Gas-Transport and the Dielectric Properties of Metathesis Polymer from the Ester of exo-5-Norbornenecarboxylic Acid and 1,1′-Bi-2-naphthol
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
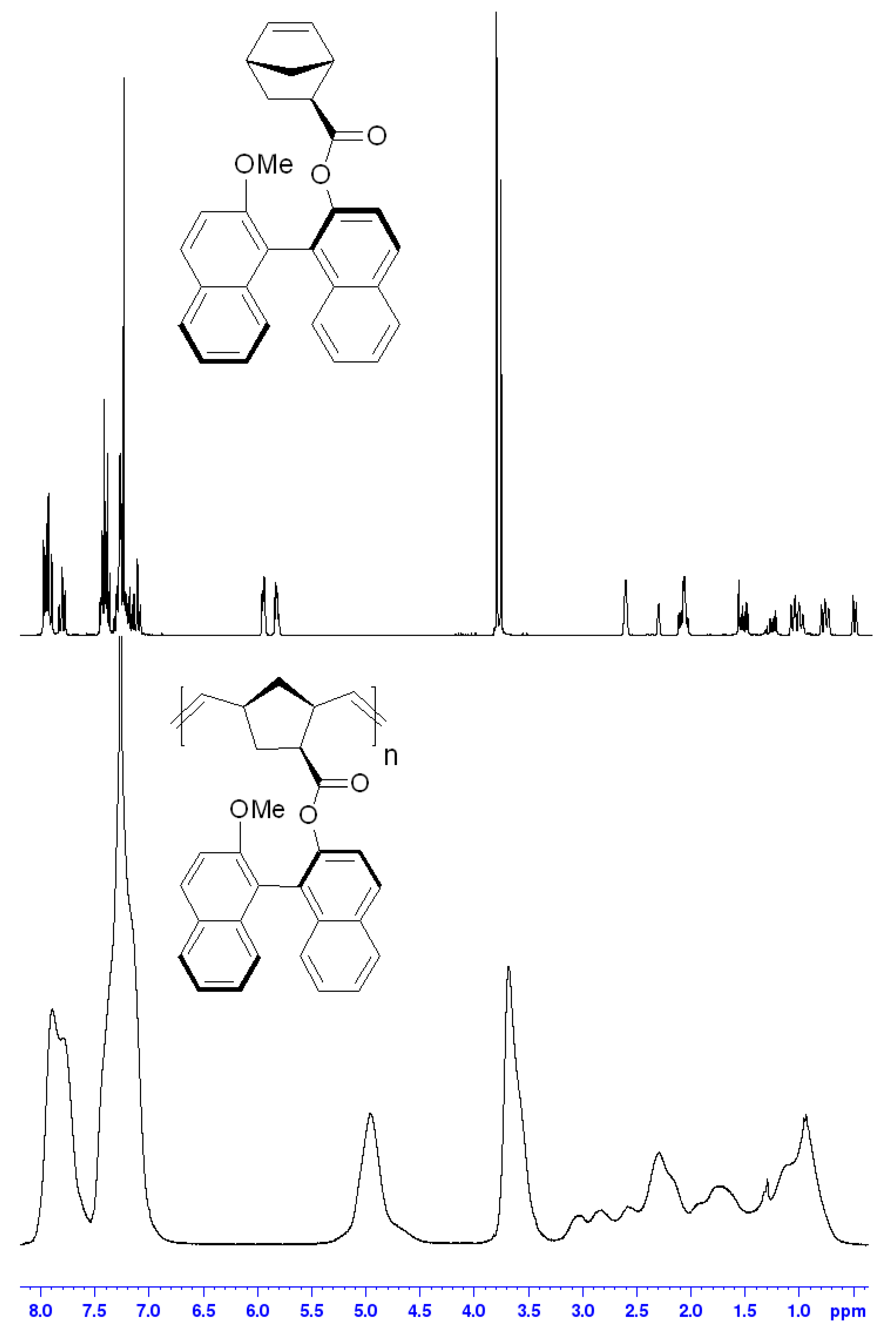
2.2. Methods of Monomer’s and Polymer’s Characterization
2.3. Film Preparation
2.4. Measurements of Gas-Transport Properties
2.5. Synthesis of the Monomer
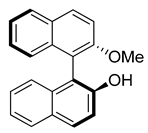
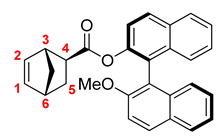
2.5.1. Diastereomer A
2.5.2. Diastereomer B
2.6. Metathesis Polymerization
3. Results and Discussion
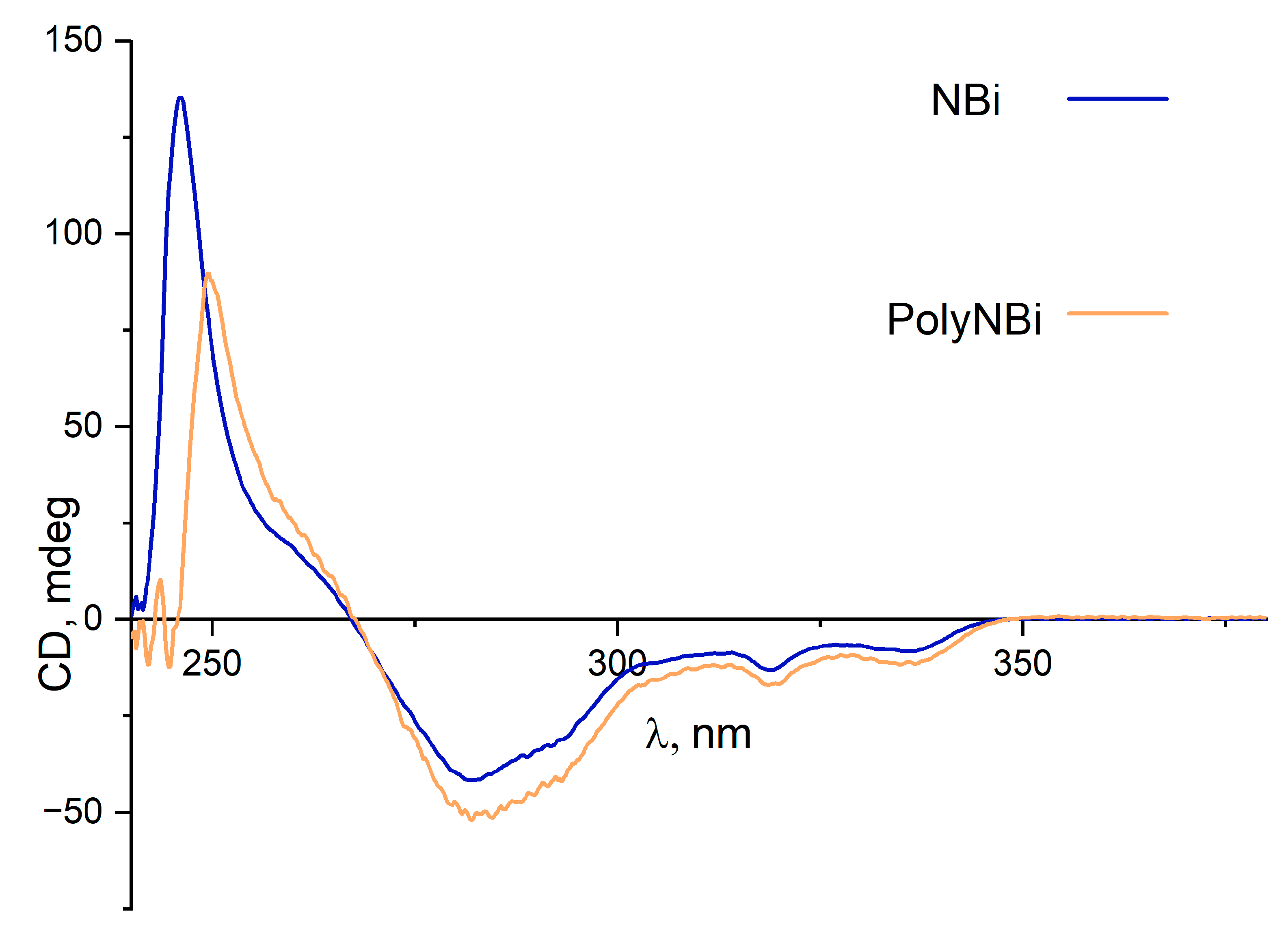
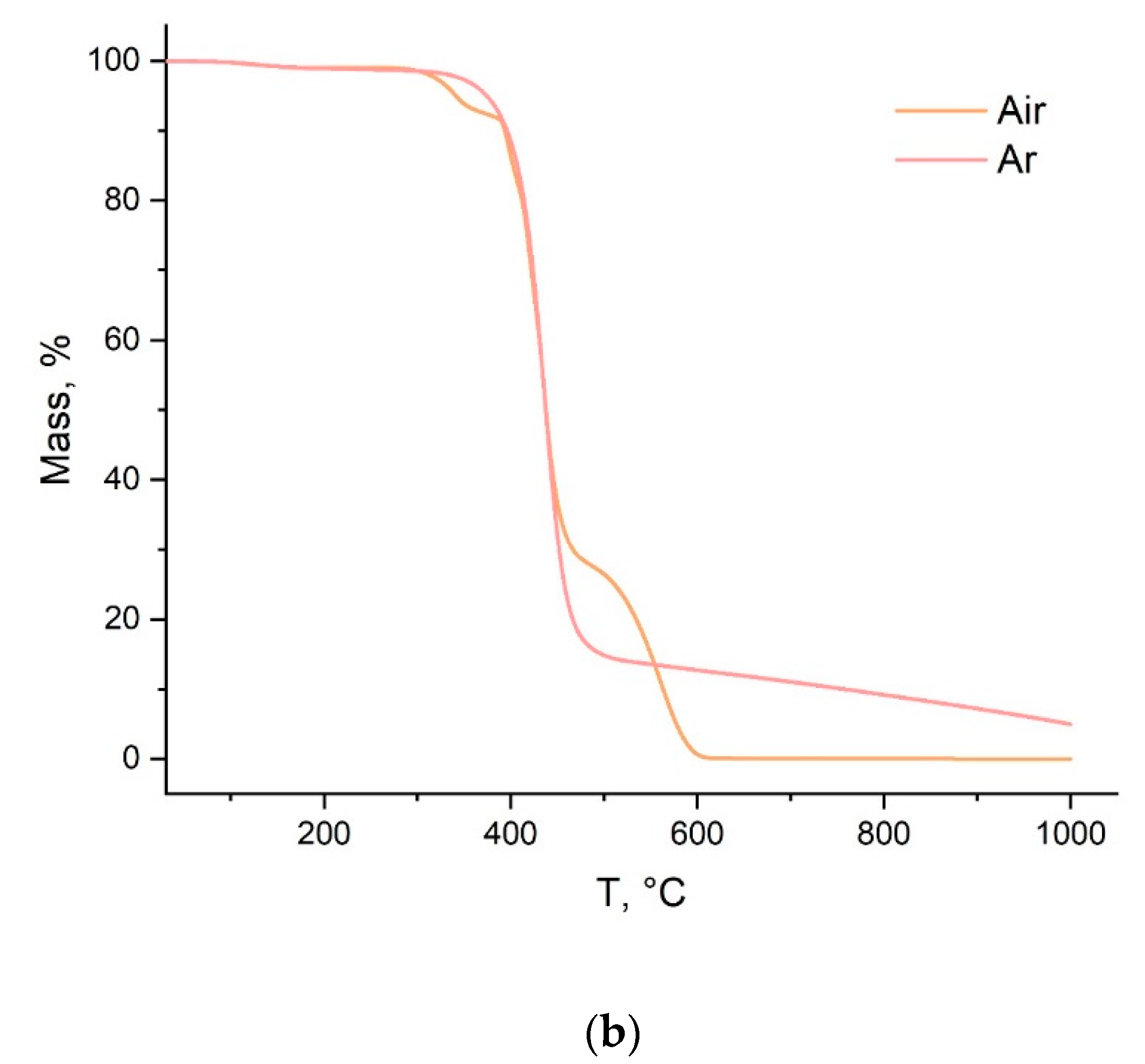
3.1. Synthesis and Physico-Chemical Properties of the Polymer
3.2. Gas-Transport Properties
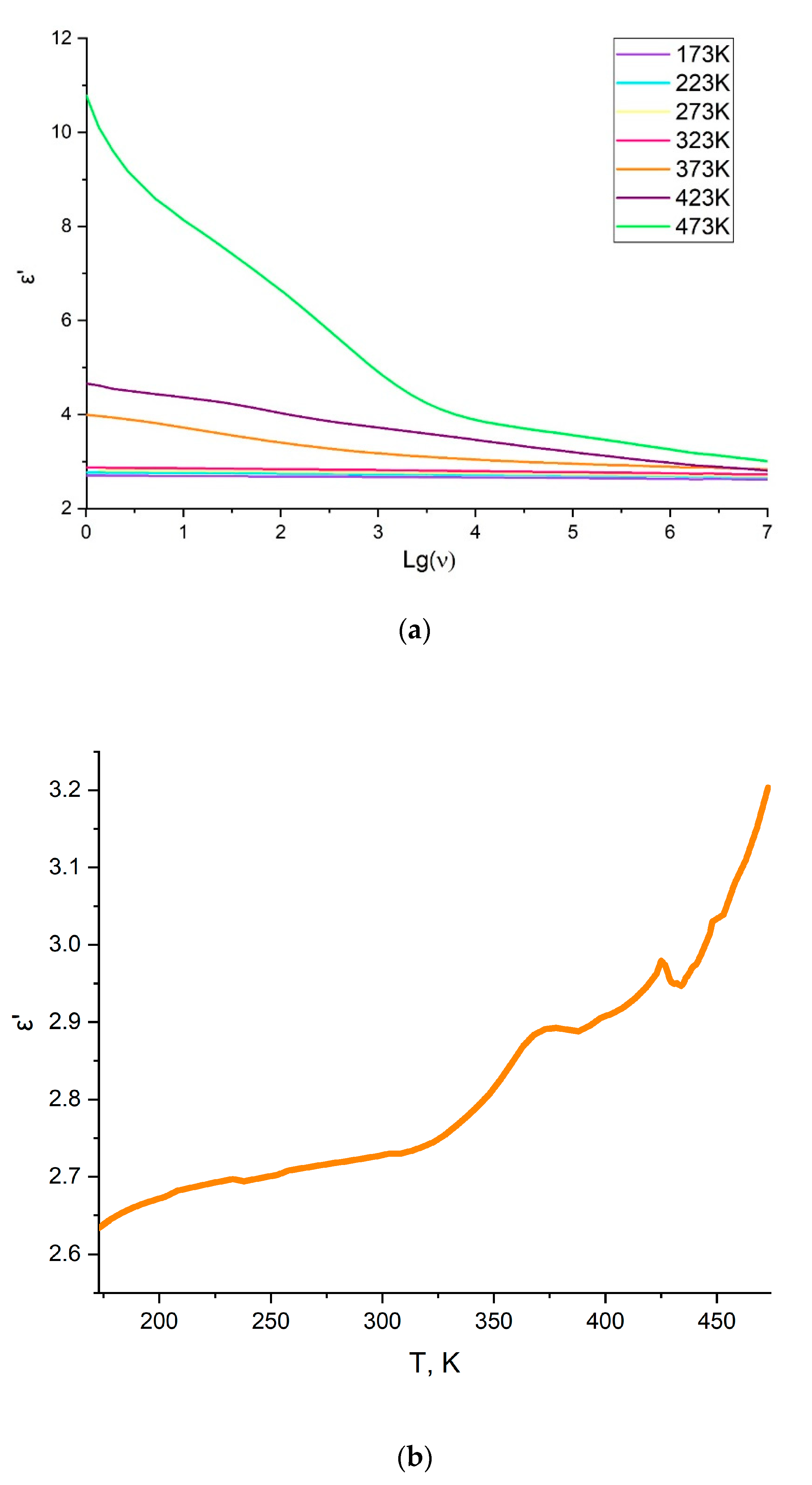
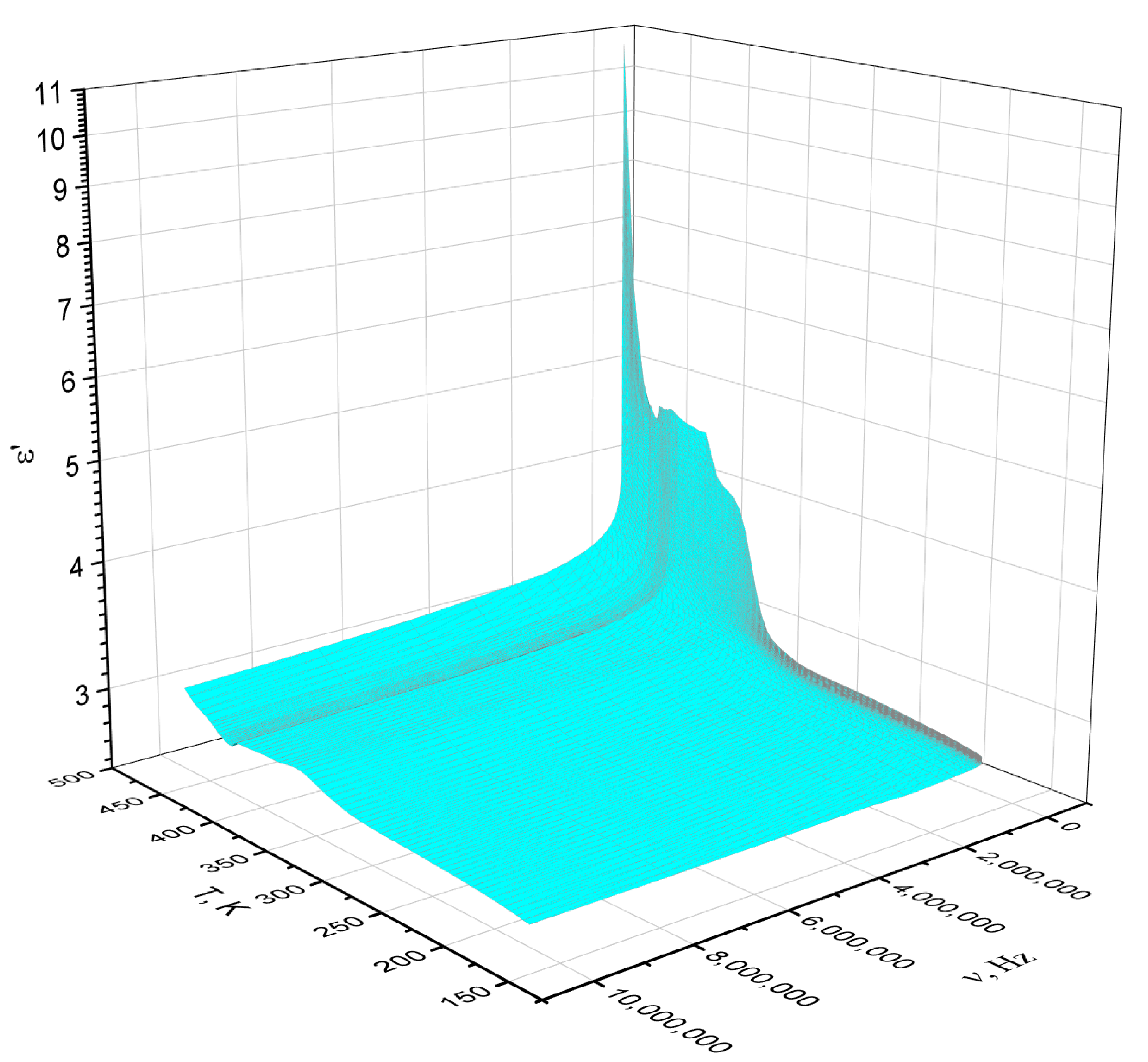
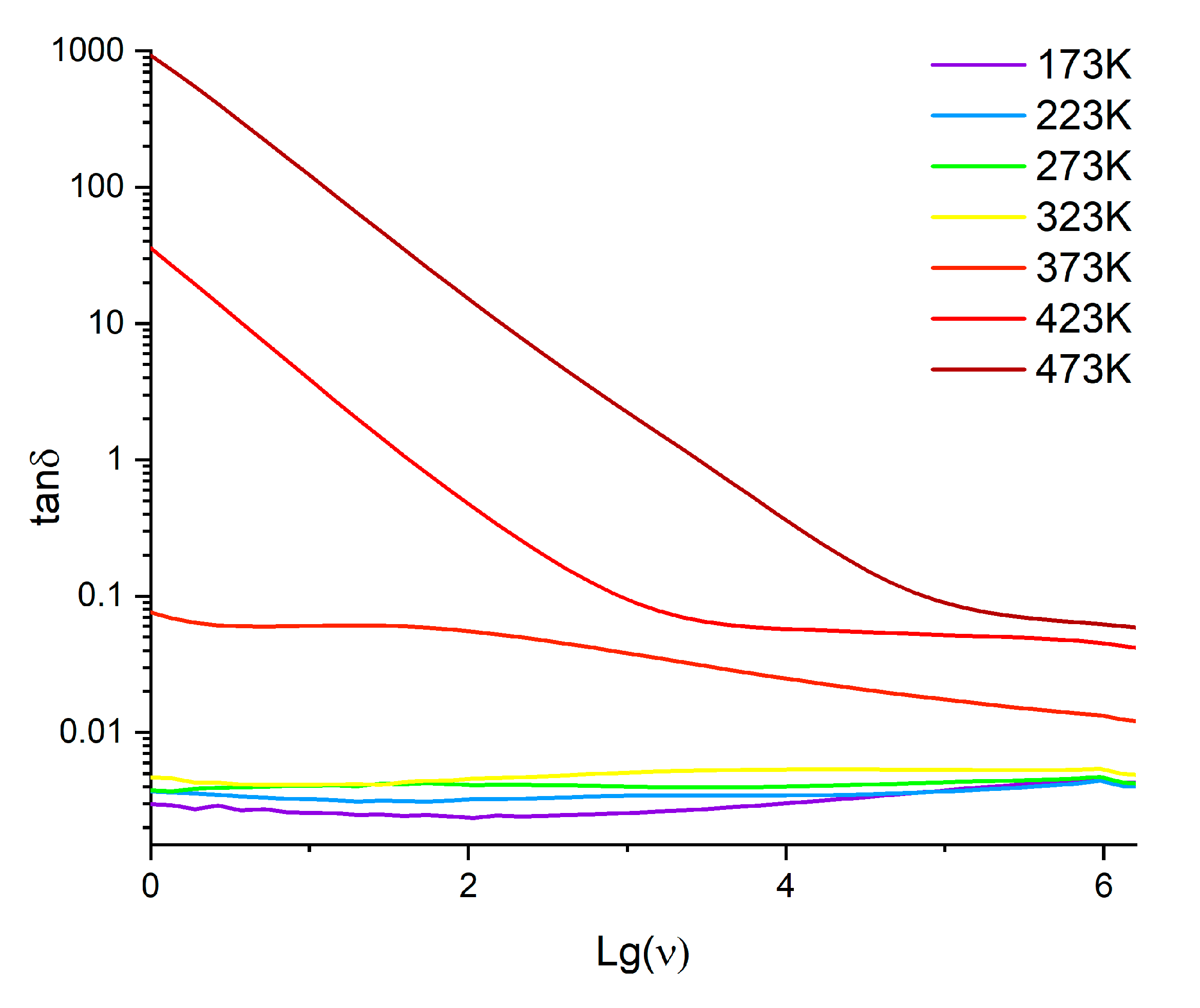
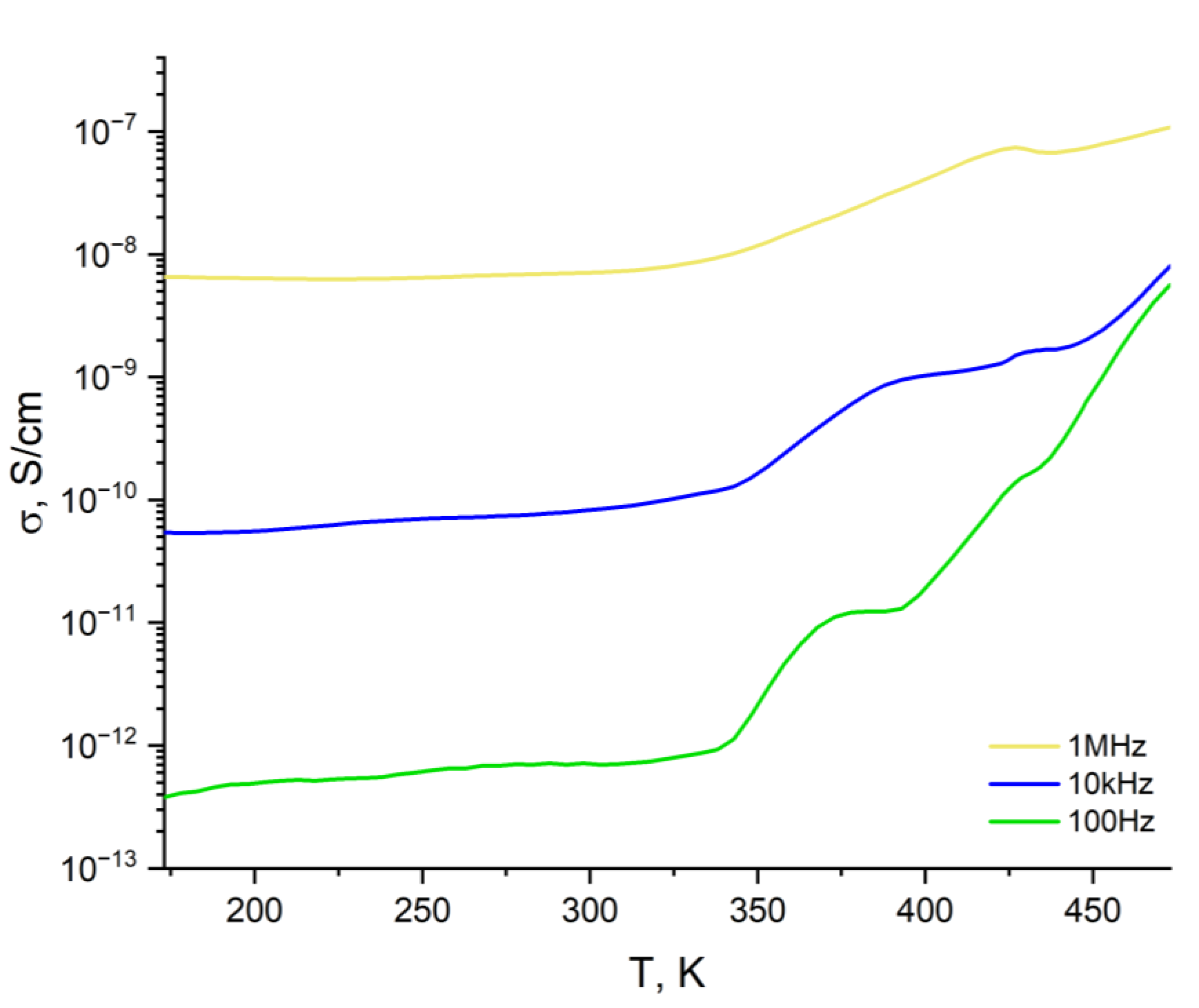
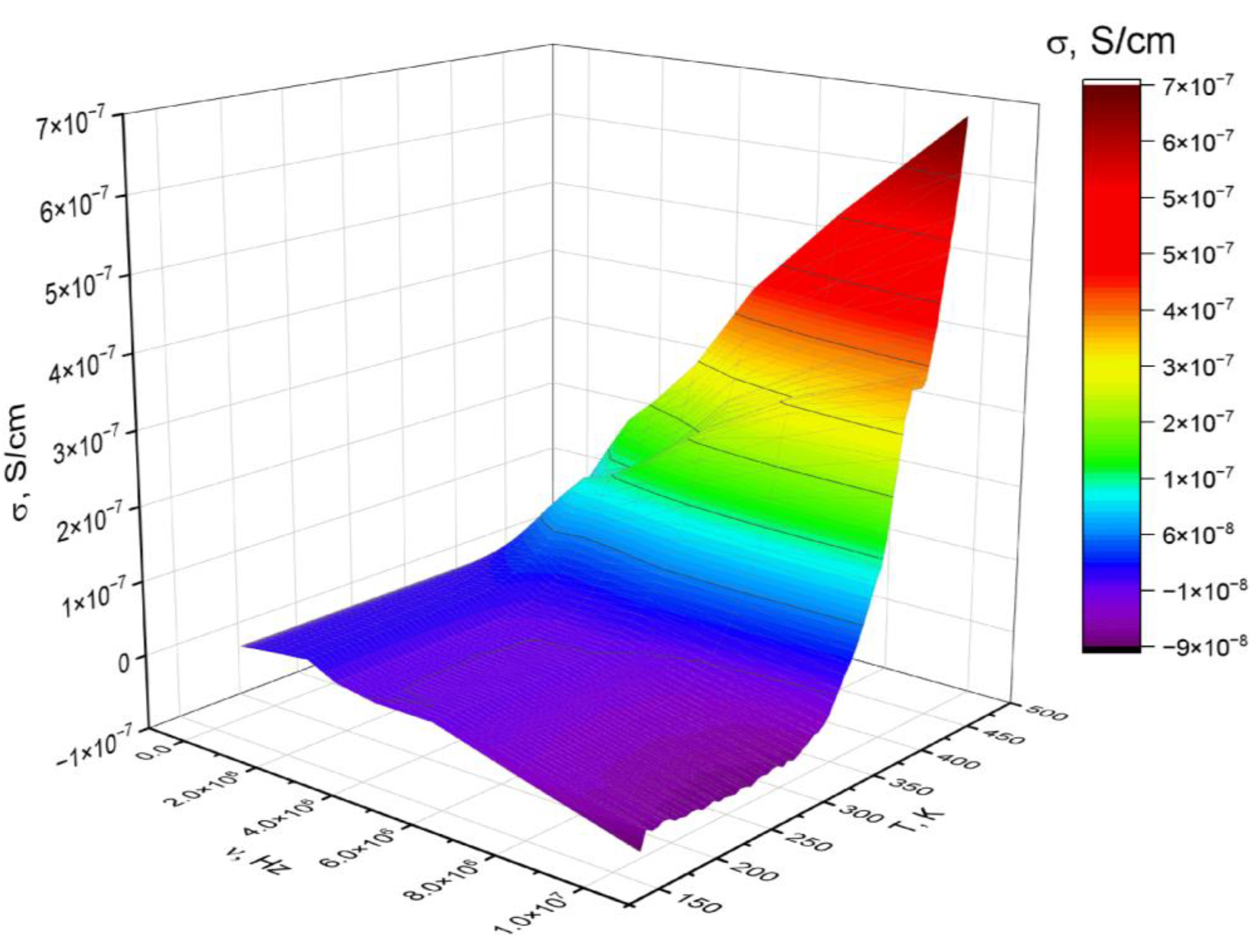
3.3. Dielectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivin, K.J.; Mol, J.C. Chapter 11. Ring-Opening Metathesis Polymerization: General Aspects. In Olefin Metathesis and Metathesis Polymerization; Mol, K.J., Ivin, J.C., Eds.; Academic Press: London, UK, 1997; pp. 224–259. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Chapala, P.P. Addition polymerization of functionalized norbornenes as a powerful tool for assembling molecular moieties of new polymers with versatile properties. Prog. Polym. Sci. 2018, 84, 1–46. [Google Scholar] [CrossRef]
- Suslov, D.S.; Bykov, M.V.; Kravchenko, O.V. Norbornene Addition Polymerization with Catalysts Based on Transition Metal Compounds: 2008–2018. Polym. Sci. Ser. C 2019, 61, 145–173. [Google Scholar] [CrossRef]
