Nitrogen Dense Distributions of Imidazole Grafted Dipyridyl Polybenzimidazole for a High Temperature Proton Exchange Membrane
Abstract
:1. Introduction
2. Experimental Section
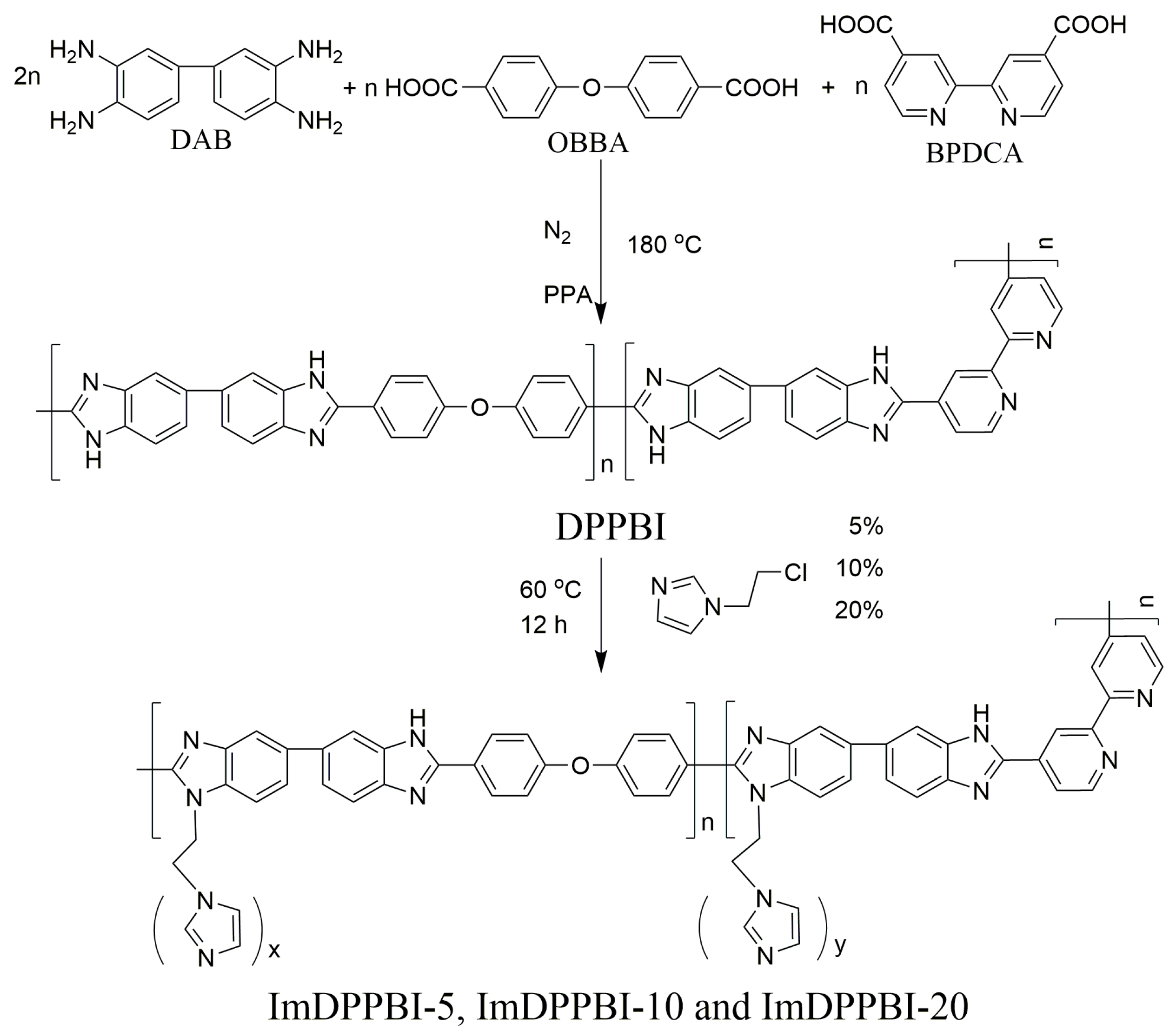
2.1. Synthesis of Dipyridyl PBI (DPPBI) Copolymers
2.2. Grafting the 1-(2-chloroethyl)-1H-imidazole to DPPBI (ImDPPBI-x)
2.3. Preparation of the PBI Composite Membranes
3. Results and Discussion
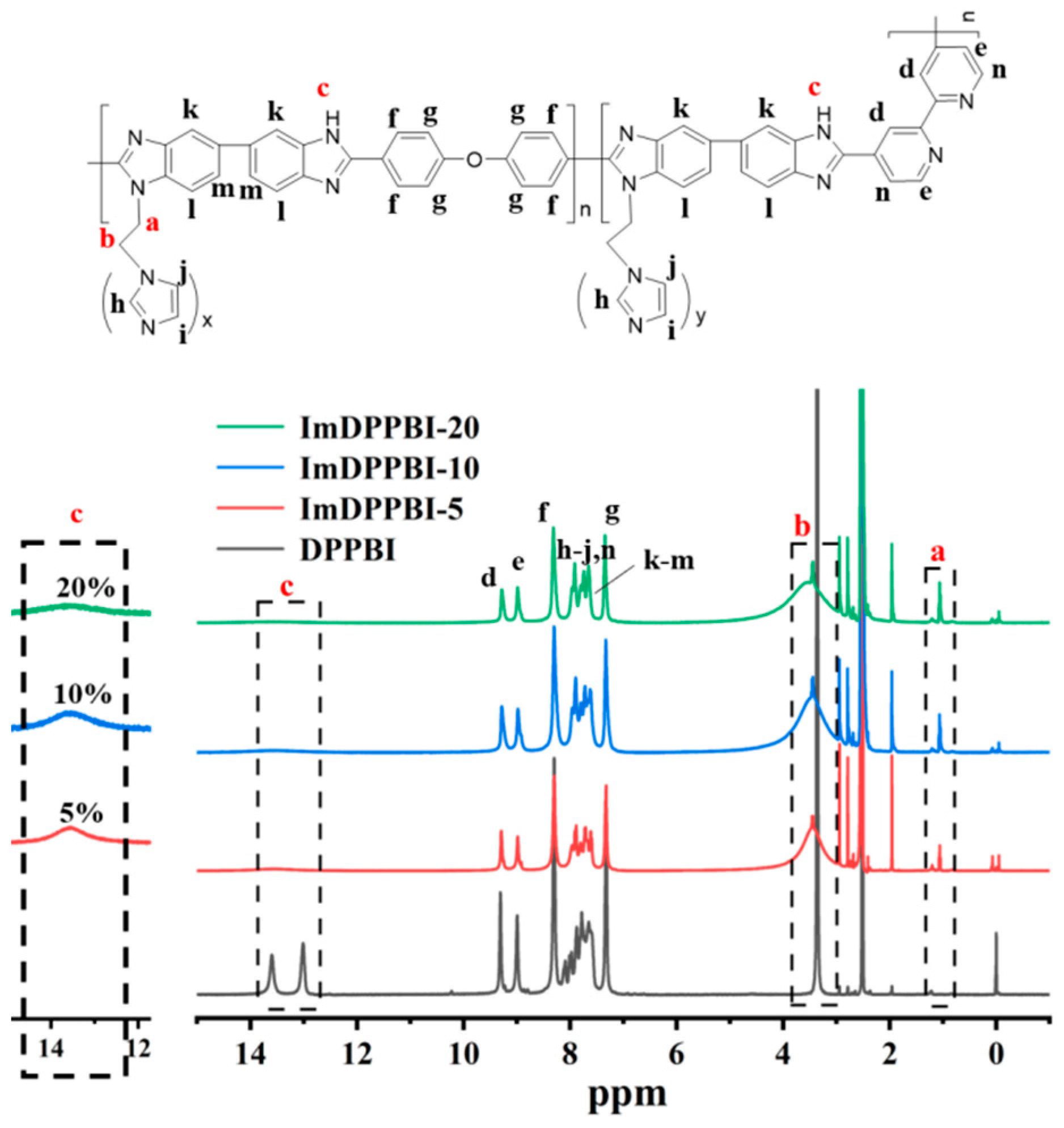
3.1. Characterization of Imidazole Grafted Dipyridyl PBI (ImDPPBI-x)
3.2. Preparation of the PBI Composite Membranes
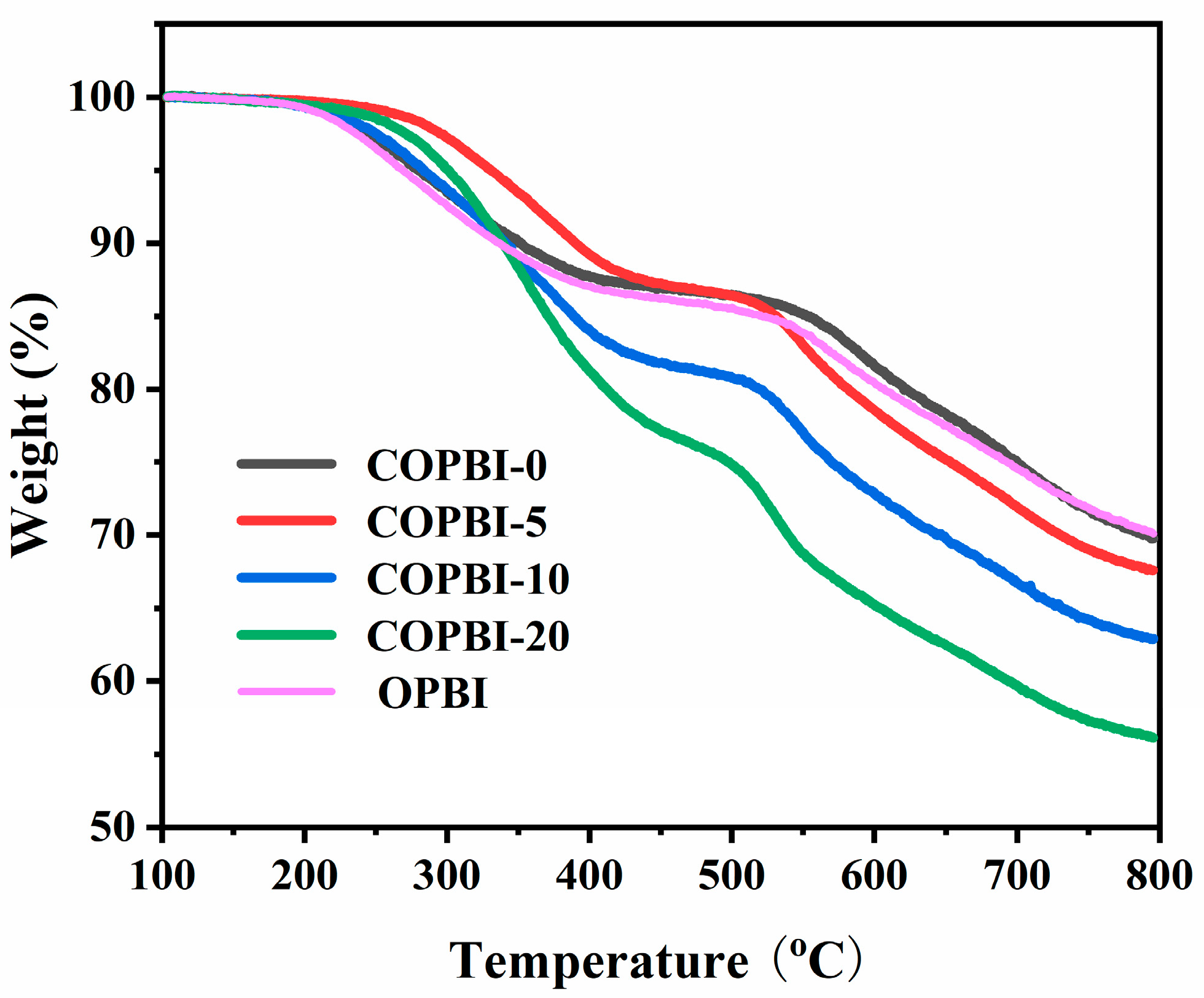
3.3. The Membranes Morphology and Thermal Stability
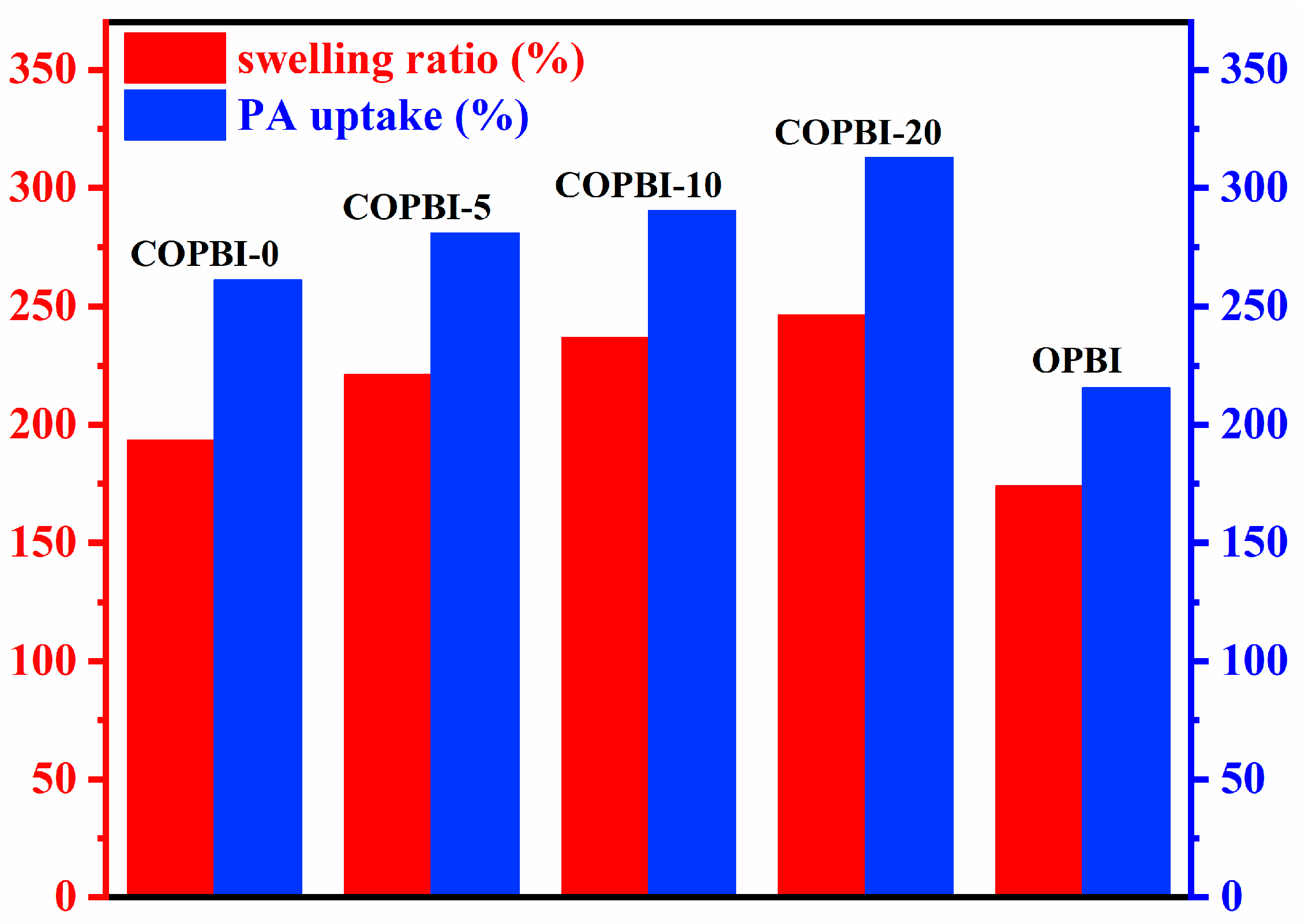
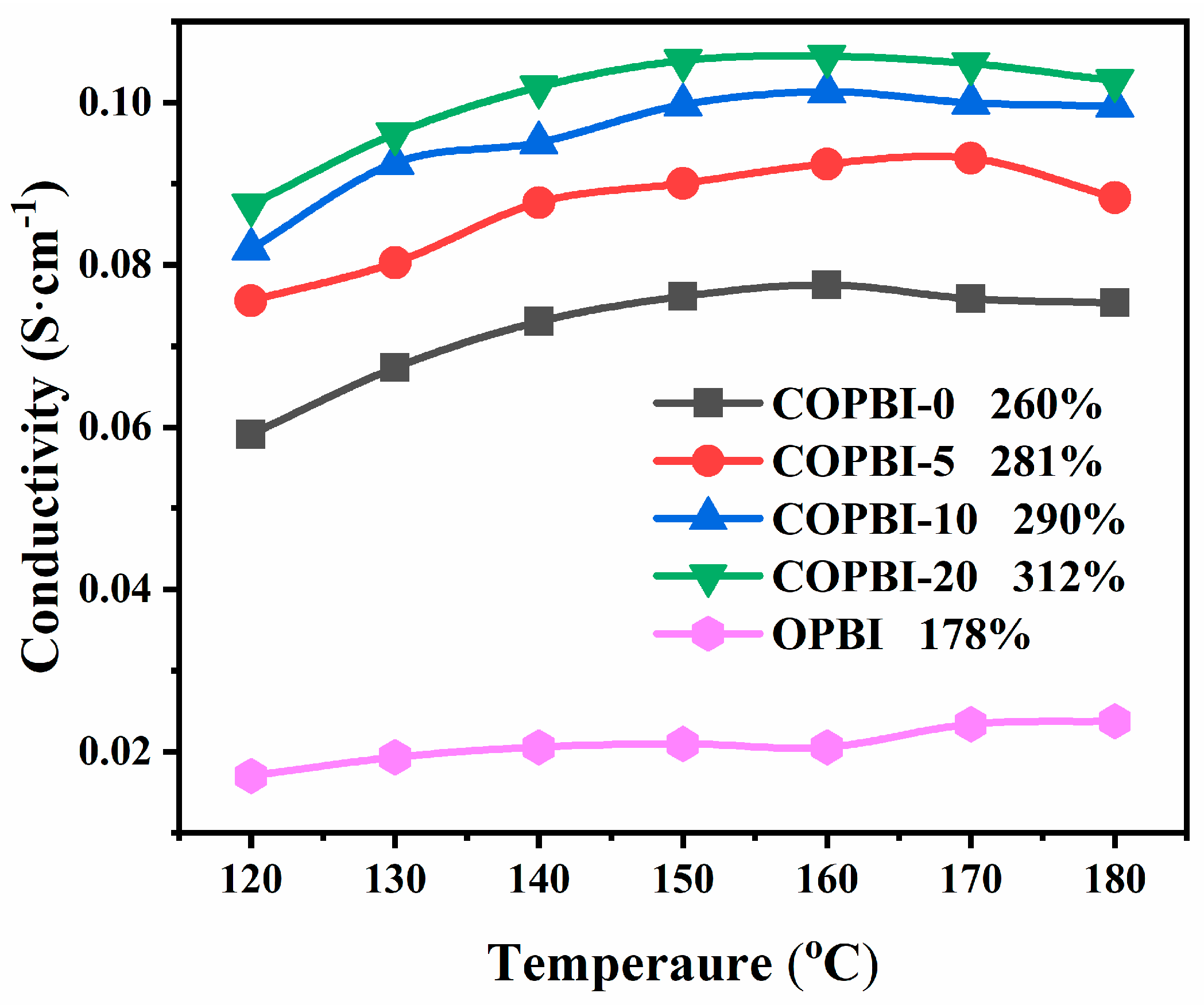
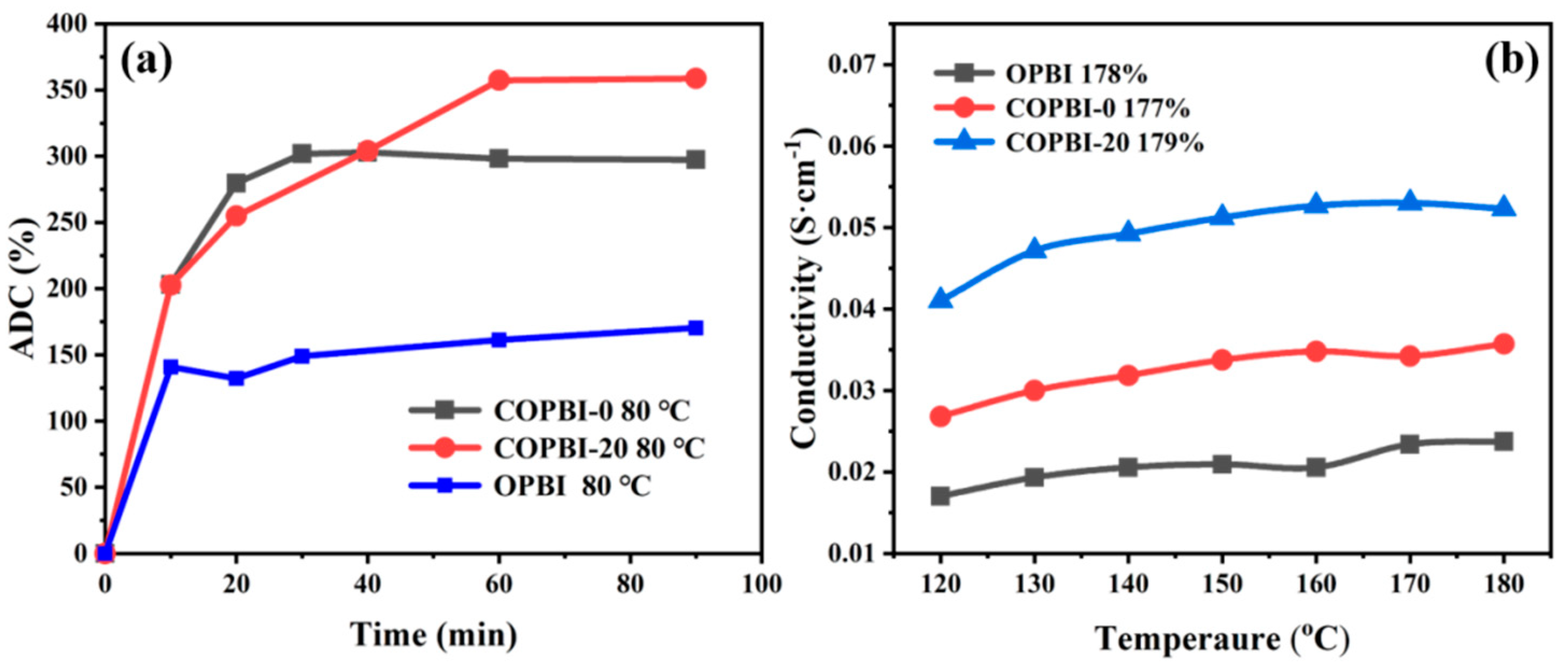
3.4. The Acid Doping Level, Swelling Ratio, and Proton Conductivity
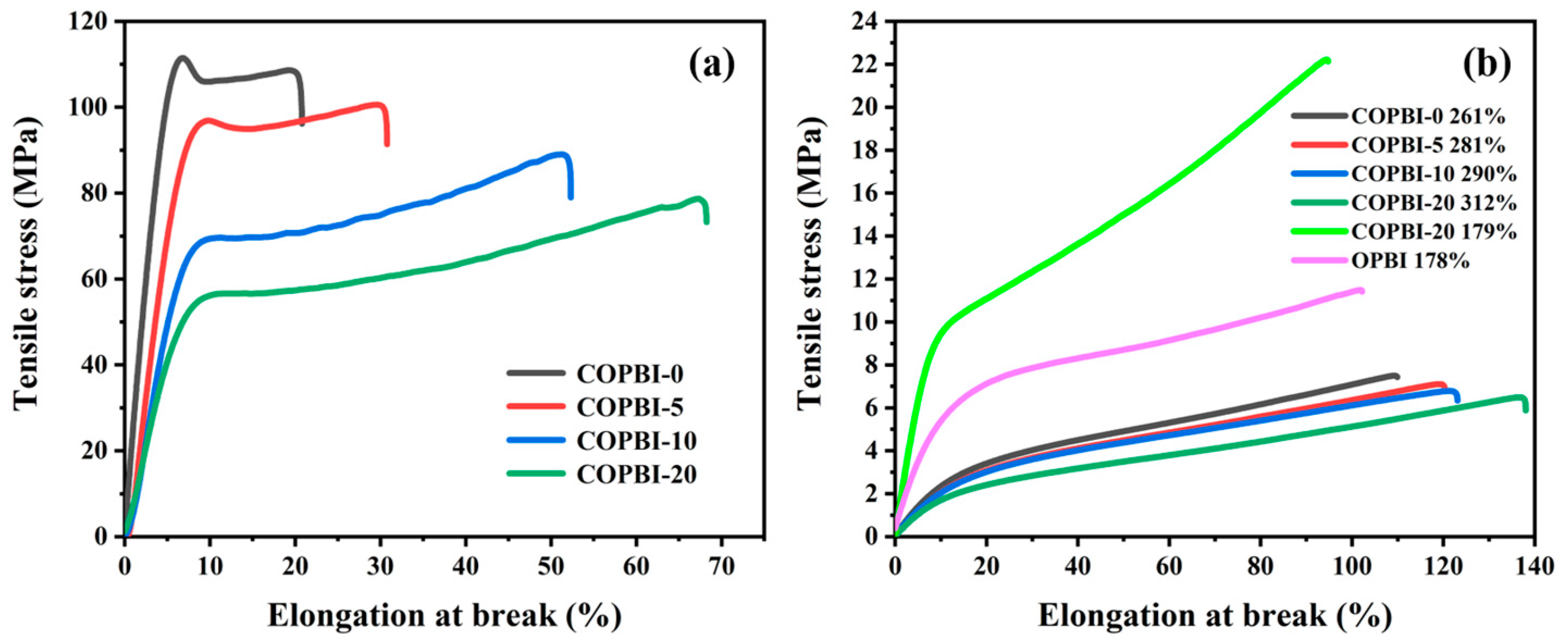
3.5. Mechanical Properties of the Membranes
3.6. Fuel Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative Polymer Systems for Proton Exchange Membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.P.; Shahi, V.K. Organic–Inorganic Nanocomposite Polymer Electrolyte Membranes for Fuel Cell Applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Zhao, J.; Wang, S.; Wu, H.; Guiver, M.D.; Jiang, Z. Nanostructured Ion-Exchange Membranes for Fuel Cells: Recent Advances and Perspectives. Adv. Mater. 