Degradation of Hydrogels Based on Potassium and Sodium Polyacrylate by Ionic Interaction and Its Influence on Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Characterizations
3. Results and Discussion
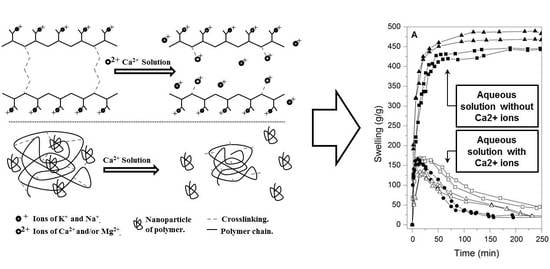
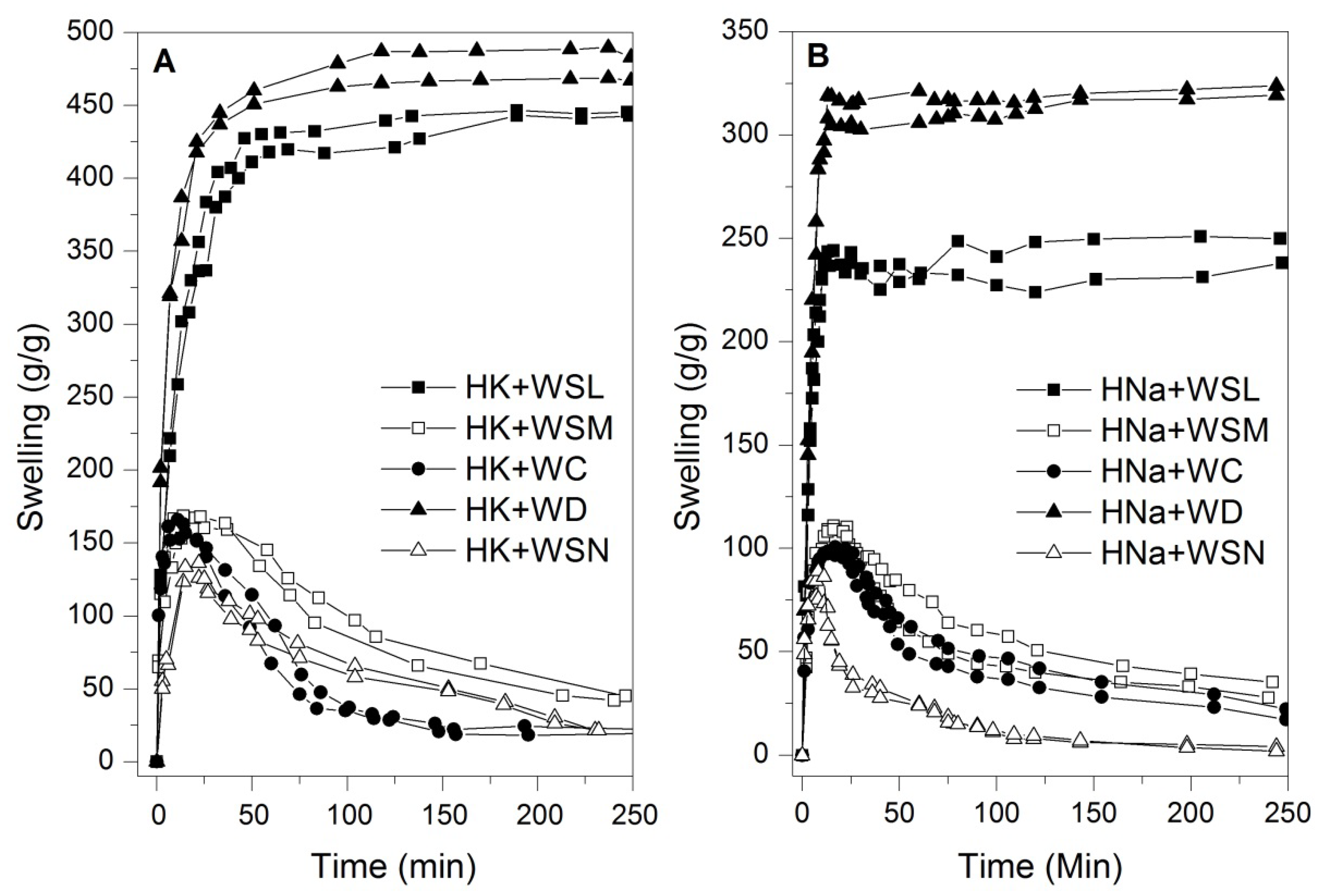
3.1. Swelling Test in Commercial Waters
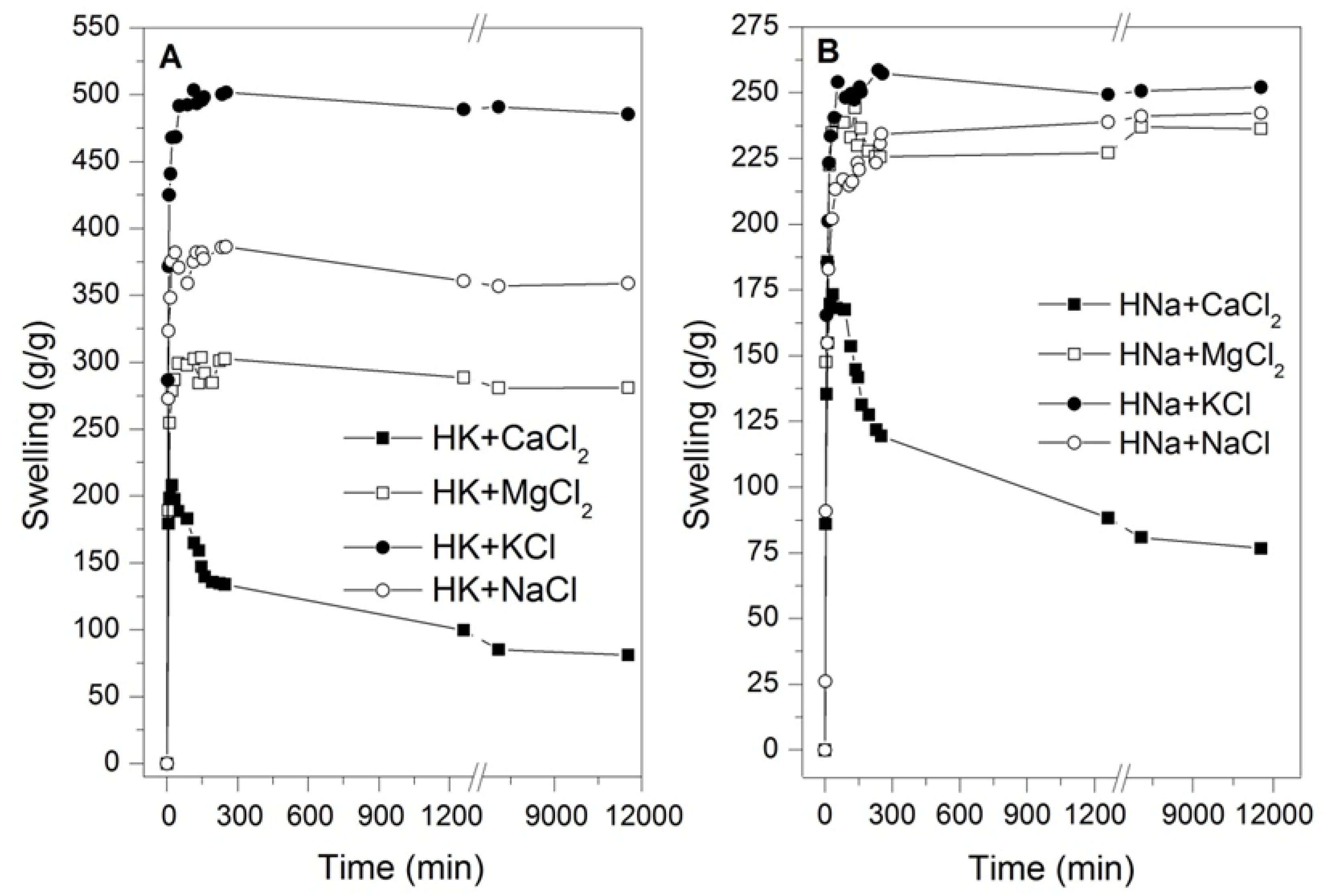
3.2. Swelling Test in Saline Solutions
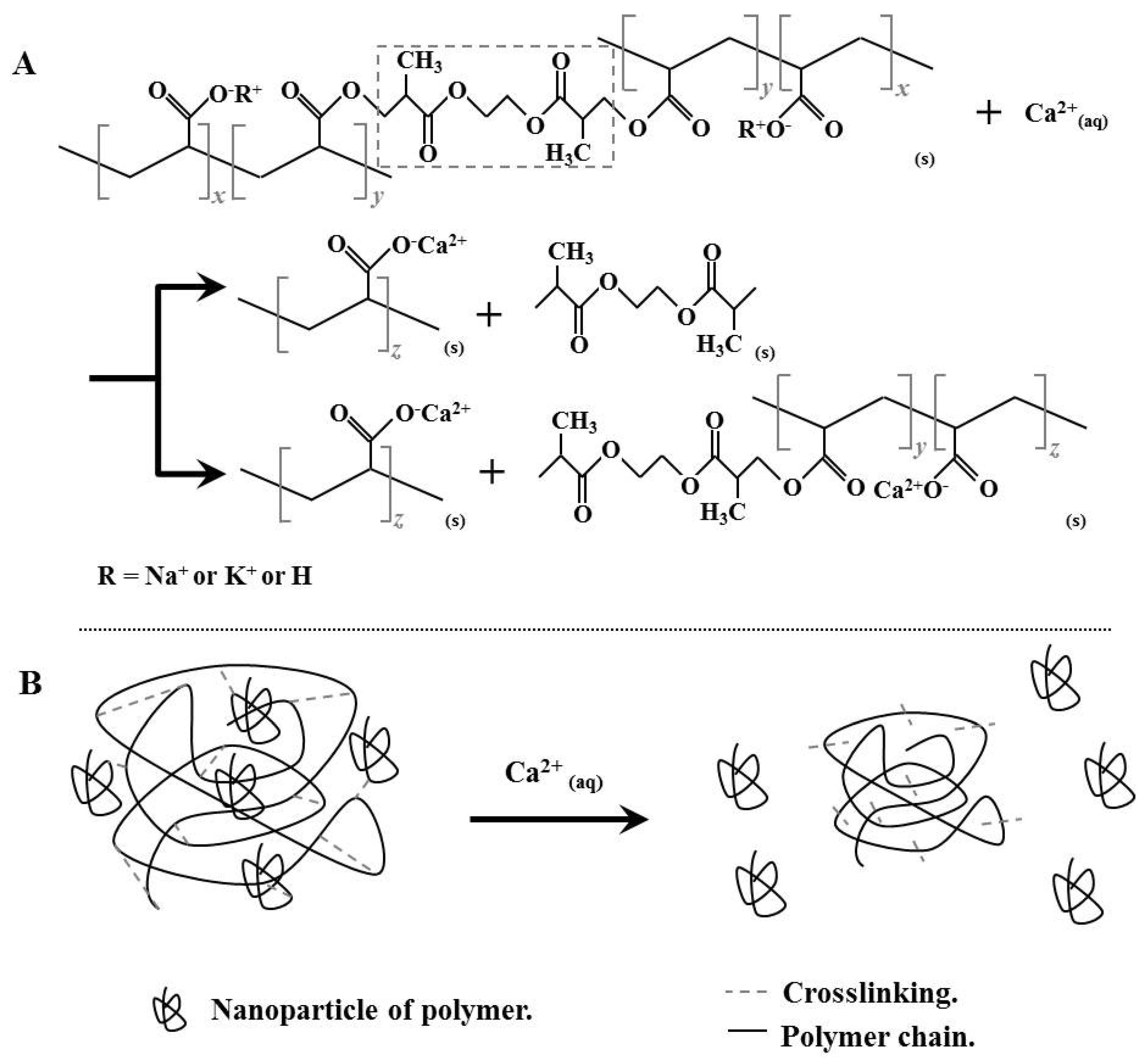
3.3. Swelling Test in Calcium-Associated Solutions
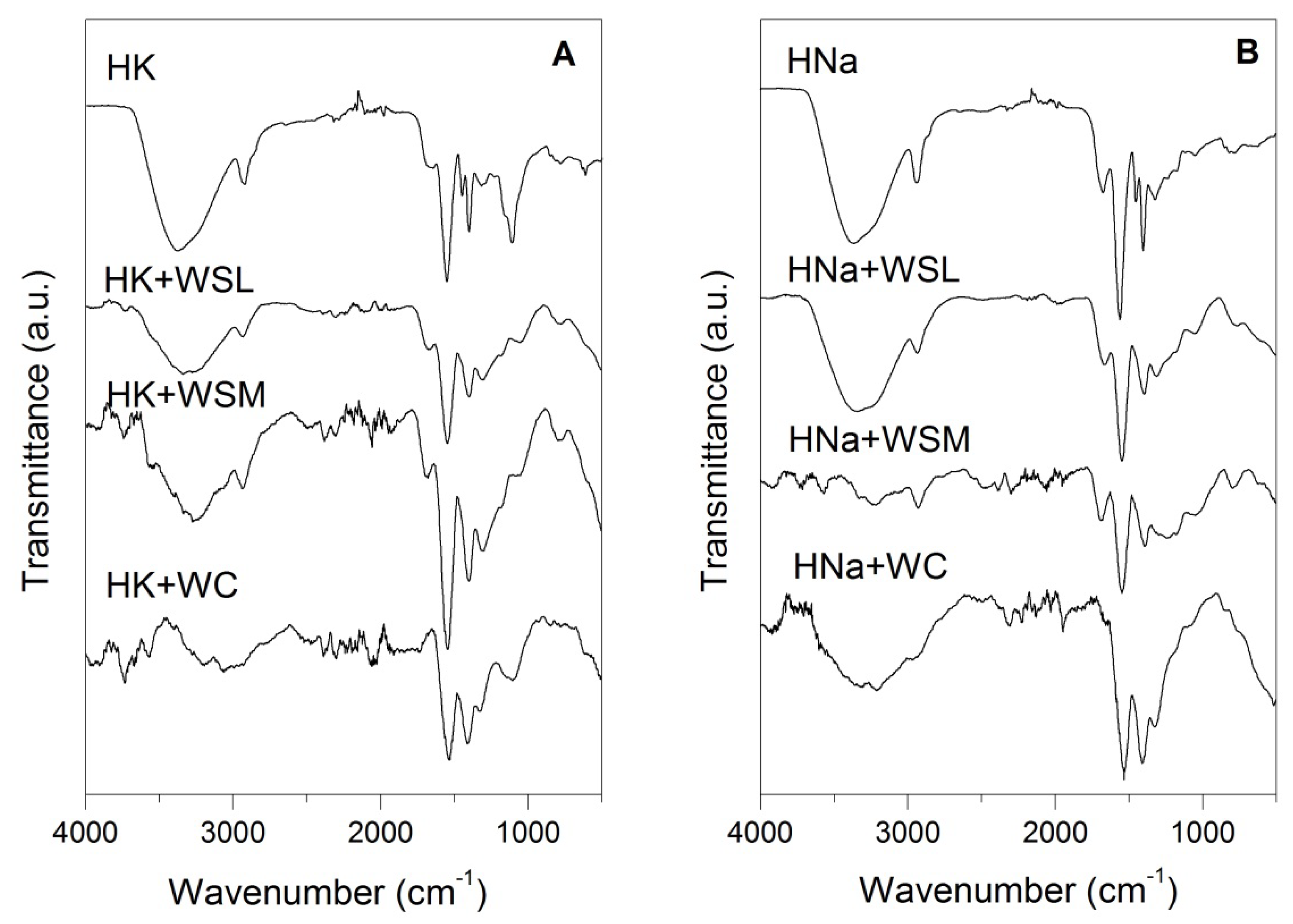
3.4. FTIR Analysis
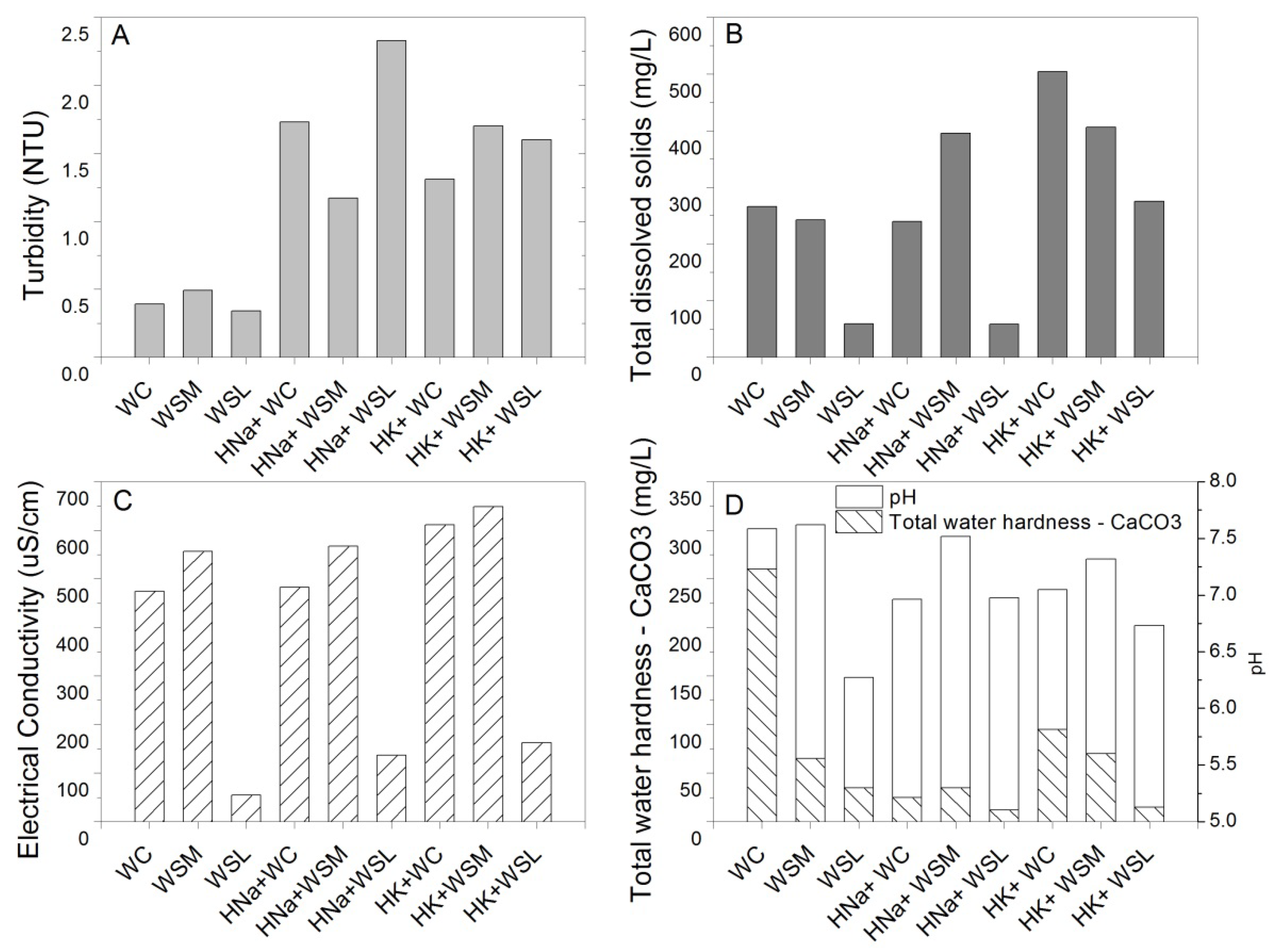
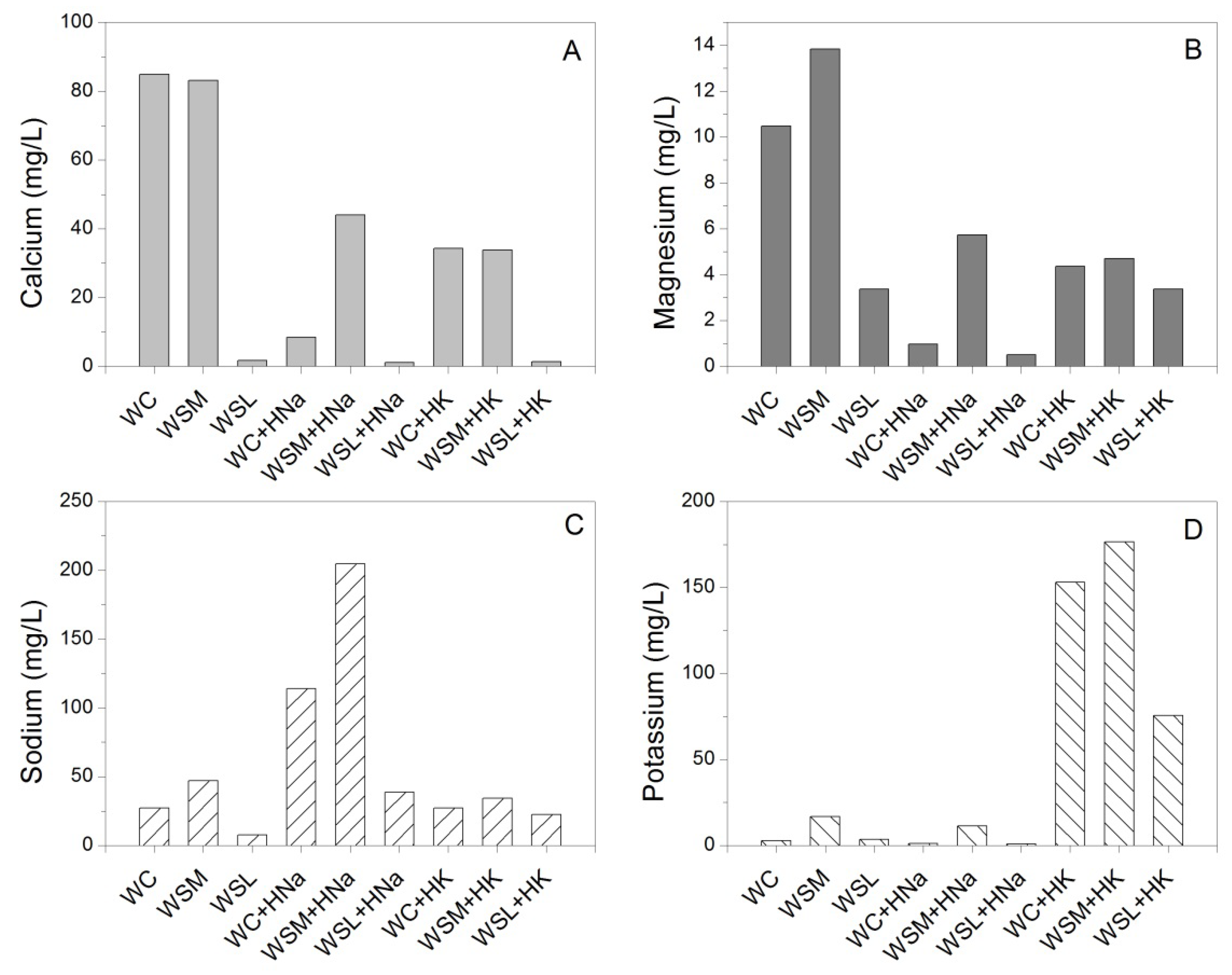
3.5. Physicochemical Analysis
3.6. Dynamic Light Scattering (DLS) and Zeta-Potential
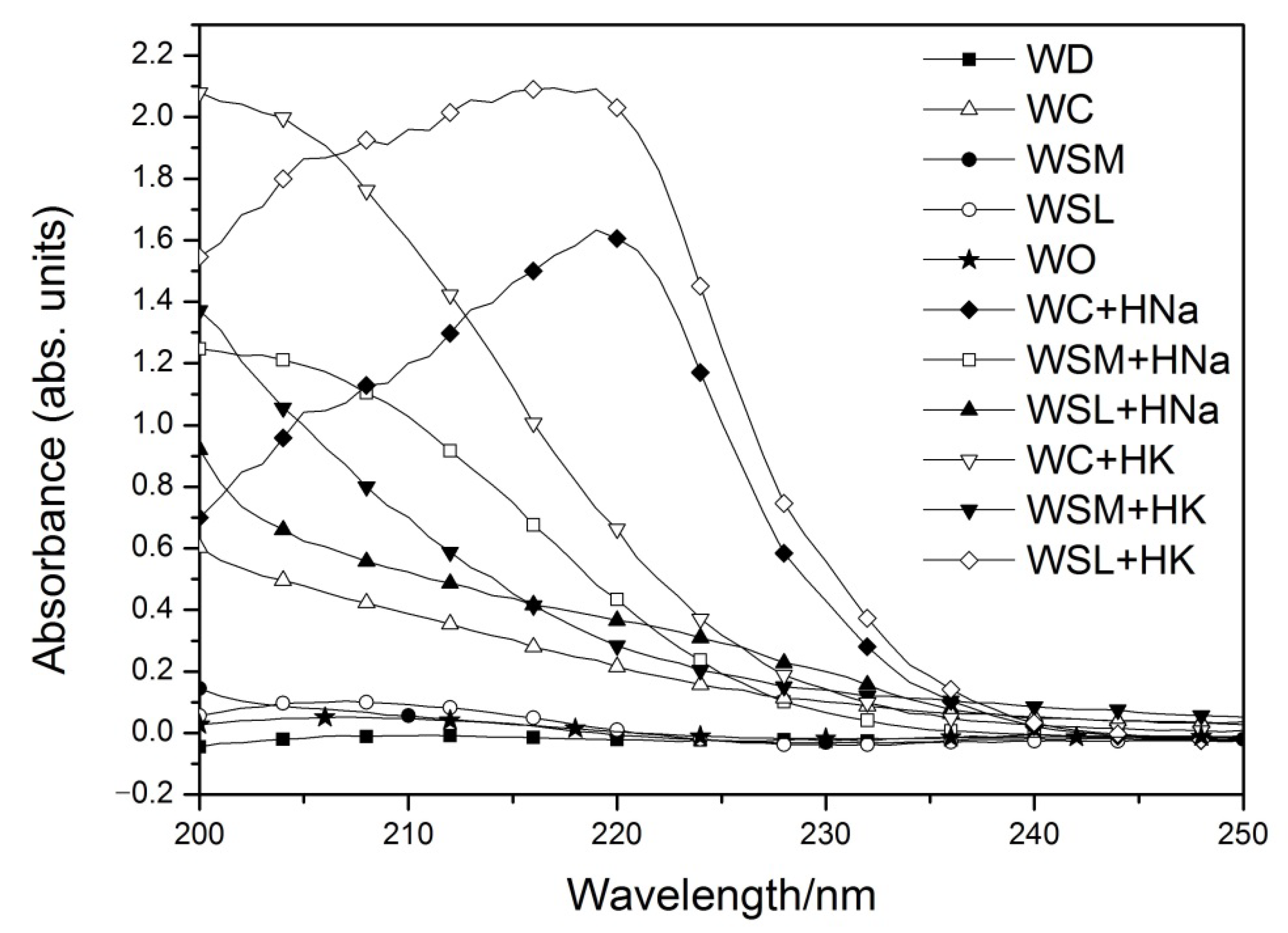
3.7. UV-vis Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ullah, F.; Bisyrul, M.; Javed, F.; Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Patel, S.V.; Yadav, S.; Singh, V.; Kumar, M.; Kumar, M. Hydrogel and its effect on soil moisture status and plant growth: A review. J. Pharm. Phytochem. 2020, 9, 1746–1753. [Google Scholar] [CrossRef]
- Yao, T.; Jia, W.; Tong, X.; Feng, Y.; Qi, Y.; Zhang, X.; Wu, J. One-step preparation of nanobeads-based polypyrrole hydrogel by a reactive-template method and their applications in adsorption and catalysis. J. Colloid Interface Sci. 2018, 527, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, M.; Ouyang, C.; Lu, T.J.; Li, F.; Xu, F. Biofriendly, Stretchable, and Reusable Hydrogel Electronics as Wearable Force Sensors. Small 2018, 14, 1801711. [Google Scholar] [CrossRef]