- Pérez-Ortega, I.; Albeniz, A.C. Highly efficient vinylic addition polymerization of 5-vinyl-2-norbornene using benzylic palladium complexes as precatalysts. Polym. Chem. 2021, 12, 5963–5969. [Google Scholar] [CrossRef]
- García-Loma, R.; Albéniz, A.C. Vinylic Addition Polynorbornene in Catalysis. Asian J. Org. Chem. 2019, 8, 304–315. [Google Scholar] [CrossRef]
- Petrov, V.A.; Vasil’ev, N.V.; Nikolai, V. Synthetic Chemistry of Quadricyclane. Curr. Org. Synth. 2006, 3, 215–259. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Kim, T.; Lim, S.; Park, S.-R.; Han, C.J.; Lee, M.H. Polynorbornene copolymer with side-chain triarylborane and iridium(III) groups: An emissive layer material with electron transporting properties for PhOLEDs. Polymer 2015, 66, 67–75. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, J.; Tao, Y.; Wang, Y.; Chen, X.; Chen, X.; Hou, J.; Sun, J.; Fang, Q. Low Dielectric Fluorinated Polynorbornene with Good Thermostability and Transparency Derived from a Biobased Allylphenol (Eugenol). ACS Sustain. Chem. Eng. 2019, 7, 4078–4086. [Google Scholar] [CrossRef]
- Feiring, A.E.; Crawford, M.K.; Farnham, W.B.; Feldman, J.; French, R.H.; Junk, C.P.; Leffew, K.W.; Petrov, V.A.; Qiu, W.; Schadt, F.L.; et al. New Amorphous Fluoropolymers of Tetrafluoroethylene with Fluorinated and Non-Fluorinated Tricyclononenes. Semiconductor Photoresists for Imaging at 157 and 193 nm. Macromolecules 2006, 39, 3252–3261. [Google Scholar] [CrossRef] [Green Version]
- Bykov, V.I.; Makovetskii, K.L.; Popov, D.S.; Bermeshev, M.; Butenko, T.A.; Filatova, M.P.; Finkel’Shtein, E.S. Binary and ternary copolymers of norbornene and its derivatives with acrylates as novel materials for optoelectronics. Polym. Sci. Ser. B 2012, 54, 99–105. [Google Scholar] [CrossRef]
