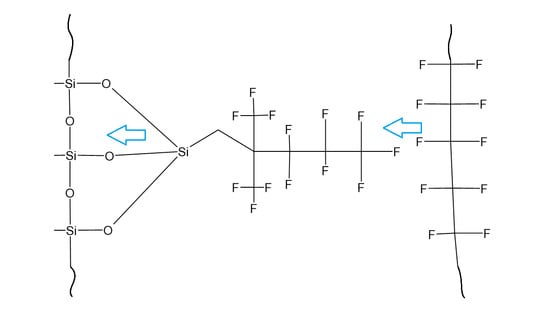
Chemical Structural Coherence Principle on Polymers for Better Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 1,1,1,2,2,3,3-Heptafluoro-4,4-bis(trifluoromethyl)pentyltriethoxysilane
2.3. Aluminum Foil Anodizing
2.4. Sample Surface Preparation
2.5. Sample Surface Modification
2.6. Polymer Films Application
2.7. Shear Strength Test Samples Preparation
2.8. Shear Strength Test
2.9. Glass Microspheres Modification
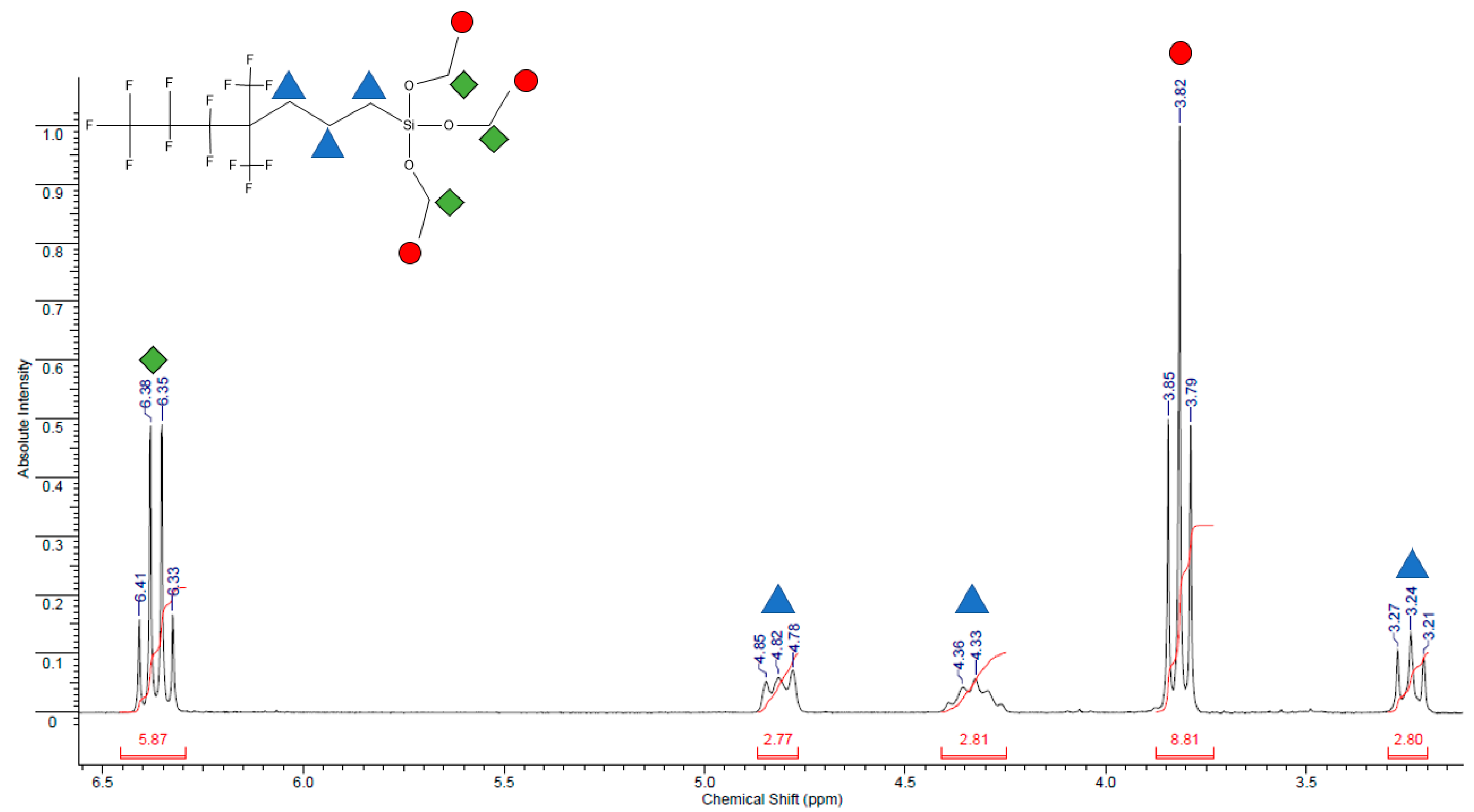