2015, 27, 5280–5295. [Google Scholar] [CrossRef] [PubMed]
- Schmittinger, W.; Vahidi, A. A Review of the Main Parameters Influencing Long-Term Performance and Durability of PEM Fuel Cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- Mahato, N.; Jang, H.; Dhyani, A.; Cho, S. Recent Progress in Conducting Polymers for Hydrogen Storage and Fuel Cell Applications. Polymers 2020, 12, 2480. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Polymer Fuel Cells Based on Polybenzimidazole/H3PO4. Energy Environ. Sci. 2012, 5, 6436–6444. [Google Scholar]
- Johnson, F.E.; Cabasso, I. Synthesis and Mechanism of PBI Phosphonate, Poly[2,2′-(-m-Phenylene)-5,5′-Bibenzimidazole Phosphonate Ester], and Its Polyphosphonic Acid Derivatives. Macromolecules 2010, 43, 3634–3651. [Google Scholar] [CrossRef]
- Scofield, M.E.; Liu, H.; Wong, S.S. A Concise Guide to Sustainable PEMFCs: Recent Advances in Improving Both Oxygen Reduction Catalysts and Proton Exchange Membranes. Chem. Soc. Rev. 2015, 44, 5836–5860. [Google Scholar]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.; Lee, J.H. Polymer Membranes for High