- Hina, M.; Bashir, S.; Kamran, K.; Ramesh, S.; Ramesh, K. Synthesis and characterization of self-healable poly (acrylamide) hydrogel electrolytes and their application in fabrication of aqueous supercapacitors. Polymer 2020, 210, 123020. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wang, J.; Hu, M.; Feng, Y.; Wang, P.; Wang, Y.; Nie, N.; Zhang, J.; Chen, H.; et al. Concentrated Hydrogel Electrolyte-Enabled Aqueous Rechargeable NiCo//Zn Battery Working from –20 to 50 °C. ACS Appl. Mater. Interfaces 2018, 11, 49–55. [Google Scholar] [CrossRef]
- Kumar, N.; Mittal, H.; Alhassan, S.M.; Ray, S.S. Bionanocomposite Hydrogel for the Adsorption of Dye and Reusability of Generated Waste for the Photodegradation of Ciprofloxacin: A Demonstration of the Circularity Concept for Water Purification. ACS Sustain. Chem. Eng. 2018, 6, 17011–17025. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Wang, X. Synthesis and characterization of an injectable ε-polylysine/carboxymethyl chitosan hydrogel used in medical application. Mater. Chem. Phys. 2020, 248, 122902. [Google Scholar] [CrossRef]
- Gradinaru, V.; Treweek, J.; Overton, K.; Deisseroth, K. Hydrogel-Tissue Chemistry: Principles and Applications. Annu. Rev. Biophys. 2018, 47, 355–376. [Google Scholar] [CrossRef] [Green Version]
- Ramiah, P.; du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. 2020, 7, 76. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [Green Version]
