Abstract
Pea starch and some legume starches are the side streams of plant-based protein production. Structural modification toward moderate digestibility and desirable functionality is a way to increase the economic values of these side-stream starches. We applied an innovative and sustainable technique, high-pressure homogenization, to alter pea starch structure, which resulted in a high level of complexation with the small phenolic acid molecule, gallic acid, to alter starch digestibility. This study showed a great level of disruption of the compact starch structure represented by the decrease in gelatinization temperature, enthalpy change, and relative crystallinity. The addition of a high concentration (10%) of gallic acid contributed to a typical V-type X-ray diffractometry pattern. Data demonstrated a significant decrease (~23%) in the susceptibility to α-amylase and an increase in resistant starch (~13%). In addition, starch functionality was improved with a reduced retrogradation rate. Pea starch responded to the high-pressure homogenization process well. Compared with the rice and maize starch reported in the literature, pea starch required a reduced amount of gallic acid to form a high level of complexation with a significant delay in starch digestion.
1. Introduction
Legumes are a primary source of plant protein, and pea (Pisum sativum) is a preferred crop as it is allergen-free and is typically not genetically modified [1,2]. The massive production of pea proteins generates much pea starch, which has low thermal stability, low acid and shear resistance, and a high tendency to retrograde [3]. Research to develop high-value-added applications with pea starch is in high demand [1,4,5,6]. Pea starch has a relatively high amount of amylose, approximately 40% (w/w), depending on the variety [7]. With its rich amylose content, pea starch has the potential to be a suitable carrier of micronutrients (e.g., phenolic acids) through complexation with amylose molecules. Among plant-derived micronutrients, gallic acid (GA) stands out as a small, natural crosslinker with three hydroxyl groups, forming either inclusion complexes, non-inclusion complexes, or both with starch molecules [8]. GA is a safe compound with healthy benefits, such as antioxidant and enzyme inhibition activities.
Complexation can also alter the functionality of pea starch to suit various food applications, such as reducing the tendency of retrogradation, improving heat and shear stability, and slowing starch digestibility [9]. However, conventional amylose-based complexation processing usually requires heating to gelatinize the starch, and starch with a high quantity of amylose, at 70% or higher, is preferred. In addition, some micronutrients used for complexation are sensitive to heat and degrade during heat-based complexation processing. Furthermore, the cost of high-amylose starch makes those products less competitive on the market. Therefore, we propose using innovative and cost-effective processing techniques, such as high-pressure homogenization (HPH), to utilize side-stream ingredients such as pea starch from plant-sourced protein production.
HPH is a sustainable food processing technique with high pressure and high shear force, and it promotes the interaction between starch and other ingredients, such as lipids and phenolic acids [10,11,12]. HPH effectively fosters the formation of starch–phenolic acid complexes by decreasing the starch molecular size, which promotes aggregation and microparticle formation. In addition, the leached starch molecules, during HPH processing, have a high tendency to react with phenolic acids under a high-speed shear force [8].
This study proposes using HPH to modify pea starch digestibility through complexation with GA. Pea starch has a C-type polymorphic structure, which is different from maize and rice starch that has been previously studied. The peripheral region of pea starch granules has an A-type polymorphic crystalline structure, which has a looser structure and is gelatinized at a lower temperature than the B-type polymorphic crystalline structure in the center area of pea starch granules. With these unique structural features, we expected HPH to stimulate increased leaching from the peripheral region due to its easy degradation, promoting the complexation of leached starch molecules with GA via the high shear force. Compared with conventional heating (~one hour or longer), the short processing time with HPH (~12 min in this study) may prevent the heat degradation of phenolic acids. In this study, we modified pea starch digestibility through complexation with GA via HPH, and the modification via HPH processing was characterized.
2. Materials and Methods
2.1. Materials
Commercial pea starch was received from Roquette (LN 30; Lestrem, France), and as noted by the manufacturer, the native pea starch contained 0.1% of crude fat and 0.5% of protein. Gallic acid (3,4,5-trihydroxybenzoic acid, 97–102%) was from Sigma-Aldrich (St. Louis, MO, USA). D-Glucose Assay Kit was purchased from Megazyme International Ltd. (Wicklow, Ireland). Pepsin from porcine gastric mucosa (371 U/mg), pancreatin from porcine pancreas (8x USP), amyloglucosidase from Aspergillus niger (319 U/mL), invertase from S. cerevisiae (334 U/mg), and α-amylase from porcine pancreas (type VI-B, 11 U/mg) were from Sigma-Aldrich (St. Louis, MO, USA). All reagents and chemicals were of analytical grade and used without further purification. Enzyme activity was defined and determined by the manufacturers, and newly purchased enzymes were used in this study.
2.2. Methods
2.2.1. Preparation of Pea Starch–Gallic Acid Complex
Pea starch slurries (10% w/w) were prepared using deionized water at approximately 27 ± 5 °C. In sequence, GA (5% and 10% weight based on pea starch) was added into the starch slurry with constant stirring for 3 min. Then, the mixture was homogenized via a high-pressure homogenizer (Panda PLUS 2000, GEA Niro Soavi S.p.A., Italy) at 180 MPa for four cycles, which was 3 min for each cycle and a total of 12 min. A control treatment without GA was prepared with the same procedure. Due to the smooth warming of the mixtures promoted by the high-pressure homogenization process, the mixtures were cooled to approximately 30 °C and then washed twice with 50% (v/v) ethanol solution. The suspension was centrifugated (2800× g for 5 min) to remove free (uncomplexed) GA. The precipitates were dehydrated in an oven at 37 °C for approximately 14 h. Dehydrated samples were ground into powder using a coffee grinder (EKM150, Rommelsbacher ElektroHausgeräte GmbH, Dinkelsbühl, Germany). Powders were passed through a 100-mesh screen before further analyses.