Temperature Proton Exchange Membrane Fuel Cell: Recent Advances and Challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Maity, S.; Jana, T. Soluble Polybenzimidazoles for PEM: Synthesized from Efficient, Inexpensive, Readily Accessible Alternative Tetraamine Monomer. Macromolecules 2013, 46, 6814–6823. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Yang, F.; Lee Zhou, L.; Yin, B.; Wang, P.; Wang, L. Achieving High Power Density and Excellent Durability for High Temperature Proton Exchange Membrane Fuel Cells Based on Crosslinked Branched Polybenzimidazole and Metal-Organic Frameworks. J. Membr. Sci. 2021, 630, 119288. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, D.; Liu, G.; Cui, Y.; Liang, D.; Wang, X.; Yong, Z.; Wang, Z. Multifunctional Poly(Ionic Liquid)s Cross-Linked Polybenzimidazole Membrane with Excellent Long-Term Stability for High Temperature-Proton Exchange Membranes Fuel Cells. J. Power Sources 2021, 494, 229732. [Google Scholar] [CrossRef]
- Rao, S.; Guo, Z.; Liang, D.; Chen, D.; Li, Y.; Xiang, Y. 3D Proton Transfer Augments Bio-Photocurrent Generation. Adv. Mater. 2015, 27, 2668–2673. [Google Scholar] [CrossRef]