- Halah, E.; López-Carrasquero, F.; Contreras, J. Applications of hydrogels in the adsorption of metallic ions. Cienc. Ing. 2018, 39, 57–70. [Google Scholar]
- Karagöz, İ.; Yücel, G. Use of super absorbent polymers with Euonymus plants (Euonymus japonicus ‘Aureomarginatus’) in ornamental plant cultivation. J Agric Sci. 2020, 26, 201–211. [Google Scholar] [CrossRef]
- Sanz-Gómez, J. Characterization and Effects of Cross-Linked Potassium Polyacrylate as Soil Amendment. Ph.D. Thesis, University of Seville, Seville, Spain, 2015. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels -A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaith, B.S.; Kalia, S.; Swart, H.C. Conducting polymer hydrogels and their applications. In Conducting Polymer Hybrids, 1st ed.; Kumar, V., Kalia, S., Swart, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 193–221. [Google Scholar] [CrossRef]
- Chatzoudis, G.K.; Rigas, F. Soil salts reduce hydration of polymeric gels and affect moisture characteristics of soil. Commun. Soil Sci. Plant. Anal. 1999, 30, 2465–2474. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Nunes, A.R.; Santos, P.; Girão, A.V.; Duarte, A.; Rocha-Santos, T. Degradation of polyethylene microplastics in seawater: Insights into the environmental degradation of polymers into the environmental degradation of polymers. J. Environ. Sci. Health Part A 2018, 53, 866–875. [Google Scholar] [CrossRef]
- Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef] [Green Version]