2.2.2. Thermal Properties by DSC
The thermal properties of the pea starch–GA complexes and of the control treatment were determined by differential scanning calorimetry (DSC; model 214 Nevio; Netzsch-Gerätebau GmbH, Selb, Germany). Samples (6 mg) were weighted in aluminum pans, mixed with 18 μL of deionized water, and then equilibrated at ambient temperature for 24 h. The pans were scanned from 10 °C to 150 °C at 10 °C/min, and onset (To), peak (Tp), and conclusion (Tc) temperatures, enthalpy change (ΔH), and the range of gelatinization temperature (calculated by subtracting To from Tc) were recorded. The starch gelatinization degree (GD) was obtained following the equation: DSG (%) = (1 − ΔHTS/ΔHNS) × 100; where ΔHTS is the enthalpy change of HPH-treated starches and ΔHNS is the enthalpy change of native pea starch.
2.2.3. Retrogradation Degree by DSC
To investigate the effects of GA on starch retrogradation, samples scanned in Section 2.2.2 were stored at 4 °C for seven days and then rescanned following the method of Jacobson and BeMiller (1998) [13]. The degree of retrogradation (DR%) was calculated following the equation: DR (%) = (ΔHr/ΔHg) × 100; where ΔHr refers to the enthalpy change of the retrograded sample after storage and ΔHg is the enthalpy change of gelatinized samples.
2.2.4. Crystalline Patterns by X-ray Diffractometry
The native pea starch, HPH-treated pea starch and starch–GA complexes were equilibrated in a chamber with 100% relative humidity for 24 h before X-ray analysis. The X-ray diffractograms were obtained using a D8 Discover X-ray diffractometer (Bruker, Karlsruhe, Germany) equipped with an IμSCu microfocus sealed tube, with a step size of 0.02°, a target voltage of 50 kV, a current of 0.8 mA, and a scan time of 300 s, and the samples were scanned from 3° to 35°. The relative crystallinity (RC) of the starches was obtained by using the software Origin 8.0 (OriginLab Corp., Northampton, MA, USA), and using the following equation as described by Vanier et al. (2019): RC (%) = (Ac/(Ac + Aa)) × 100; where Ac is the crystalline area and Aa is the amorphous area on the X-ray diffractograms [14].
2.2.5. Complex Index by Folin–Ciocalteu Analysis
The GA content in the complexes was estimated by the Folin–Ciocalteu procedure as described by Chi et al. (2019) [15] and Sanchez-Rangel et al. (2013) [16]. The pea starch–GA complex (10 mg) was weighed into a 50 mL Falcon tube and suspended in 10 mL of dimethyl sulfoxide. In the sequence, the mixture was stirred in a vortex for 2 min and then centrifuged at 1800× g for 10 min. An aliquot of the mixture (15 μL) was diluted with deionized water (240 μL) in a 96-well microplate, followed by adding the Folin–Ciocalteu reagent (0.25 N, 15 μL). The mixture was kept on the bench for 3 min, and then 30 μL of sodium carbonate (1N) was added into the mixture. The final mixtures were kept at proximately 27 °C for 90 min in the dark. The absorbance at 760 nm was recorded using a plate reader and compared against a GA standard curve. The complex index was defined as the percentage of the detected GA amount in treatments to the amount of GA added in the modification.
2.2.6. α-Amylase Hydrolysis
Starch–GA samples (500 mg) were mixed with a 25 mL solution (pH 6.9), which contained calcium chloride (0.1 mM), glycerol (0.2 mg/mL), and sodium azide (0.2 mg/mL). The substrate mixtures were prewarmed at 37 °C for 5 min, and then 5 mL of α-amylase suspension (0.33 U/mg starch) was added into the mixture. The hydrolysis was conducted in a water bath controlled at 37 °C. An aliquot of 0.5 mL was taken after 0, 5, 10, 15, 20, 40, 60, 80, 100, and 120 min and heated in a boiling water bath for 10 min to inactivate enzyme activity. The concentration of reducing sugars released by α-amylase hydrolysis was determined by the miniaturized Somogyi–Nelson assay [17].
2.2.7. In Vitro Starch Digestibility Assessment
To determine the rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) fractions, in vitro digestibility of pea starch–GA complexes was carried out following the method of Englyst and Cummings (1992) [18]. Pepsin (2.65 g) was suspended in 0.05 mol/L hydrochloric acid (120 mL) containing ethanol (1 mL) and guar gum (0.6 g) to obtain the enzyme working solution A. The enzyme working solution B was prepared by suspending 9 g of porcine pancreatin in 60 mL of 0.1 mol/L calcium chloride solution, followed by centrifugation at 9000× g for 10 min. Subsequently, amyloglucosidase (1.75 mL diluted into 2.25 mL of 0.1 mol/L calcium chloride) and invertase (18 mg dissolved into 2 mL of 0.1 mol/L calcium chloride) were added to working solution B.
For the gastric phase, the starch–GA complexes (0.5 g) were dispersed in 5 mL of sodium acetate buffer 0.05 mol/L (pH 5.2) and equilibrated at 37 °C for 5 min. Then, 10 mL of enzyme working solution A and three glass marbles were added to the samples, and the mixture was incubated in a shaking water bath (150 rpm) at 37 °C for 30 min. In sequence, to start the intestinal phase, 5 mL of enzyme solution B was added to the mixture, and it was further incubated at 37 °C for 120 min. Aliquots of the hydrolysates (1 mL) were collected at times of 0, 20, and 120 min of intestinal phase simulation and placed in a boiling water bath (10 min) to inactivate the enzymes. The samples were centrifuged at 10,800× g for 20 min, and glucose content present in the supernatant was analyzed using a GOPOD reagent. The released glucose after 20 and 120 min was labeled as G20 and G120, respectively, by which RDS, SDS, and RS fractions were calculated as described in the formulas below:
RDS = G20 × 0.9/TS
SDS = (G120-G20) × 0.9/TS
RS = [TS-RDS-SDS]/TS
TS means the total starch (TS) content of the complexes used for digestibility measurement, equal to 0.5 g.