- Finkelshtein, E.; Gringolts, M.; Bermeshev, M.; Chapala, P.; Rogan, Y. Polynorbornenes. In Membrane Materials for Gas and Vapor Separation; Yampolskii, Y., Finkelshtein, E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 143–221. [Google Scholar]
- He, X.; Liu, J.; Zhu, H.; Zheng, Y.; Chen, D. Novel quaternary ammonium functional addition-type norbornene copolymer as hydroxide-conductive and durable anion exchange membrane for direct methanol fuel cells. RSC Adv. 2015, 5, 63215–63225. [Google Scholar] [CrossRef]
- Kang, B.-G.; Kim, D.-G.; Register, R.A. Vinyl Addition Copolymers of Norbornylnorbornene and Hydroxyhexafluoroisopropylnorbornene for Efficient Recovery of n-Butanol from Dilute Aqueous Solution via Pervaporation. Macromolecules 2018, 51, 3702–3710. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Alentiev, D.A.; Moskalets, A.P.; Bermesheva, M.V. New Adhesive Materials Based on Silicon-Substituted Polynorbornenes. Polym. Sci. Ser. B 2019, 61, 314–322. [Google Scholar] [CrossRef]
- Cosnier, S.; Szunerits, S.; Marks, R.S.; Novoa, A.; Puech, L.; Perez, E.; Rico-Lattes, I. A comparative physical study of two different hydrophilic synthetic latex matrices for the construction of a glucose biosensor. Talanta 2001, 55, 889–897. [Google Scholar] [CrossRef]
- De la Torre, J.A.M.; Albéniz, A.C. Vinylic Addition Polynorbornene as Support for N-Heterocyclic Carbene Palladium Complexes: Use as Reservoir of Active Homogeneous Catalytic Species in C−C Cross-Coupling Reactions. ChemCatChem 2016, 8, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Hu, Y.; Shiotsuki, M.; Sanda, F.; Freeman, B.; Masuda, T. Synthesis and Properties of Indan-Based Polyacetylenes That Feature the Highest Gas Permeability among All the Existing Polymers. Macromolecules 2008, 41, 8525–8532. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Rigid and microporous polymers for gas separation membranes. Prog. Polym. Sci. 2015, 43, 1–32. