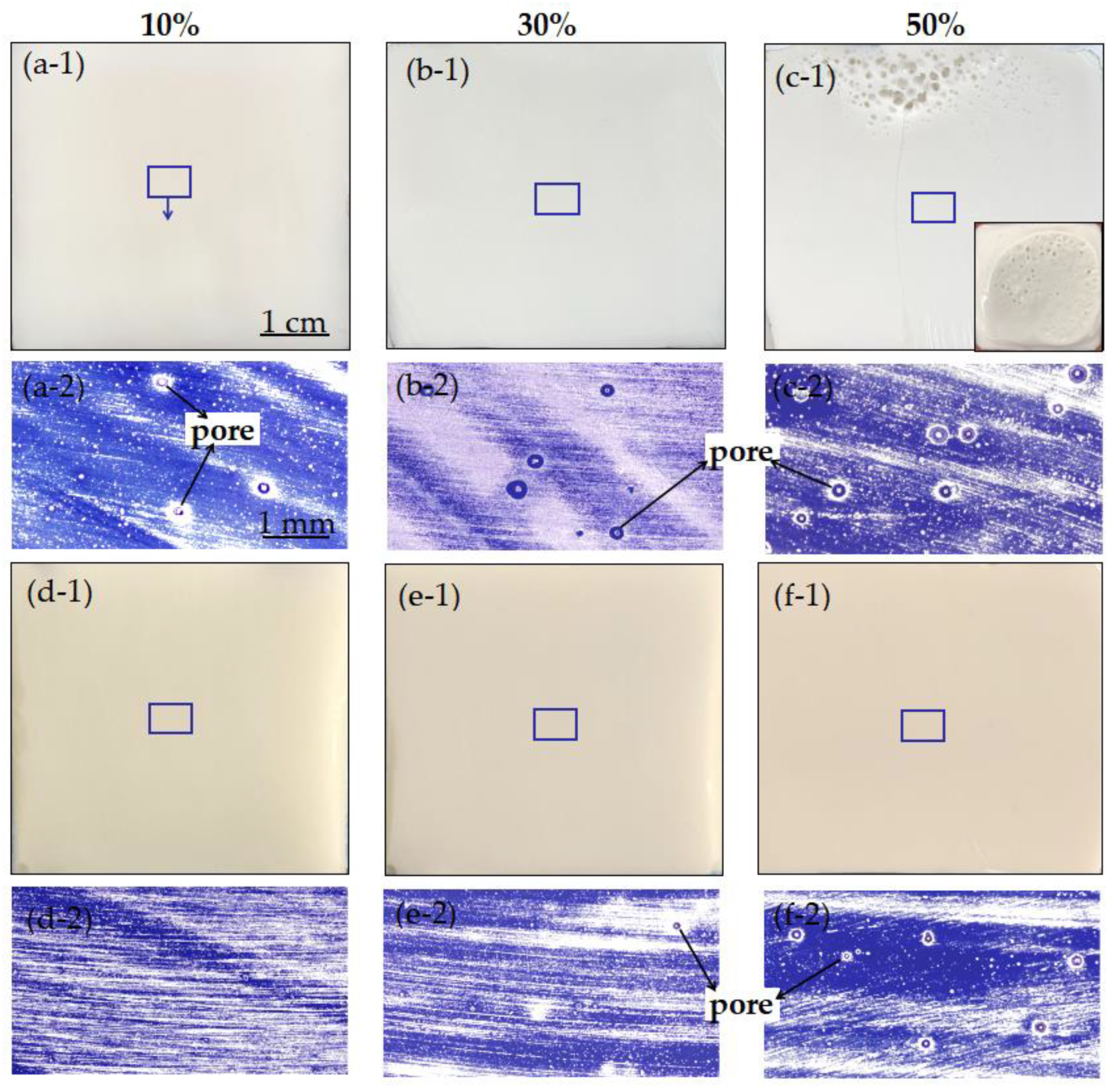
3.1. Microwave Curing Characteristics of UPR
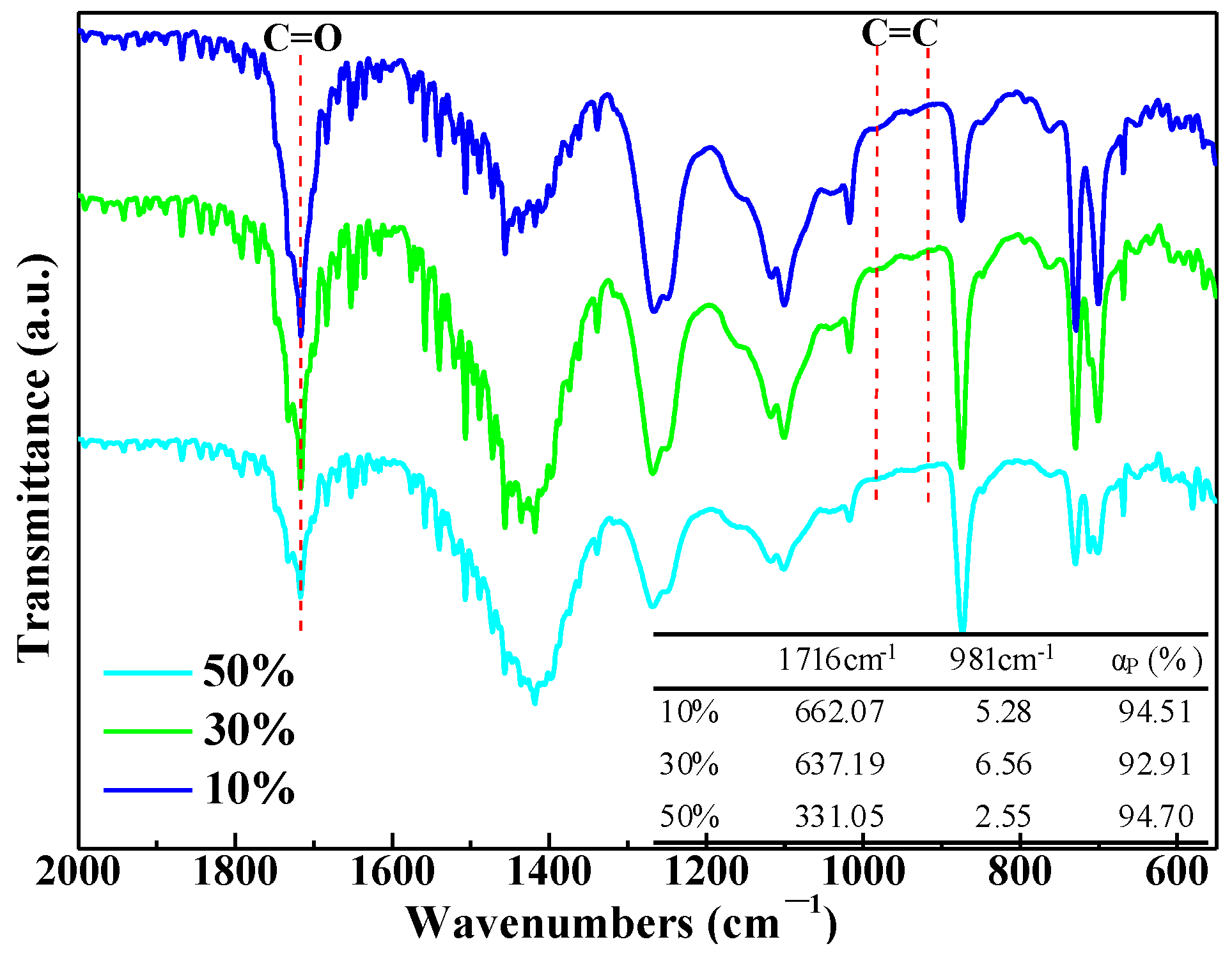
3.2. Microwave Curing Characteristics of UPR Composites with CaCO3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Zhang, H.; Huang, M.; Lai, F. Unsaturated polyester resin concrete: A review. Constr. Build. Mater. 2019, 228, 116709. [Google Scholar] [CrossRef]
- Davallo, M.; Pasdar, H.; Mohseni, M. Mechanical properties of unsaturated polyester resin. Int. J. ChemTech Res. 2010, 2, 2113–2117. [Google Scholar]
- Adeodu, A.O.; Anyaeche, C.O.; Oluwole, O.O. Modeling of Microwave Curing of Unsaturated Polyester Based Composite Materials as Production Process Guide. Int. J. Mater. Sci. Appl. 2014, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Penczek, P.; Czub, P.; Pielichowski, J. Unsaturated polyester resins: Chemistry and technology. Crosslink. Mater. Sci. 2005, 184, 1–95. [Google Scholar] [CrossRef]
- Jones, F.R. Unsaturated polyester resins. In Brydson’s Plastics Materials; Butterworth-Heinemann: Oxford, UK, 2017; pp. 743–772. [Google Scholar] [CrossRef]
- Athawale, A.A.; Pandit, J.A. Unsaturated polyester resins, blends, interpenetrating polymer networks, composites, and nanocomposites: State of the art and new challenges. In Unsaturated Polyester Resins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–42. [Google Scholar] [CrossRef]