2.2.8. Statistical Analysis
Data analysis was carried out by analysis of variance (ANOVA). Tukey’s test compared the means at the 5% level of significance using SAS® software (Statistical Analysis System, Cary, NC, USA).
3. Results
3.1. Thermal Properties and Retrogradation of Pea Starch–GA Complex
The thermal properties of the pea starch, HPH-treated pea starch (HPH-PS), and HPH-treated pea starch–gallic acid complex (HPH-PS-GA) were examined with DSC, and the results are presented in Table 1. We observed a relatively low enthalpy change in native pea starch in this study. The pea starch used in this study was a by-product from pea protein isolation, which might not reserve the integrity of starch granules as the commercial starch isolation, and had a disorganized granular structure.

Table 1.
Thermal properties and gelatinization degree of native and modified pea starch.
HPH-treated samples had a reduction in onset (To), peak (Tp), and conclusion (Tc) temperatures and in enthalpy change (ΔH) compared to native pea starch, indicating that HPH facilitated starch gelatinization. The addition of GA, both at 5% and 10%, did not significantly change gelatinization temperature, although both treatments had a slightly higher peak temperature (Tp) than HPH-treated starch without GA (Table 1). The presence of GA in both concentrations significantly decreased the enthalpy change (ΔH) (p < 0.05), indicating a lower thermostability of the starch structure. HPH-treated samples had a narrower gelatinization temperature range than native pea starch, suggesting a higher homogeneity of amylopectin crystals in the HPH-treated samples compared to the native pea starch. With the presence of GA, HPH-PS-GA had a higher gelatinization degree than HPH-PS.
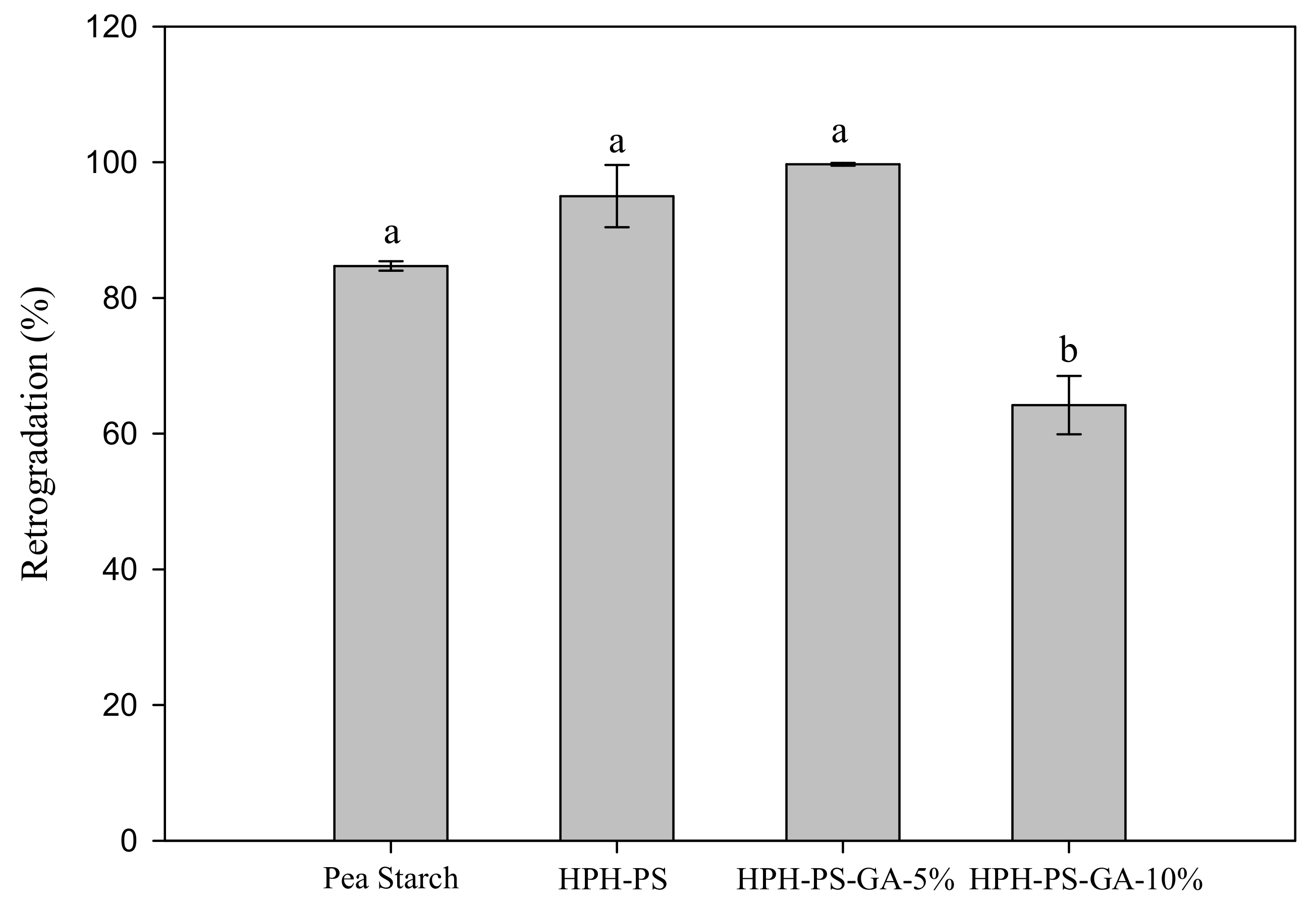
After cold storage for seven days, HPH-PS and HPH-PS-GA-5% samples had a higher retrogradation percentage (95% and 99%, respectively) than untreated pea starch (84.7%; Figure 1). Samples made with 10% of GA had the lowest retrogradation rate (64.3%), 20% lower than the untreated pea starch.