- Sollehudin, I.M.; Heerwan, P.M.; Ishak, M.I.; Beams, C.; Chin, S.C.; Tong, F.S. Degradation and stability of polymer: A mini review. In Proceedings of the IOP Conference Series: Materials Science and Engineering 788, Atlanta, GA, USA, 22 April 2020; p. 012048. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kondo, S.; Chung, U.-I.; Sakai, T. Degradation behavior of polymer gels caused by nonspecific cleavages of network strands. Chem. Mater. 2014, 26, 5352–5357. [Google Scholar] [CrossRef]
- Bankeeree, W.; Samathayanon, C.; Prasongsuk, S.; Lotrakul, P.; Kiatkamjornwong, S. Rapid Degradation of Superabsorbent Poly(Potassium Acrylate) and its Acrylamide Copolymer Via Thermo-Oxidation by Hydrogen Peroxide. J. Polym. Environ. 2021, 29, 3964–3976. [Google Scholar] [CrossRef]
- Wang, Y.; Delgado-Fukushima, E.; Fu, R.X.; Doerk, G.S.; Monclare, J.K. Controlling drug absorption, release, and erosion of photopatterned protein engineered hydrogels. Biomacromolecules 2020, 21, 3608–3619. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Tiwari, N.; Badiger, M.V. Enhanced drug release by selective cleavage of cross-links in a double-cross-linked hydrogel. RSC Adv. 2016, 6, 102453–102461. [Google Scholar] [CrossRef]
- Ahmadian, Y.; Bakravi, A.; Hashemi, H.; Namazi, H. Synthesis of polyvinyl alcohol/CuO nanocomposite hydrogel and its application as drug delivery agent. Polym. Bull. 2019, 74, 1967–1983. [Google Scholar] [CrossRef]