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Andreyanov, F.A.; Borisov, I.L.; Zarezin, D.P.; Bakhtin, D.S.; Gavrilova, N.N.; Ilyasov, I.R.; Nechaev, M.S.; Asachenko, A.F.; et al. Modifications of addition poly(5-vinyl-2-norbornene) and gas-transport properties of the obtained polymers. React. Funct. Polym. 2020, 149, 104513. [Google Scholar] [CrossRef]
- Karpov, G.O.; Alentiev, D.A.; Wozniak, A.I.; Bermesheva, E.V.; Lounev, I.V.; Gusev, Y.A.; Shantarovich, V.P.; Bermeshev, M.V. Dielectric properties of addition and metathesis polynorbornenes with bulky side-substituents. Polymer 2020, 203, 122759. [Google Scholar] [CrossRef]
- Liu, B.; Haw, K.-G.; Zhang, C.; Yu, G.; Li, J.; Zhang, P.; Li, S.; Wu, S.; Li, J.; Zou, X. Flexible films derived from PIM-1 with ultralow dielectric constants. Microporous Mesoporous Mater. 2020, 294, 109887. [Google Scholar] [CrossRef]
- Konnertz, N.; Ding, Y.; Harrison, W.J.; Budd, P.M.; Schönhals, A.; Böhning, M. Molecular Mobility of the High Performance Membrane Polymer PIM-1 as Investigated by Dielectric Spectroscopy. ACS Macro Lett. 2016, 5, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chapala, P.; Bermeshev, M.; Schönhals, A.; Böhning, M. Molecular Mobility and Physical Aging of a Highly Permeable Glassy Polynorbornene as Revealed by Dielectric Spectroscopy. ACS Macro Lett. 2017, 6, 813–818. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Fang, L.; Sun, J.; Fang, Q. A novel post-polymerizable polynorbornene prepared via ROMP: Easy synthesis and conversion into a free-standing film with high Tg and low dielectric constant. Mater. Chem. Front. 2018, 2, 1467–1474. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, T.J.; Alentiev, D.; Gringolts, M.; Finkelshtein, E.; Bermeshev, M.; Long, B.K. Substituted polynorbornene membranes: A modular template for targeted gas separations. Polym. Chem. 2021, 12, 2947–2977. [Google Scholar] [CrossRef]