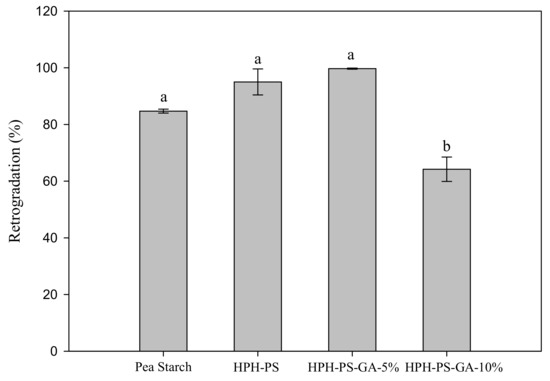
Figure 1.
Retrogradation percentage of native and modified pea starch. HPH-PS: high-pressure homogenization-treated pea starch; HPH-PA-GA-5% and HPH-PA-GA-10%: high-pressure homogenization-treated pea starch with 5% or 10% gallic acid. The letters a and b indicate statistically significant difference at p < 0.05 between groups.
3.2. Crystalline Patterns by X-ray Diffractometry
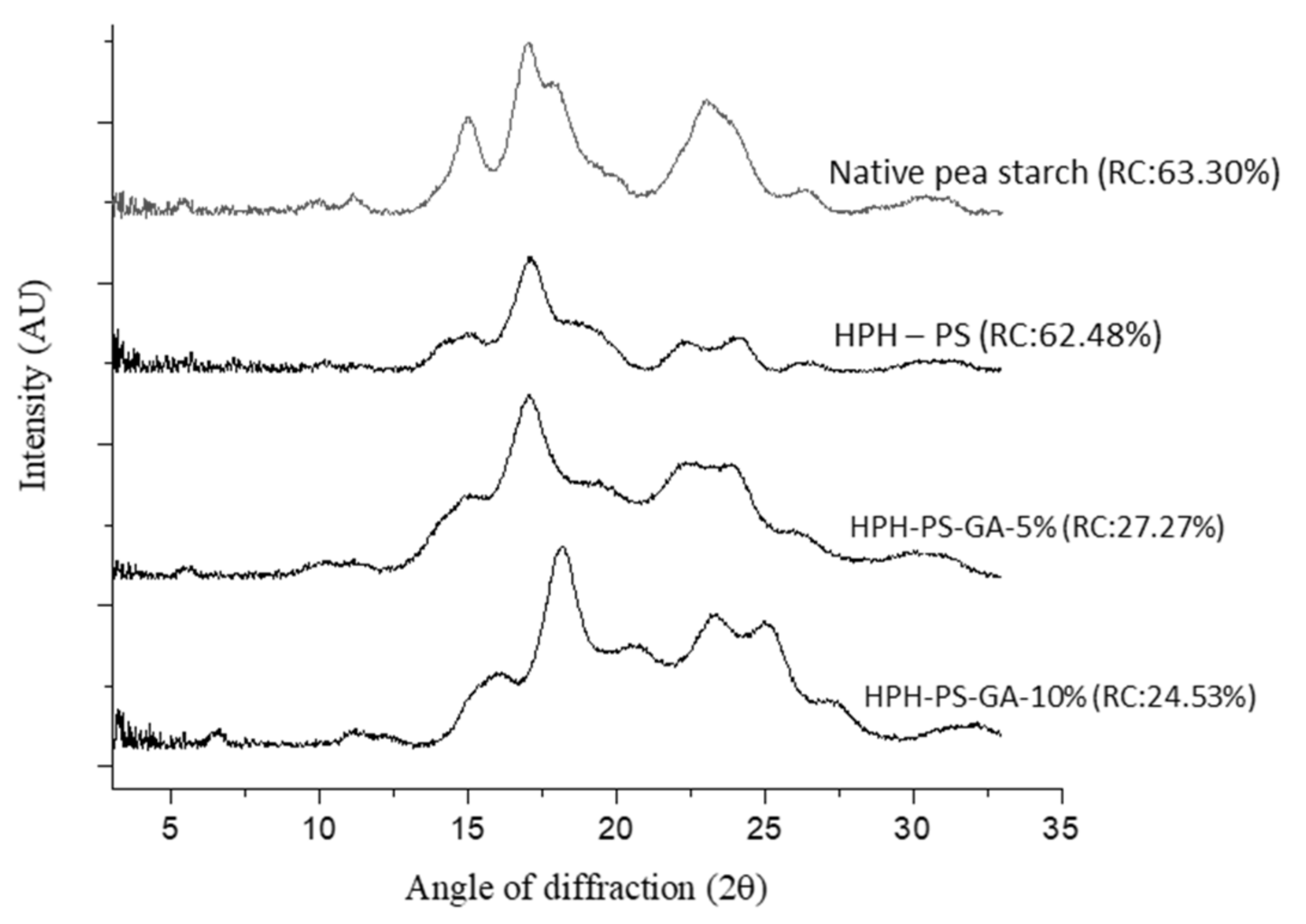
Figure 2 shows a typical C-type crystalline pattern in native pea starch with signature peaks at 5°, 15°, 17°, 18°, and 23°. The pattern of HPH-treated pea starch (without adding GA) shifted to a B-type pattern with peaks at 2θ of 15°, 17°, 22.4°, and 24°. With 10% of GA (HPH-PS-GA-10%), a peak at 19.8° was observed, suggesting a V-type inclusion complex formation.
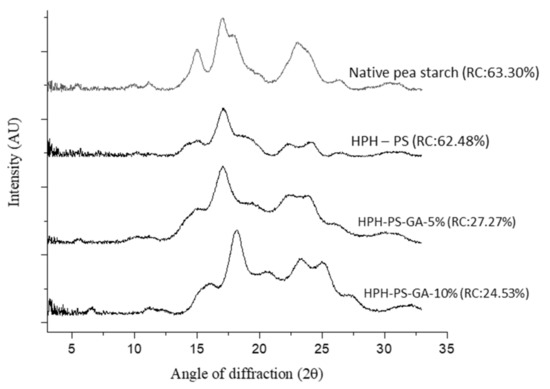
Figure 2.
X-ray diffractograms of native and modified pea starch. HPH-PS: high-pressure homogenization-treated pea starch; HPH-PS-GA-5% and HPH-PS-GA-10%: high-pressure homogenization-treated pea starch with 5% and 10% gallic acid, respectively; RC: relative crystallinity.