- Delahaye, N.; Marais, S.; Saiter, J.M.; Metayer, M. Characterization of unsaturated polyester resin cured with styrene. J. Appl. Polym. Sci. 1998, 67, 695–703. [Google Scholar] [CrossRef]
- Stuck, M.; Krenz, I.; Schulze Kökelsum, B.; Boye, S.; Voit, B.; Lorenz, R. Improving glass transition temperature of unsaturated polyester thermosets: Conventional unsaturated polyester resins. J. Appl. Polym. Sci. 2021, 138, 49825. [Google Scholar] [CrossRef]
- Hong, C.; Wang, X.; Pan, Z.; Zhang, Y. Curing thermodynamics and kinetics of unsaturated polyester resin with different chain length of saturated aliphatic binary carboxylic acid. J. Therm. Anal. Calorim. 2015, 122, 427–436. [Google Scholar] [CrossRef]
- Cao, X.; Lee, L.J. Control of shrinkage and residual styrene of unsaturated polyester resins cured at low temperatures: I. Effect of curing agents. Polymer 2003, 44, 1893–1902. [Google Scholar] [CrossRef]
- Matuskova, E.; Vinklarek, J.; Honzicek, J. Effect of Accelerators on the Curing of Unsaturated Polyester Resins: Kinetic Model for Room Temperature Curing. Ind. Eng. Chem. Res. 2021, 60, 14143–14153. [Google Scholar] [CrossRef]
- Kozioł, M.; Gradoń, P. Effect of glass fibre presence on curing process of unsaturated polyester resin. Compos. Theory Pract. 2018, 18, 191–195. [Google Scholar]
- Ramis, X.; Salla, J.M. Effect of the initiator content and temperature on the curing of an unsaturated polyester resin. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 751–768. [Google Scholar] [CrossRef]