- Mulpuri, S.V.; Shin, J.; Shin, B.-G.; Greiner, A.; Yoon, D.Y. Synthesis and characterization of substituted polynorbornene derivatives. Polymer 2011, 52, 4377–4386. [Google Scholar] [CrossRef]
- Shin, B.-G.; Cho, T.-Y.; Yoon, D.Y.; Liu, B. Structure and properties of polynorbornene derivatives: Poly(norbornene dicarboxylic acid dialkyl ester)s and poly(norbornene dimethyl dicarboxylate)s. Macromol. Res. 2007, 15, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Owusu, F.; Tress, M.; Nüesch, F.A.; Lehner, S.; Opris, D.M. Synthesis of polar polynorbornenes with high dielectric relaxation strength as candidate materials for dielectric applications. Mater. Adv. 2022, 3, 998–1006. [Google Scholar] [CrossRef]
- Alaboalirat, M.; Qi, L.; Arrington, K.J.; Qian, S.; Keum, J.K.; Mei, H.; Littrell, K.C.; Sumpter, B.G.; Carrillo, J.-M.Y.; Verduzco, R.; et al. Amphiphilic Bottlebrush Block Copolymers: Analysis of Aqueous Self-Assembly by Small-Angle Neutron Scattering and Surface Tension Measurements. Macromolecules 2018, 52, 465–476. [Google Scholar] [CrossRef]
- Tayama, E.; Sugawara, T. Chiral Tetraaryl- and Tetraalkynylborates as Chiral Solvating Agents for Tetraalkylammonium Salts. Eur. J. Org. Chem. 2018, 2019, 803–811. [Google Scholar] [CrossRef]
- Wilks, B.R.; Chung, W.J.; Ludovice, P.J.; Rezac, M.R.; Meakin, P.; Hill, A.J. Impact of average free-volume element size on transport in stereoisomers of polynorbornene. I. Properties at 35 °C. J. Polym. Sci. B Polym. Phys. 2003, 41, 2185–2199. [Google Scholar] [CrossRef]