- He, G.; Xu, M.; Zhao, J.; Jiang, S.; Wang, S.; Li, Z.; He, X.; Huang, T.; Cao, M.; Wu, H.; et al. Bioinspired Ultrastrong Solid Electrolytes with Fast Proton Conduction along 2D Channels. Adv. Mater. 2017, 29, 1605898. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Liu, J.; Wang, J.; He, Y.; Davey, K.; Qiao, S.-Z. Molecular-Level Hybridization of Nafion with Quantum Dots for Highly Enhanced Proton Conduction. Adv. Mater. 2018, 30, 1707516. [Google Scholar] [CrossRef]
- Song, M.-K.; Li, H.; Li, J.; Zhao, D.; Wang, J.; Liu, M. Tetrazole-Based, Anhydrous Proton Exchange Membranes for Fuel Cells. Adv. Mater. 2014, 26, 1277–1282. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Duong, N.M.H.; Brela, M.; Dyduch, K.; Michalak, A.; Jankova, K.; Cho, H.; Jang, J.H.; Kim, H.-J.; Cleemann, L.N.; et al. Tetrazole Substituted Polymers for High Temperature Polymer Electrolyte Fuel Cells. J. Mater. Chem. A 2015, 3, 14389–14400. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Lin, X.; Cai, D.; He, N.; Zhao, J. Preparation and Characterization of Novel Pyridine-Containing Polybenzimidazole Membrane for High Temperature Proton Exchange Membrane Fuel Cells. J. Membr. Sci. 2016, 502, 29–36. [Google Scholar] [CrossRef]