- Feng, H.; Yin, Y.; Tang, J. Microwave drying of food and agricultural materials: Basics and heat and mass transfer modeling. Food Eng. Rev. 2012, 4, 89–106. [Google Scholar] [CrossRef]
- Saltiel, C.; Datta, A.K. Heat and mass transfer in microwave processing. In Advances in Heat Transfer; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1–94. [Google Scholar] [CrossRef]
- Haghi, A.K.; Amanifard, N. Analysis of heat and mass transfer during microwave drying of food products. Braz. J. Chem. Eng. 2008, 25, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, R.; Aroua, M.K.; Nesbitt, A.; Day, R.J. Curing of polymeric composites using microwave resin transfer moulding (RTM). J. Eng. Sci. Technol. 2007, 2, 151–163. [Google Scholar]
- Wallace, M.; Attwood, D.; Day, R.J.; Heatley, F. Investigation of the microwave curing of the PR500 epoxy resin system. J. Mater. Sci. 2006, 41, 5862–5869. [Google Scholar] [CrossRef] [Green Version]
- Rahmat, A.R.; Day, R.J. Curing characteristics of unsaturated polyester/aramid reinforced composite: Microwave vs. thermal energy. J. Teknol. 2003, 39, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Rahmat, A.R.; Heatley, F.; Day, R.J. Comparison of microwave and thermal cure of unsaturated polyester resin. Plast. Rubber Compos. 2003, 32, 257–264. [Google Scholar] [CrossRef]
- Feng, T.; Ding, H.; Gao, D.; Zhang, Z. Numerical simulation and experiment of hardening behaviors in unsaturated polyester resin artificial marble blocks under microwave radiation. IEEE Trans. Plasma Sci. 2016, 44, 2485–2492. [Google Scholar] [CrossRef]