- Wilks, B.R.; Chung, W.J.; Ludovice, P.J.; Rezac, M.E.; Meakin, P.; Hill, A.J. Structural and free-volume analysis for alkyl-substituted palladium-catalyzed poly(norbornene): A combined experimental and Monte Carlo investigation. J. Polym. Sci. B Polym. Phys. 2006, 44, 215–233. [Google Scholar] [CrossRef]
- Belov, N.A.; Gringolts, M.L.; Morontsev, A.A.; Starannikova, L.E.; Yampolskii, Y.P.; Finkelstein, E.S. Gas-transport properties of epoxidated metathesis polynorbornenes. Polym. Sci. Ser. B 2017, 59, 560–569. [Google Scholar] [CrossRef]
- Finkelshtein, E.S.; Bermeshev, M.; Gringolts, M.L.; Starannikova, L.E.; Yampolskii, Y.P. Substituted polynorbornenes as promising materials for gas separation membranes. Russ. Chem. Rev. 2011, 80, 341–361. [Google Scholar] [CrossRef]
- Aitken, C.L.; Koros, W.J.; Paul, D.R. Gas transport properties of biphenol polysulfones. Macromolecules 1992, 25, 3651–3658. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Borisov, I.L.; Volkov, A.V.; Petukhov, D.I.; Gavrilova, N.N.; Shantarovich, V.P.; Asachenko, A.F.; Topchiy, M.A.; Finkelshtein, E.S.; et al. Switching on/switching off solubility controlled permeation of hydrocarbons through glassy polynorbornenes by the length of side alkyl groups. J. Membr. Sci. 2022, 641, 119848. [Google Scholar] [CrossRef]
- Guseva, M.A.; Alentiev, D.A.; Bakhtin, D.S.; Borisov, I.L.; Borisov, R.S.; Volkov, A.V.; Finkelshtein, E.S.; Bermeshev, M.V. Polymers based on exo-silicon-substituted norbornenes for membrane gas separation. J. Membr. Sci. 2021, 638, 119656. [Google Scholar] [CrossRef]
- Yalçınkaya, E.E. Polynorbornene/MMT nanocomposites via surface-initiated ROMP: Synthesis, characterization, and dielectric and thermal properties. J. Mater. Sci. 2013, 49, 749–757. [Google Scholar] [CrossRef]
- Asandulesa, M.; Kostromin, S.; Podshivalov, A.; Tameev, A.; Bronnikov, S. Relaxation processes in a polymer composite for bulk heterojunction: A dielectric spectroscopy study. Polymer 2020, 203, 122785. [Google Scholar] [CrossRef]