- Lin, K.; Wang, C.; Qiu, Z.; Yan, Y. Enhancement of Proton Conductivity Performance in High Temperature Polymer Electrolyte Membrane, Processed the Adding of Pyridobismidazole. Polymers 2022, 14, 1283. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, H.; Jana, T.; Scanlon, E.; Chen, R.; Choe, E.-W.; Ramanathan, L.S.; Yu, S.; Benicewicz, B.C. Synthesis and Characterization of Pyridine-Based Polybenzimidazoles for High Temperature Polymer Electrolyte Membrane Fuel Cell Applications. Fuel Cells 2005, 5, 287–295. [Google Scholar] [CrossRef]
- Berber, M.R.; Nakashima, N. Bipyridine-Based Polybenzimidazole Membranes with Outstanding Hydrogen Fuel Cell Performance at High Temperature and Non-Humidifying Conditions. J. Membr. Sci. 2019, 591, 117354. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, S.; Tian, G.; Xiang, J.; Zhang, L.; Cheng, P.; Zhang, J.; Tang, N. Preparation and Molecular Simulation of Grafted Polybenzimidazoles Containing Benzimidazole Type Side Pendant as High-Temperature Proton Exchange Membranes. J. Membr. Sci. 2021, 620, 118858. [Google Scholar] [CrossRef]
- Li, X.; Ma, H.; Wang, P.; Liu, Z.; Peng, J.; Hu, W.; Jiang, Z.; Liu, B.; Guiver, M.D. Highly Conductive and Mechanically Stable Imidazole-Rich Cross-Linked Networks for High-Temperature Proton Exchange Membrane Fuel Cells. Chem. Mater. 2020, 32, 1182–1191. [Google Scholar] [CrossRef]