Figure 2 also demonstrated data on the relative crystallinity, in which native pea starch had the highest degree of relative crystallinity (63.30%), followed by HPH-PS (62.48%), HPH-PS-GA-5% (27.27%), and HPH-PS-GA-10% (24.53%). The addition of GA significantly reduced the relative crystallinity by approximately 35% compared with the native pea starch, indicating that the crystalline was less organized with the presence of GA.
3.3. Complex Index
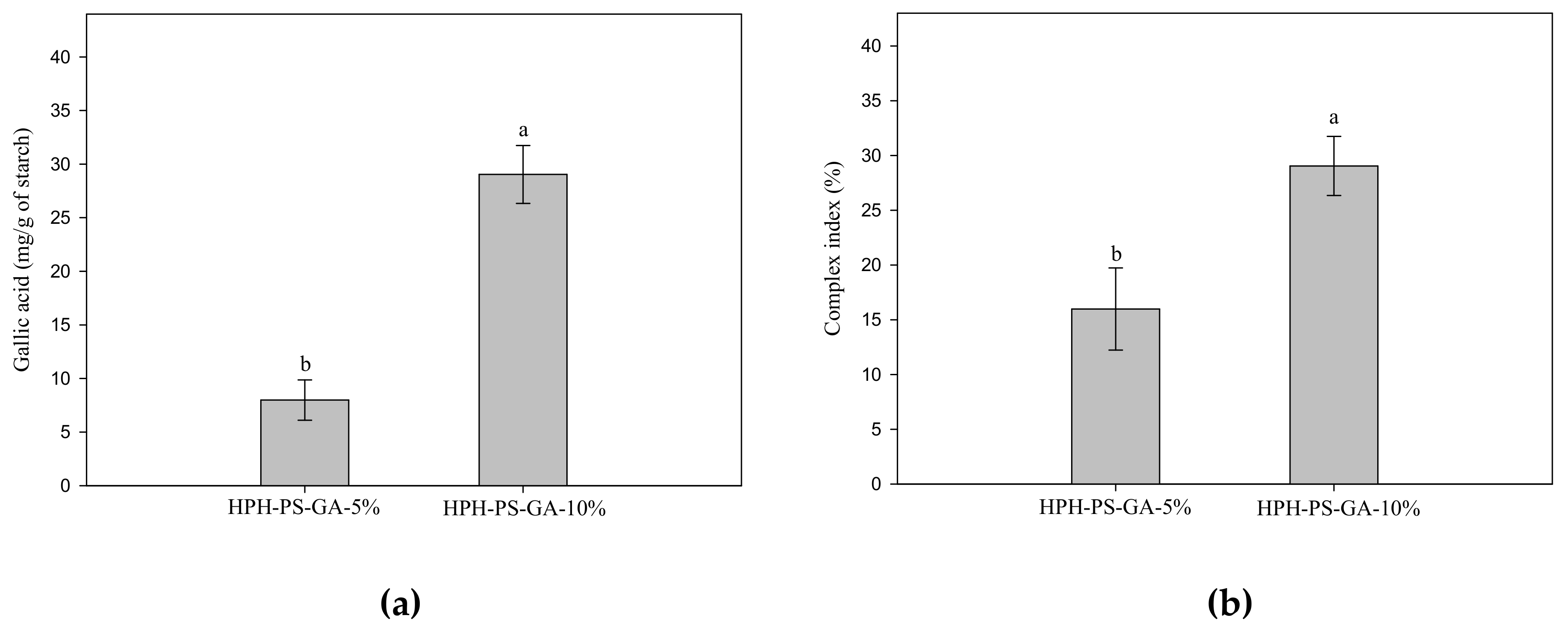
Figure 3 shows the results of GA content and complex index of samples. Samples processed with 10% GA had a high level of the complex index (p < 0.05).
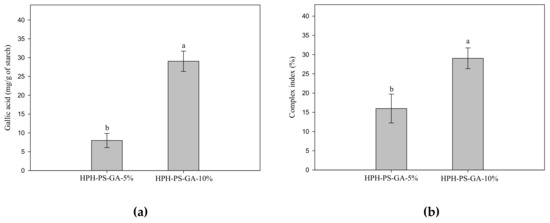
Figure 3.
Gallic acid content (a) and complex index (b) of pea–gallic acid complexes made by high-pressure homogenization with 5% and 10% (w/w) gallic acid. The letters a and b indicate statistically significant difference at p < 0.05 between groups in the same graph.