- Adeodu, A.; Daniyan, I.; Azeez, T.; Omohimoria, C. Effect of microwave curing on the tensile property of particulate reinforced polymer matrix composites. Adv. Mater. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.S.; Lee, L.J. Microstructure formation in the cure of unsaturated polyester resins. Polymer 1988, 29, 1793–1800. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Khalina, A.; Sapuan, S.M.; Laila, A.D.; Rahmah, M. Curing behaviour of unsaturated polyester resin and interfacial shear stress of sugar palm fibre. J. Mech. Eng. Sci. 2017, 11, 2650. [Google Scholar] [CrossRef]
- Baskaran, R.; Sarojadevi, M.; Vijayakumar, C.T. Mechanical and thermal properties of unsaturated polyester/calcium carbonate nanocomposites. J. Reinf. Plast. Compos. 2011, 30, 1549–1556. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, S. Microvoids in unsaturated polyester resins containing poly (vinyl acetate) and composites with calcium carbonate and glass fibers. Polymer 2000, 41, 3861–3870. [Google Scholar] [CrossRef]
- Johari, A.N.; Ishak, M.R.; Leman, Z.; Yusoff, M.; Asyraf, M. Influence of CaCO3 in pultruded glass fiber/unsaturated polyester resin composite on flexural creep behavior using conventional and time-temperature superposition principle methods. Polimery 2020, 65, 792–800. [Google Scholar] [CrossRef]