- Berber, M.R.; Rosa, F.; Iranzo, A. Mechanically Robust and Highly Conductive Polymer Electrolyte Membranes Comprising High Molecular Weight Poly[2,2′-(Bipyridyl)-Bibenzimidazole] and Graphene Oxide. Polymer 2021, 233, 124223. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Wang, D.; Ni, H.; Wang, Z. Benzimidazole Grafted Polybenzimidazole Cross-Linked Membranes with Excellent PA Stability for High-Temperature Proton Exchange Membrane Applications. Appl. Surf. Sci. 2019, 465, 332–339. [Google Scholar] [CrossRef]
- Yin, B.; Wu, Y.; Liu, C.; Wang, P.; Wang, L.; Sun, G. An Effective Strategy for the Preparation of a Wide-Temperature-Range Proton Exchange Membrane Based on Polybenzimidazoles and Polyacrylamide Hydrogels. J. Mater. Chem. A 2021, 9, 3605–3615. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, C.; Gao, L.; Xu, Y.; Che, Q.; He, R. Influences of the Structure of Imidazolium Pendants on the Properties of Polysulfone-Based High Temperature Proton Conducting Membranes. J. Membr. Sci. 2015, 493, 80–87. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Liu, Z.; Peng, J.; Shi, C.; Hu, W.; Jiang, Z.; Liu, B. Arylether-type polybenzimidazoles bearing benzimidazolyl pendants for high-temperature proton exchange membrane fuel cells. J. Power Sources 2018, 393, 99–107. [Google Scholar] [CrossRef]