- Namazi, H.; Hasani, M.; Yadollahi, M. Antibacterial oxidized starch/ZnO nanocomposite hydrogel: Synthesis and evaluation of its swelling behaviours in various pHs and salt solutions. Int. J. Biol. Macromol. 2019, 126, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, G.; Li, Z.; Hua, J.; Wu, M.; Gong, J.; Zhang, J.; Ban, L.-T.; Huang, L. Novel biological hydrogel: Swelling behaviors study in salt solutions with different ionic valence number. Polymers 2018, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Jiang, G.; Li, B.; Han, P. Effects of high-concentration salt solutions and pH on swelling behavior of physically and chemically cross-linked hybrid hydrophobic association hydrogels with good mechanical strength. Soft Mater. 2018, 16, 249–264. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.; Liu, L.; Yao, J. Synthesis and characterization of a novel cellulose-g-poly(acrylic acid-co-acrylamide) superabsorbent composite based on flax yarn waste. Carbohydr. Polym. 2012, 87, 2519–2525. [Google Scholar] [CrossRef]
- Deraman, N.F.; Mohamed, N.R.; Romli, A.Z. Swelling kinetics and characterization of novel superabsorbent polymer composite based on mung bean starch-filled poly(acrylic acid)-graft-waste polystyrene. Int. J. Plast Technol. 2019, 23, 188–194. [Google Scholar] [CrossRef]




| Potassium Polyacrylate (HK) | Sodium Polyacrylate (HNa) | |
|---|---|---|
| Generic chemical formula | [-CH2-CH(COOK)-]n | [-CH2-CH(COONa)-]n |
| Purity (%) | ~96 | ~95 |
| Molecular weight by GPC (Mw) | ~4000 | ~5100 |
| Particle size (Mesh) | 20–40 | 5–10 |
| Crosslinker | Ethylene glycol dimethacrylate (EGDMA) | Ethylene glycol dimethacrylate (EGDMA) |
| Crosslinking density * (Croslinking unit per Monomer units) | 950–1350 | 700–1100 |