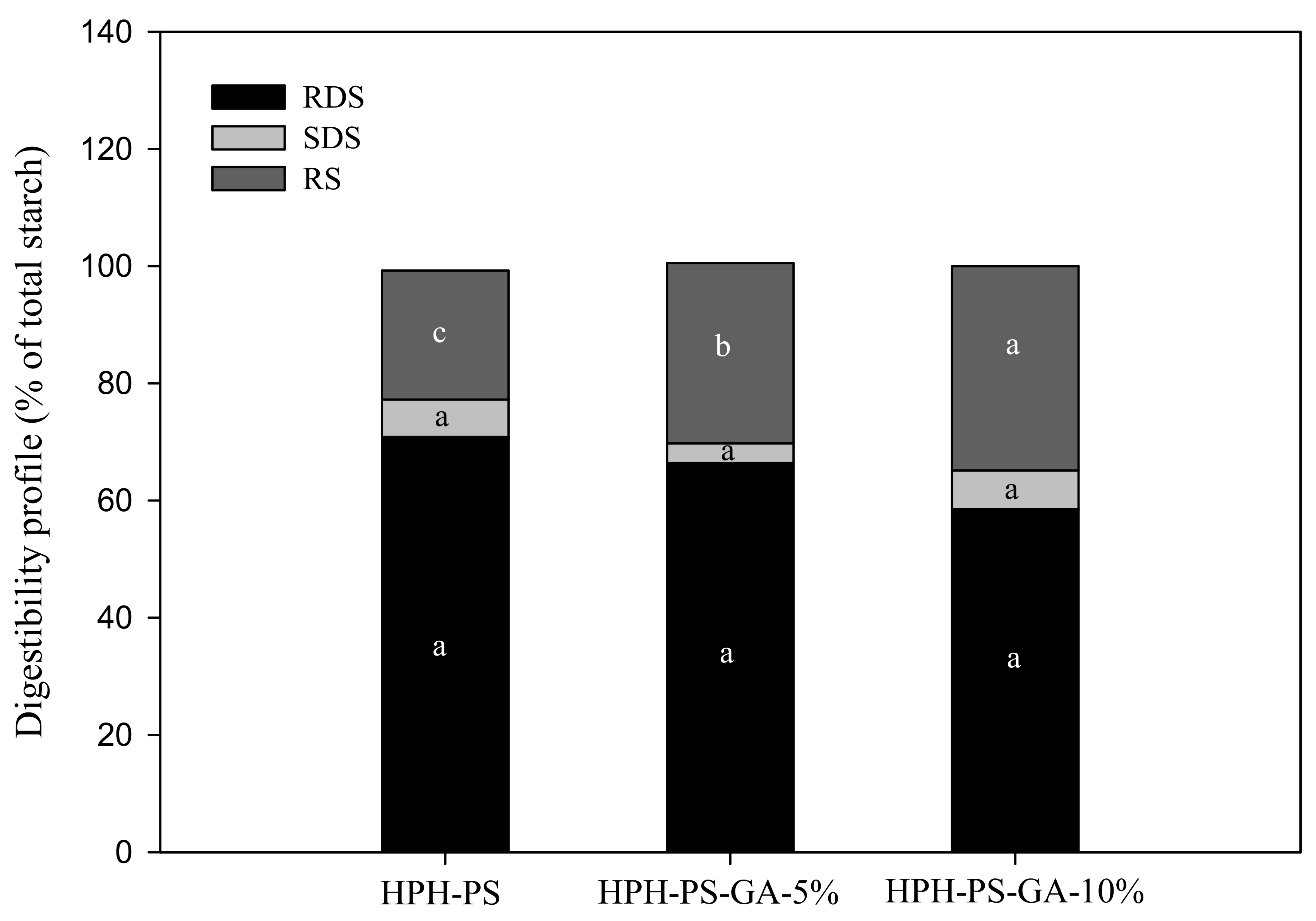
3.4. In Vitro Starch Digestibility
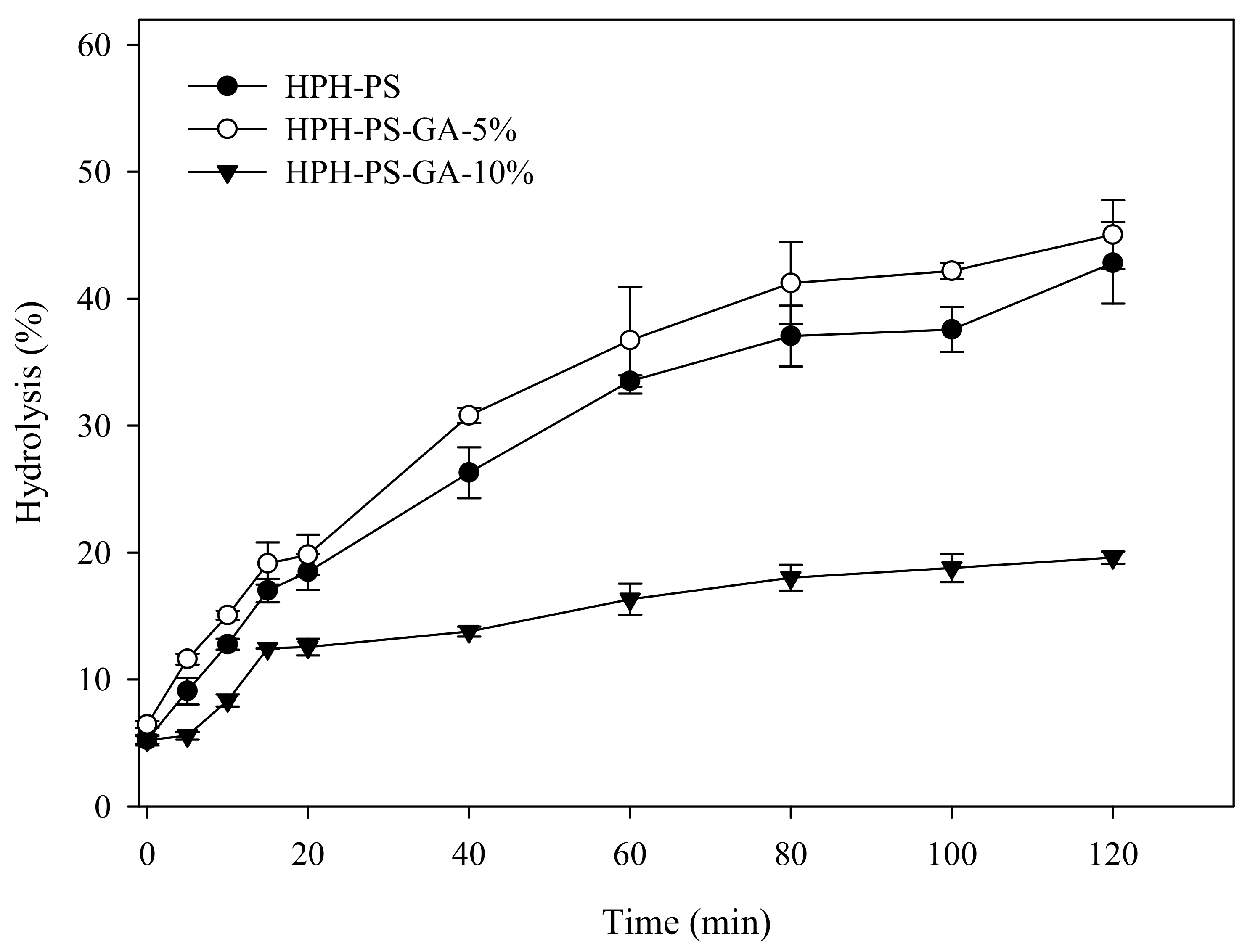
Results of the α-amylase hydrolysis kinetic study showed HPH-PS-GA-10% reduced approximately 23% of the digestion rate compared with HPH-PS (Figure 4). The addition of GA (in both HPH-PS-GA-5% and HPH-PS-GA-10%) increased RS levels (Figure 5) when compared with HPH-PS (control sample). The highest concentration of GA (HPH-PS-GA-10%) promoted an increase of 13% in RS. This study did not observe significant changes in rapidly digestible starch and slowly digestible starch. The substantial reduction in the susceptibility to α-amylase (Figure 4) was not reflected in the glucose production by fungal glucoamylase (amyloglucosidase; Figure 5). The α-amylolysis might release some GA and inhibit fungal glucoamylase from generating glucose effectively. An advanced study in quantifying the free GA of α-amylase hydrolysates and examining enzyme activities would provide a disclosure.