- Larsson, O.; Said, E.; Berggren, M.; Crispin, X. Insulator Polarization Mechanisms in Polyelectrolyte-Gated Organic Field-Effect Transistors. Adv. Funct. Mater. 2009, 19, 3334–3341. [Google Scholar] [CrossRef]
- Han, H.-J.; Zhang, S.; Sun, R.-Y.; Wu, J.-H.; Xie, M.-R.; Liao, X.-J. Photocrosslinkable polynorbornene-based block copolymers with enhanced dielectric and thermal properties. Chin. J. Polym. Sci. 2016, 34, 378–389. [Google Scholar] [CrossRef]
- You, Z.; Gao, D.; Jin, O.; He, X.; Xie, M. High dielectric performance of tactic polynorbornene derivatives synthesized by ring-opening metathesis polymerization. J. Polym. Sci. A Polym. Chem. 2012, 51, 1292–1301. [Google Scholar] [CrossRef]
- You, Z.; Song, W.; Zhang, S.; Jin, O.; Xie, M. Polymeric microstructures and dielectric properties of polynorbornenes with 3,5-bis(trifluoromethyl)biphenyl side groups by ring-opening metathesis polymerization. J. Polym. Sci. A Polym. Chem. 2013, 51, 4786–4798. [Google Scholar] [CrossRef]



| Monomer/[Ru] Molar Ratio | C, M | Yield, % | Mw·10−3 | Mn·10−3 | Mw/Mn | Tg, °C | [α]D,°,b |
|---|---|---|---|---|---|---|---|
| 1000/1 | 0.5 | 97 | 786 | 236 | 3.3 | 161 | −28 |
| 500/1 | 0.5 | 98 | 537 | 135 | 3.9 | ||
| 1000/1 | 0.05 | 94 | 1500 | 438 | 3.4 |
| Polymer | 2θ1, ° | 2θ2, ° | d1, Å | d2, Å |
|---|---|---|---|---|
| polyNBi | 11.8 | 19.7 | 7.5 | 4.5 |
| polyNB [35] | 18.1 | - | 4.9 | - |
| Polymer | Permeability (P), Barrer | ||||||
|---|---|---|---|---|---|---|---|
| He | H2 | N2 | O2 | CO2 | CH4 | C2H6 | |
| polyNBi | 8.4 | 8.1 | 0.10 | 0.61 | 2.67 | 0.13 | 0.046 |
| polyNB [36] | 19.4 | 13.0 | 0.75 | 2.6 | 13.9 | 1.9 | - |
| PSF [37] | 13 | - | 0.25 | 1.4 | 5.6 | 0.27 | - |
| Diffusivity coefficients (D), D·108, cm2/s | |||||||