- Wahono, S.; Irwan, A.; Syafri, E.; Asrofi, M. Preparation and characterization of ramie cellulose nanofibers/CaCO3 unsaturated polyester resin composites. ARPN J. Eng. Appl. Sci. 2018, 13, 746–751. [Google Scholar]
- Aktas, A.; Krishnan, L.; Kandola, B.; Boyd, S.W.; Shenoi, R.A. A cure modelling study of an unsaturated polyester resin system for the simulation of curing of fibre-reinforced composites during the vacuum infusion process. J. Compos. Mater. 2015, 49, 2529–2540. [Google Scholar] [CrossRef]
- Lima, M.S.; Costa, C.S.; Coelho, J.F.; Fonseca, A.C.; Serra, A.C. A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem. 2018, 20, 4880–4890. [Google Scholar] [CrossRef]
- Chi, J.; Wu, S.; Shu, C. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J. Hazard. Mater. 2009, 171, 1145–1149. [Google Scholar] [CrossRef]
- Lin, W.; Wu, S.; Shiu, G.; Shieh, S.; Shu, C. Self-accelerating decomposition temperature (SADT) calculation of methyl ethyl ketone peroxide using an adiabatic calorimeter and model. J. Therm. Anal. Calorim. 2009, 95, 645–651. [Google Scholar] [CrossRef]
- Waigaonkar, S.; Babu, B.; Rajput, A. Curing studies of unsaturated polyester resin used in FRP products. Indian J. Eng. Mater. Sci. 2011, 18, 31–39. [Google Scholar]
- He, C.L.; Ma, S.J.; Su, X.J.; Mo, Q.H.; Yang, J.L. Comparison of the microwave absorption characteristics of hematite, magnetite and pyrite. J. Microw. Power Electromagn. Energy 2015, 49, 131–146. [Google Scholar] [CrossRef]