| Description | Deformation | Wavenumber (cm−1) | |
|---|---|---|---|
| HK | Stretching vibration of the hydroxyl group. | O-H | ~3369 |
| Asymmetric and Symmetric stretch. | CH2 | ~ 2935 & ~2860 | |
| Deformation vibrations. | C-OH | ~ 1674 | |
| Asymmetric and symmetric stretching and another associated deformation of the group. | COO- | ~ 1555, ~1451, ~1404, ~1317 & ~1169 | |
| Stretching vibrations of C-O bond and deformation vibrations of C-O-H group. | C-O & C-O-H | ~1239 | |
| Bond deformation. | C–C | 1162 | |
| Bond stretching in the carboxyl acid structure. | C=O | ~1113 | |
| Other characteristic deformations of polymeric hydrogel based on potassium. | -- | ~855, ~820, ~784~638 & ~616 | |
| HNa | Stretching vibration of the hydroxyl group. | O-H | ~3383 |
| Asymmetric and Symmetric stretch. | CH2 | ~ 2930 & ~2865 | |
| Deformation vibrations. | C-OH | ~1659 | |
| Asymmetric and symmetric stretching and another associated deformation of the group. | COO- | ~ 1555, ~1451, ~1404, ~1322 & ~1162 | |
| Stretching vibrations of C-O bond and deformation vibrations of C-O-H group. | C-O & C-O-H | −1235 | |
| Bond deformation. | C–C | 1162 | |
| Bond stretching in the carboxyl acid structure. | C=O | ~1128 | |
| Other characteristic deformations of hydrogel based on sodium. | -- | ~1047, ~815, ~774 & ~621 |
| References Sample | Type of Sample | References Sample | Type of Sample |
|---|---|---|---|
| HK | Hydrogel based on potassium | HNa | Hydrogel based on sodium |