| Membrane | ADC | Swelling | Tensile Strength (MPa) | Elongation at Break (%) | ||
|---|---|---|---|---|---|---|
| (%) | V (%) | Undoped | Doped | Undoped | Doped | |
| OPBI | 178 ± 6 | 174 ± 9 | 65.6 ± 0.6 | 11.5 ± 0.3 | 26.0 ± 0.6 | 100.9 ± 1.9 |
| COPBI-0 | 261 ± 2 | 193 ± 4 | 112.9 ± 6.2 | 8.2 ± 0.7 | 21.0 ± 7.5 | 119.1 ± 9.1 |
| COPBI-5 | 281 ± 6 | 221 ± 2 | 102.5 ± 5.1 | 7.4 ± 0.3 | 28.2 ± 7.3 | 128.8 ± 8.5 |
| COPBI-10 | 290 ± 1 | 237 ± 2 | 86.0 ± 3.0 | 6.9 ± 0.1 | 43.6 ± 8.7 | 133.0 ± 9.9 |
| COPBI-20 | 313 ± 3 | 246 ± 4 | 74.7 ± 4.0 | 6.5 ± 0 | 59.5 ± 8.8 | 138.1 ± 0 |
| COPBI-20 | 179 ± 5 | 198 ± 5 | 74.7 ± 4.0 | 22.2 ± 0.9 | 59.5 ± 8.8 | 94.8 ± 8.2 |
| Membrane | ADC | Tensile Strength | Proton Conductivity | Power Density | Single Cell Test Conditions | Reference | ||
|---|---|---|---|---|---|---|---|---|
| (%) | (MPa) | (S cm−1) | (mW cm−2) | Test Gas | Pt Catalyst Concentration (mg cm−2) Anode/Cathode | Temperature (°C) | ||
| COPBI-0 | 261 ± 2 | 8.2 ± 0.7 | 0.077 (160 °C) | 622 | H2/O2 | 1.0/1.0 | 160 | This work |
| COPBI-20 | 313 ± 3 | 6.5 ± 0 | 0.105 (160 °C) | 836 | H2/O2 | 1.0/1.0 | 160 | This work |