| polyNBi | 550 | 140 | 0.87 | 2.9 | 0.82 | 0.22 | 0.011 |
| Solubility coefficients (S), S·103, cm3/(cm3·cm Hg) | |||||||
| polyNBi | 0.15 | 0.58 | 1.2 | 2.1 | 33 | 5.9 | 42 |
| Permeability Selectivity | ||||||
|---|---|---|---|---|---|---|
| Polymer | O2/N2 | CO2/N2 | CO2/CH4 | He/N2 | C2H6/CH4 | He/CH4 |
| polyNBi | 6.1 | 26.7 | 20.5 | 84 | 0.35 | 64 |
| polyNB [36] | 3.5 | 18.5 | 7.3 | 30 | - | 10.2 |
| PSF [37] | 5.6 | 22 | 22 | 52 | - | 48 |
| Diffusivity selectivity | ||||||
| polyNBi | 3.33 | 0.94 | 4.00 | 161 | 0.050 | 636 |
| Solubility selectivity | ||||||
| polyNBi | 1.75 | 27.5 | 5.6 | 0.48 | 7.1 | 0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, I.V.; Bakhtin, D.S.; Gorlov, I.V.; Potapov, K.V.; Borisov, I.L.; Lounev, I.V.; Makarov, I.S.; Volkov, A.V.; Finkelshtein, E.S.; Bermeshev, M.V. Gas-Transport and the Dielectric Properties of Metathesis Polymer from the Ester of exo-5-Norbornenecarboxylic Acid and 1,1′-Bi-2-naphthol. Polymers 2022, 14, 2697. https://doi.org/10.3390/polym14132697
Nazarov IV, Bakhtin DS, Gorlov IV, Potapov KV, Borisov IL, Lounev IV, Makarov IS, Volkov AV, Finkelshtein ES, Bermeshev MV. Gas-Transport and the Dielectric Properties of Metathesis Polymer from the Ester of exo-5-Norbornenecarboxylic Acid and 1,1′-Bi-2-naphthol. Polymers. 2022; 14(13):2697. https://doi.org/10.3390/polym14132697
Chicago/Turabian StyleNazarov, Ivan V., Danila S. Bakhtin, Ilya V. Gorlov, Konstantin V. Potapov, Ilya L. Borisov, Ivan V. Lounev, Igor S. Makarov, Alexey V. Volkov, Eugene Sh. Finkelshtein, and Maxim V. Bermeshev. 2022. "Gas-Transport and the Dielectric Properties of Metathesis Polymer from the Ester of exo-5-Norbornenecarboxylic Acid and 1,1′-Bi-2-naphthol" Polymers 14, no. 13: 2697. https://doi.org/10.3390/polym14132697