| WD | Distilled Water | HK + WD | Hydrogel after swelling process |
| WO | Osmosis Water | HK + WO | |
| WC | Commercial water 1 | HK + WC | |
| WSM | Commercial water 2 | HK + WSM | |
| WSL | Commercial water 3 | HK + WSL | |
| WSN | Water of supply net | HNa + WD | |
| CaCl2+ HK | Saline solutions with distilled water after of swelling process | HNa + WO | |
| MgCl + HK | HNa + WC | ||
| KCl + HK | HNa + WSM | ||
| NaCl + HK | HNa + WSL | ||
| CaCl2 + HNa | Saline solutions with distilled water after of swelling process | WD + HK | Wastewater after the swelling process |
| MgCl + HNa | WO + HK | ||
| KCl + HNa | WC + HK | ||
| NaCl + HNa | WSM+ HK | ||
| Ca + HK | Calcium-associated solutions with distilled water after of swelling process | WSL + HK | |
| Ca(NO3)2 + HK | WD + HNa | ||
| CaCO3 + HK | WO+ HNa | ||
| Ca + HNa | Calcium-associated solutions with distilled water after of swelling process | WC + HNa | |
| Ca(NO3)2 + HNa | WSM+ HNa | ||
| CaCO3 + HNa | WSL + HNa |
| Water Reference | Turbidity | Total Dissolved Solids | Total Water Hardness CaCO3 | pH | Electrical Conductivity | Anions | Cations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorides (Cl-) | Sulfates (SO4−2) | Nitrates (NO3-) | Nitrites (NO2-) | Calcium | Magnesium | Sodium | Potassium | ||||||
| NTU | mg/L | mg/L | -- | uS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| WC | 0.39 | 266 | 260 | 7.56 | 478.34 | 35.09 | 177.71 | 8.8 | 0.96 | 84.86 * | 2.69 | 10.49 | 27.1 |
| WSM | 0.49 | 242 | 65 | 7.62 | 553.67 | 59.88 | 142.55 | 1.3 | 0.28 | 83.14 | 16.79 * | 13.84 * | 47.01 * |