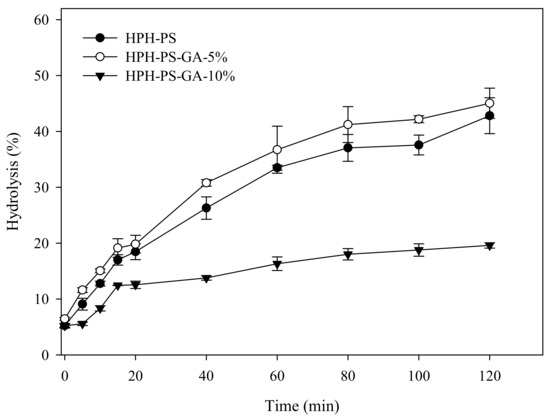
Figure 4.
α-Amylase hydrolysis kinetics of high-pressure homogenization-processed pea starch with 0% (HPH-PS), 5% (HPH-PA-GA-5%), and 10% (HPH-PA-GA-10%) gallic acid.
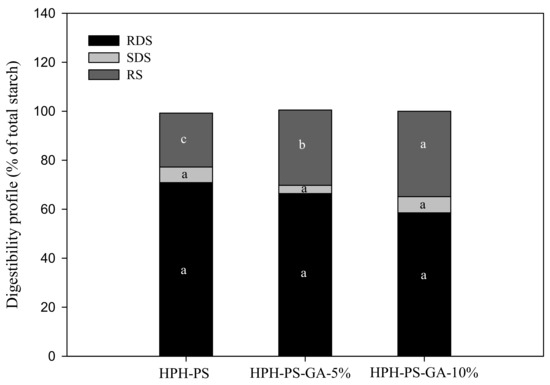
Figure 5.
The in vitro starch digestibility of high-pressure homogenization-processed pea starch with 0% (HPH-PS), 5% (HPH-PA-GA-5%), and 10% (HPH-PA-GA-10%) gallic acid. Different letters in the same digestion category (i.e., RDS) indicate a significant difference at p < 0.05.
4. Discussion
HPH modification with GA greatly disrupted the highly organized starch molecular structure. The structural disruption was caused by the mechanical change, including high pressure and high shear force, during HPH processing. The outcome of such structural disruption was the enhancement of hydration during heating [19], which promoted gelatinization and reduced crystallinity, as observed in this study. The interaction between GA and starch molecules further weakened the interactions between starch molecular chains, which decreased the enthalpy change. The acidic environment caused a relatively homogenous structure with a narrower temperature change [20,21], which was also observed in this study (Table 1).
HPH modification with GA improved pea starch functionality with a lower retrogradation rate. HPH alone increased the retrogradation rate from 85% to 99%, and such a significant change (p < 0.05) might be due to the molecular leaching promoted by HPH. However, the complexation with GA reduced retrogradation, and this desirable outcome was achieved only with a high concentration of GA. The bonding between GA and starch molecules may hinder the interaction between starch molecules and delay retrogradation.
HPH modification with a high GA concentration significantly decreased the susceptibility of pea starch to α-amylase. However, modification with a low concentration of GA did not have much influence on starch digestion. The difference between the two treatments was in the level of complexation, which interrupts the binding of α-amylase onto starch molecules [22]. The high GA modification formed a V-type complex, a mutual structural characteristic of resistant starch type 5. The α-amylase hydrolysis demonstrated a high level of indigestible residues, and an assessment based on Englyst et al. showed an increase in resistant starch fraction.
Conventionally, HPH has neither generated a high degree of complexation [10,15,23] nor a high amount of resistant starch as achieved in this study. Liu et al. (2019) reported a 1% complex index of rice processed via preheating gelatinization and HPH with 10% GA [15]. Chi et al. (2019) produced a near 13% complex index of rice via HPH with a high quantity of GA, 50% (w/w) [15]. Chi et al. (2017) also generated a near 14% complex index of maize starch via HPH with 40% GA [23]. In this study, we hypothesized that the unique C-type polymorphic structure of pea starch might generate more leaching molecules and small starch fragments for complexation during HPH processing. Data of this study supported the hypothesis and reached nearly 30% complexation with only 10% of GA. In terms of starch digestibility, rice starch modified with 29% GA via the HPH process only generated 30% resistant starch, whereas in this study, only 10% GA was needed to produce 35% resistant starch [10].
HPH was selected for this study to overcome a few disadvantages in conventional heat-based treatments, such as heat damage to phenolic compounds and higher energy demand for a long heating process. Findings of the change of digestibility and functionalities in this study suggest HPH is an effective method for the industry to utilize pea starch. Researchers may consider extending the study to other legume starches with similar granular structures.