| COPBI-20 | 179 ± 5 | 22.2 ± 0.9 | 0.054 (160 °C) | 540 | H2/O2 | 1.0/1.0 | 160 | This work |
| OPBI | 178 ± 6 | 11.5 ± 0.3 | 0.023 (170 °C) | 358 | H2/O2 | 1.0/1.0 | 160 | This work |
| PDA-PBI | 237 | 12.3 | 0.082 (160 °C) | 460 | H2/O2 | 0.6/0.6 | 160 | [18] |
| g3-OPBI | 252 | 4.1 | 0.101 (160 °C) | 305 | H2/O2 | 0.6/0.6 | 160 | [22] |
| g-PBI-20 | 381 | 6.5 | 0.117 (120 °C) | 443 | H2/O2 | 0.6/0.6 | 160 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Q.; Liu, J.; Wu, H.; Wang, W.; Ji, J.; Li, K.; Gong, C.; Wang, L. Nitrogen Dense Distributions of Imidazole Grafted Dipyridyl Polybenzimidazole for a High Temperature Proton Exchange Membrane. Polymers 2022, 14, 2621. https://doi.org/10.3390/polym14132621
Pei Q, Liu J, Wu H, Wang W, Ji J, Li K, Gong C, Wang L. Nitrogen Dense Distributions of Imidazole Grafted Dipyridyl Polybenzimidazole for a High Temperature Proton Exchange Membrane. Polymers. 2022; 14(13):2621. https://doi.org/10.3390/polym14132621
Chicago/Turabian StylePei, Qi, Jianfa Liu, Hongchao Wu, Wenwen Wang, Jiaqi Ji, Keda Li, Chenliang Gong, and Lei Wang. 2022. "Nitrogen Dense Distributions of Imidazole Grafted Dipyridyl Polybenzimidazole for a High Temperature Proton Exchange Membrane" Polymers 14, no. 13: 2621. https://doi.org/10.3390/polym14132621