| WSL | 0.34 | 59 | 35 | 6.27 | 54,33 | 29.99 | 12.12 | 1.43 | 0.31 | 1.66 | 3.58 | 3.36 | 7.55 |
| WSN | 1.85 | 1880 | 378.33 | 7.09 | 793.4 | 51.98 | 276.08 | 51.85 | 0.01 | 79.95 | 9.84 | 37.71 | 4.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinzon-Moreno, D.D.; Maurate-Fernandez, I.R.; Flores-Valdeon, Y.; Neciosup-Puican, A.A.; Carranza-Oropeza, M.V. Degradation of Hydrogels Based on Potassium and Sodium Polyacrylate by Ionic Interaction and Its Influence on Water. Polymers 2022, 14, 2656. https://doi.org/10.3390/polym14132656
Pinzon-Moreno DD, Maurate-Fernandez IR, Flores-Valdeon Y, Neciosup-Puican AA, Carranza-Oropeza MV. Degradation of Hydrogels Based on Potassium and Sodium Polyacrylate by Ionic Interaction and Its Influence on Water. Polymers. 2022; 14(13):2656. https://doi.org/10.3390/polym14132656
Chicago/Turabian StylePinzon-Moreno, Diego David, Isabel Rosali Maurate-Fernandez, Yury Flores-Valdeon, Antony Alexander Neciosup-Puican, and María Verónica Carranza-Oropeza. 2022. "Degradation of Hydrogels Based on Potassium and Sodium Polyacrylate by Ionic Interaction and Its Influence on Water" Polymers 14, no. 13: 2656. https://doi.org/10.3390/polym14132656
APA StylePinzon-Moreno, D. D., Maurate-Fernandez, I. R., Flores-Valdeon, Y., Neciosup-Puican, A. A., & Carranza-Oropeza, M. V. (2022). Degradation of Hydrogels Based on Potassium and Sodium Polyacrylate by Ionic Interaction and Its Influence on Water. Polymers, 14(13), 2656. https://doi.org/10.3390/polym14132656