5. Conclusions
In modern diets, many processed starchy foods are rapidly digested into glucose. Much research has demonstrated various modifications of starch digestibility. This study aimed to demonstrate an innovative technique and “clean-label” approach (e.g., without using synthetic chemicals) utilizing side-stream starch to alter starch digestibility. We believe that the selection of initial material (starch) is critical because each starch from different botanical sources responds to technology in different manners. Pea starch and many other legume starches that primarily originate from the massive production of plant-sourced proteins have a distinguished granular architecture that varies from commercial cereal (i.e., maize, rice) and tuber starch (i.e., potato, cassava). This study demonstrated a successful and effective complexation of pea starch with GA through an innovative and sustainable manufacturing technique, high-pressure homogenization. A V-type inclusion complex was formed, the susceptibility to α-amylase was greatly reduced, and the retrogradation rate was significantly hindered with a relatively low amount of gallic acid compared with the complexation of cereal starches. Utilizing the natural starch structure with a suitable technique is an effective way to produce desirable functionality.
Author Contributions
Conceptualization, F.A.V. and A.H.-M.L.; methodology, F.A.V. and A.H.-M.L.; investigation, F.A.V. and A.H.-M.L.; data curation, F.A.V. and A.H.-M.L.; writing—original draft preparation, F.A.V.; writing—review and editing, A.H.-M.L.; supervision, A.H.-M.L.; project administration, A.H.-M.L.; funding acquisition, A.H.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Agency for Science, Technology and Research (A*STAR) Biomedical Research Council (BMRC) on Industry Alignment Fund—Pre-Positioning Programme (IAF PP) in the Health and Biomedical Sciences (HBMS) Domain, Grant ID No: H17/01/a0/A11 & H18/01/a0/B11 Food Structure Engineering for Nutrition and Health Programme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Roquette Corporate (Lestrem, France) for providing pea starch for the study.
Conflicts of Interest
The authors declare no conflict of interest. Roquette Corporate (Lestrem, France) provided pea starch for the study as a gift and had no knowledge of the research content before publication.
References
- Oyeyinka, S.A.; Singh, S.; Amonsou, E.O. A review on structural, digestibility and physicochemical properties of legume starch-lipid complexes. Food Chem. 2021, 349, 129165. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, Z.; Sigvaldson, M.; Soladoye, O.; Gaudette, N. Utilization of pea starch and fibre fractions for replacement of wheat crumb in beef burgers. Meat Sci. 2020, 161, 107974. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Yin, X.; Hu, X.; Boye, J.I. Structural characteristics and physicochemical properties of field pea starch modified by physical, enzymatic, and acid treatments. Food Hydrocoll. 2019, 93, 93386–93394. [Google Scholar] [CrossRef]
- Hermansson, A.-M.; Svegmark, K. Developments in the understanding of starch functionality. Trends Food Sci. Technol. 1996, 7, 345–353. [Google Scholar] [CrossRef]
- Singh, N. Functional and physicochemical properties of pulse starch. In Pulse Foods: Processing, Quality and Nutraceutical Applications, 2nd ed.; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 87–112. [Google Scholar]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Xu, H.; Liang, Y.; Zheng, B. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization. Int. J. Biol. Macromol. 2019, 134, 856–863. [Google Scholar] [CrossRef]
- Fang, K.; He, W.; Jiang, Y.; Li, K.; Li, J. Preparation, characterization and physicochemical properties of cassava starch-ferulic acid complexes by mechanical activation. Int. J. Biol. Macromol. 2020, 160, 482–488. [Google Scholar] [CrossRef]
- Guo, T.; Hou, H.; Liu, Y.; Chen, L.; Zheng, B. In vitro digestibility and structural control of rice starch-unsaturated fatty acid complexes by high-pressure homogenization. Carbohydr. Polym. 2021, 256, 117607. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, B.; Zheng, B.; Chen, L.; Guo, Z. Effects and mechanism of high-pressure homogenization on the characterization and digestion behavior of lotus seed starch–green tea polyphenol complexes. J. Funct. Foods 2019, 57, 173–181. [Google Scholar] [CrossRef]
- Chen, B.; Jia, X.; Miao, S.; Zeng, S.; Guo, Z.; Zhang, Y.; Zheng, B. Slowly digestible properties of lotus seed starch-glycerine monostearin complexes formed by high pressure homogenization. Food Chem. 2018, 252, 115–125. [Google Scholar] [CrossRef]
- Jacobson, M.R.; BeMiller, J.N. Method for determining the rate and extent of accelerated starch retrogradation. Cereal Chem. 1998, 75, 22–29. [Google Scholar] [CrossRef]
- Vanier, N.L.; de Oliveira, J.P.; Bruni, G.P.; El Halal, S.L.M.; Villanova, F.A.; Zavareze, E.d.R.; Dias, A.R.G.; Bassinello, P.Z. Characteristics of starch from different bean genotypes and its effect on biodegradable films. J. Sci. Food Agric. 2019, 99, 1207–1214. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Xie, F.; Li, L.; Bai, G. Modulating the in vitro digestibility and predicted glycemic index of rice starch gels by complexation with gallic acid. Food Hydrocoll. 2019, 89, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Shao, Y.; Lin, A.H.-M. Improvement in the quantification of reducing sugars by miniaturizing the Somogyi-Nelson assay using a microtiter plate. Food Chem. 2018, 240, 898–903. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Wang, B.; Li, D.; Wang, L.-j.; Chiu, Y.L.; Chen, X.D.; Mao, Z.-h. Effect of high-pressure homogenization on the structure and thermal properties of maize starch. J. Food Eng. 2008, 87, 436–444. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Corke, H. Effect of phenolic compounds on the pasting and textural properties of wheat starch. Starch-Stärke 2008, 60, 609–616. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Tsao, R.; Yada, R.Y.; Liu, Q. Physicochemical properties and in vitro digestibility of potato starch after inclusion with vanillic acid. LWT-Food Sci. Technol. 2017, 85, 218–224. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L.; Wang, Z. Digestibility and supramolecular structural changes of maize starch by non-covalent interactions with gallic acid. Food Funct. 2017, 8, 720–730. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).