- He, C.L.; Ma, S.J.; Su, X.J.; Chen, Y.Q.; Liang, Y.S. Calorimetry study of microwave absorption of some solid materials. J. Microw. Power Electromagn. Energy 2013, 47, 251–261. [Google Scholar] [CrossRef] [PubMed]
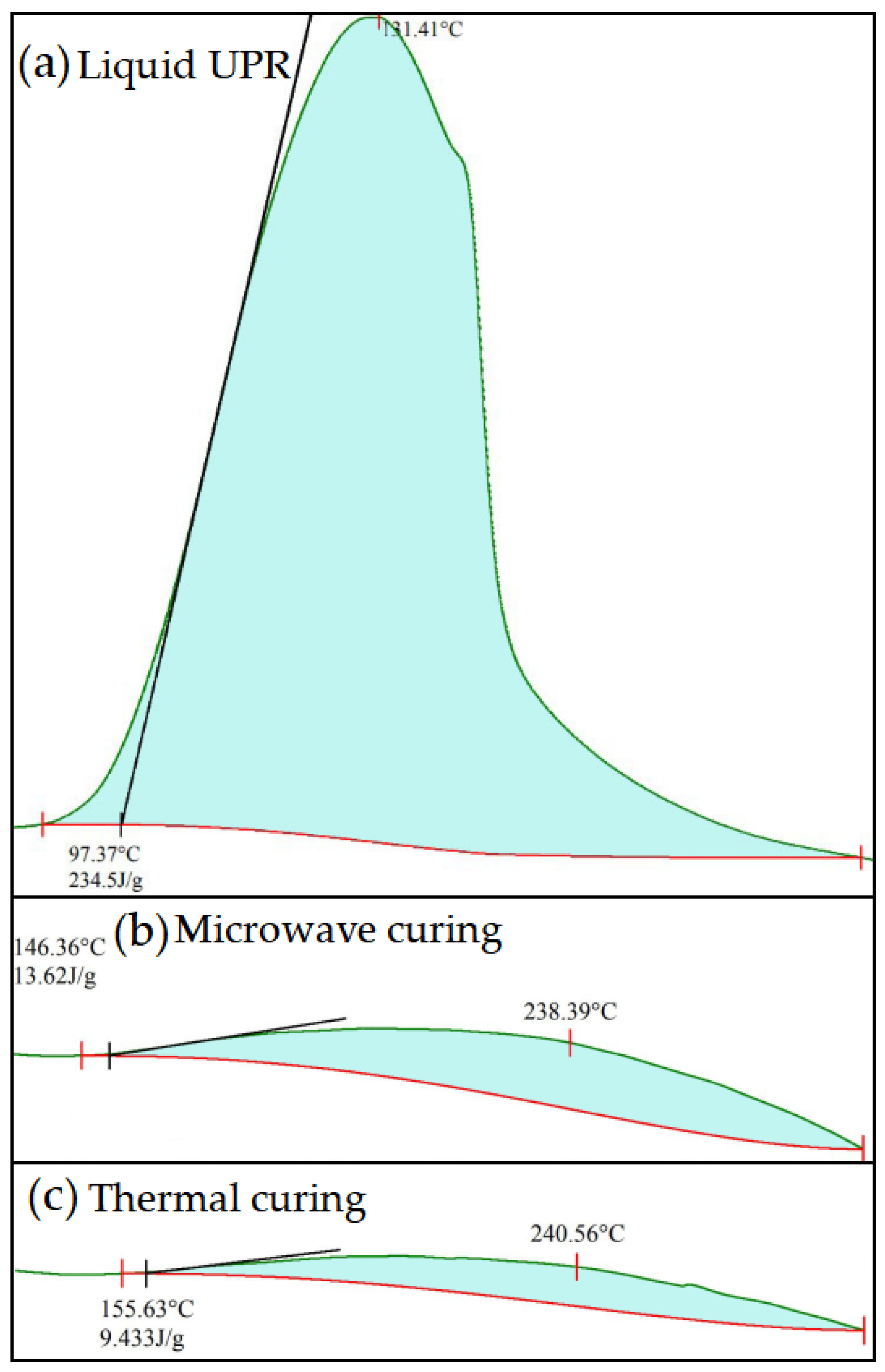
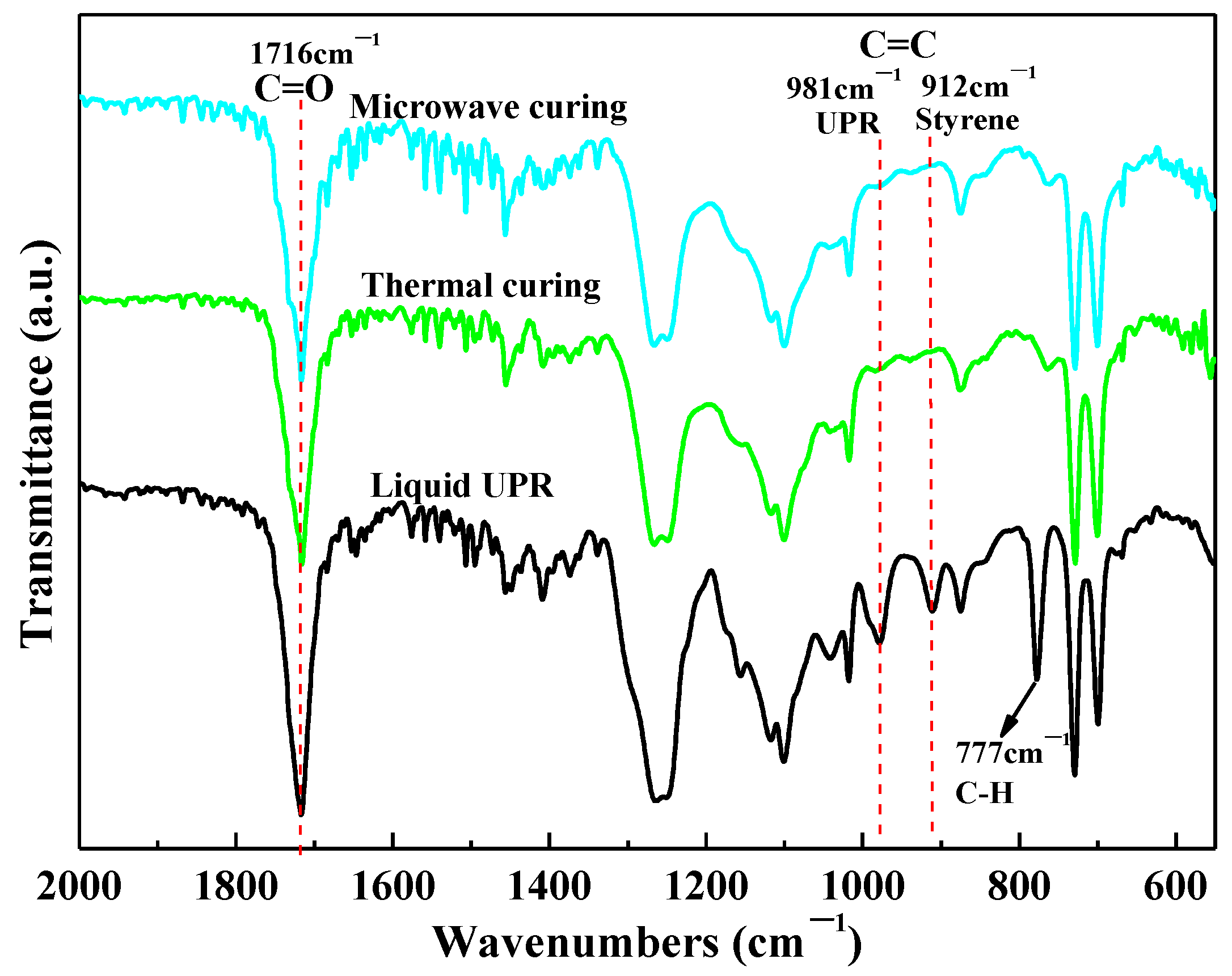

| Sample | H (J/g) | α (%) |
|---|---|---|
| Liquid UPR | 234.50 | |
| Microwave curing | 13.62 | 94.19 |
| Thermal curing | 9.43 | 95.98 |
| Sample | 1716 cm−1 | 981 cm−1 | αP (%) |
|---|---|---|---|
| Liquid UPR | 2450 | 356 | / |
| Microwave curing | 2250 | 22 | 93.27 |
| Thermal curing | 2143 | 24 | 92.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Q.; Huang, Y.; Ma, L.; Lai, W.; Zheng, Y.; Li, Y.; Xu, M.; Huang, Z. Study on Microwave Curing of Unsaturated Polyester Resin and Its Composites Containing Calcium Carbonate. Polymers 2022, 14, 2598. https://doi.org/10.3390/polym14132598
Mo Q, Huang Y, Ma L, Lai W, Zheng Y, Li Y, Xu M, Huang Z. Study on Microwave Curing of Unsaturated Polyester Resin and Its Composites Containing Calcium Carbonate. Polymers. 2022; 14(13):2598. https://doi.org/10.3390/polym14132598
Chicago/Turabian StyleMo, Qiufeng, Yifeng Huang, Lanyu Ma, Wenqin Lai, Yihua Zheng, Yanming Li, Mengxue Xu, and Zhimin Huang. 2022. "Study on Microwave Curing of Unsaturated Polyester Resin and Its Composites Containing Calcium Carbonate" Polymers 14, no. 13: 2598. https://doi.org/10.3390/polym14132598