2.10. 1,1,1,2,2,3,3-Heptafluoro-4,4-bis(trifluoromethyl)pentyltriethoxysilane Characterization
2.11. Surfaces Characterization
2.11.1. Glass Microspheres Surface Characterization
2.11.2. Aluminum Surface Characterization
3. Results and Discussion
3.1. 1.1,1,2,2,3,3-Heptafluoro-4,4-bis(trifluoromethyl)pentyltriethoxysilane Characterization
3.2. Glass Surface Characterization
3.3. Anodized Aluminum Characterization
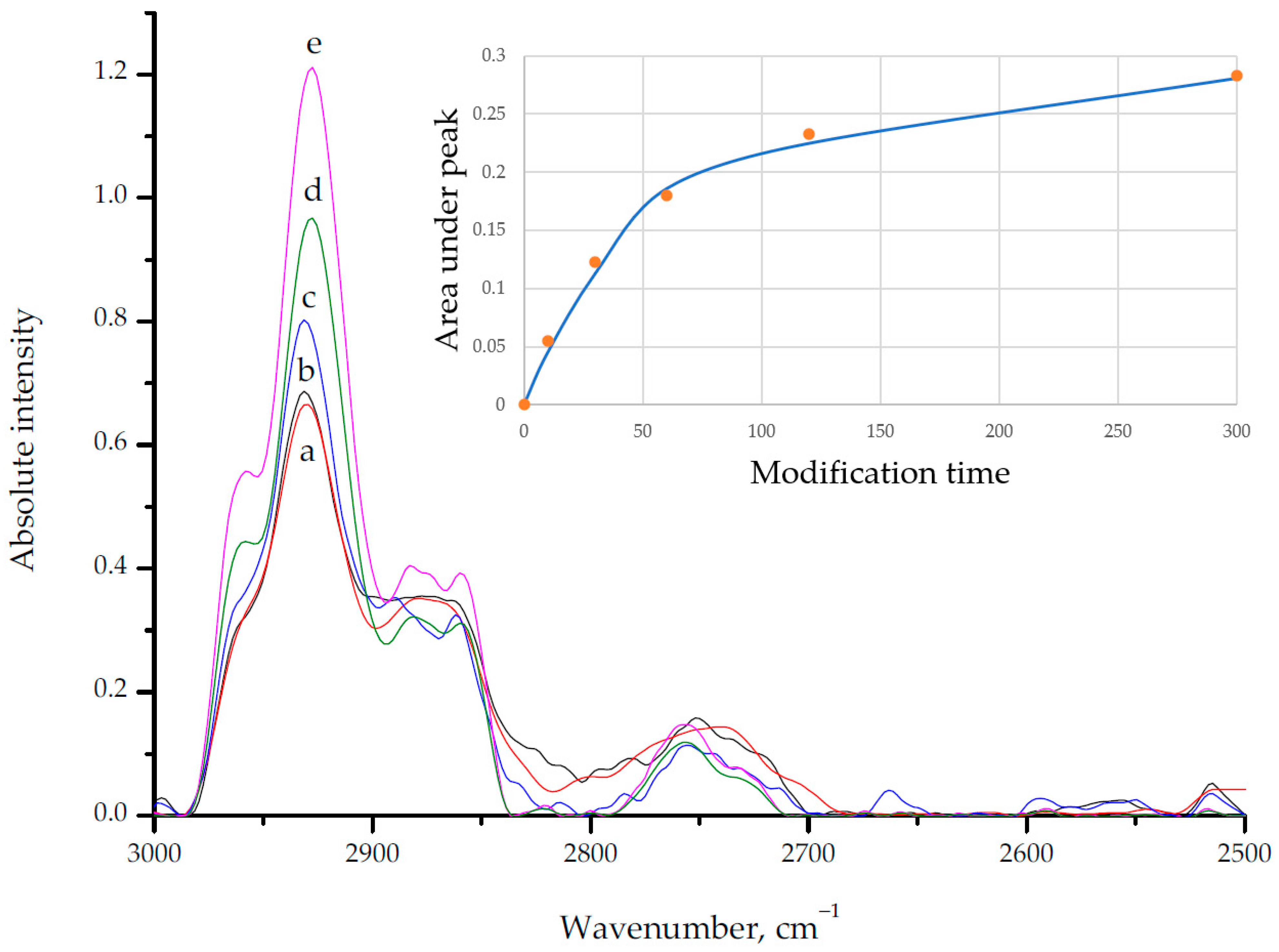
3.4. Modified Aluminum Surface Characterization
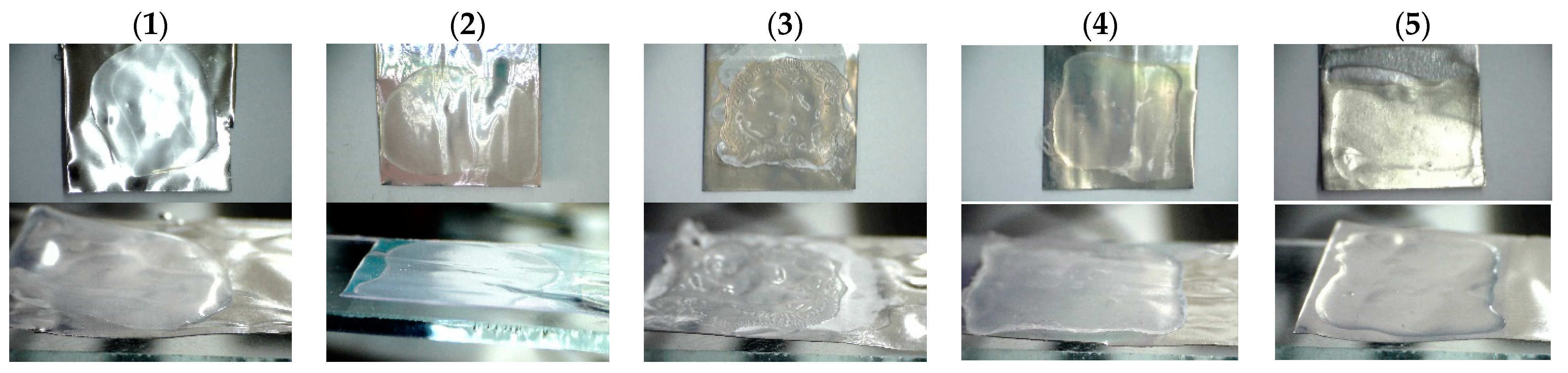
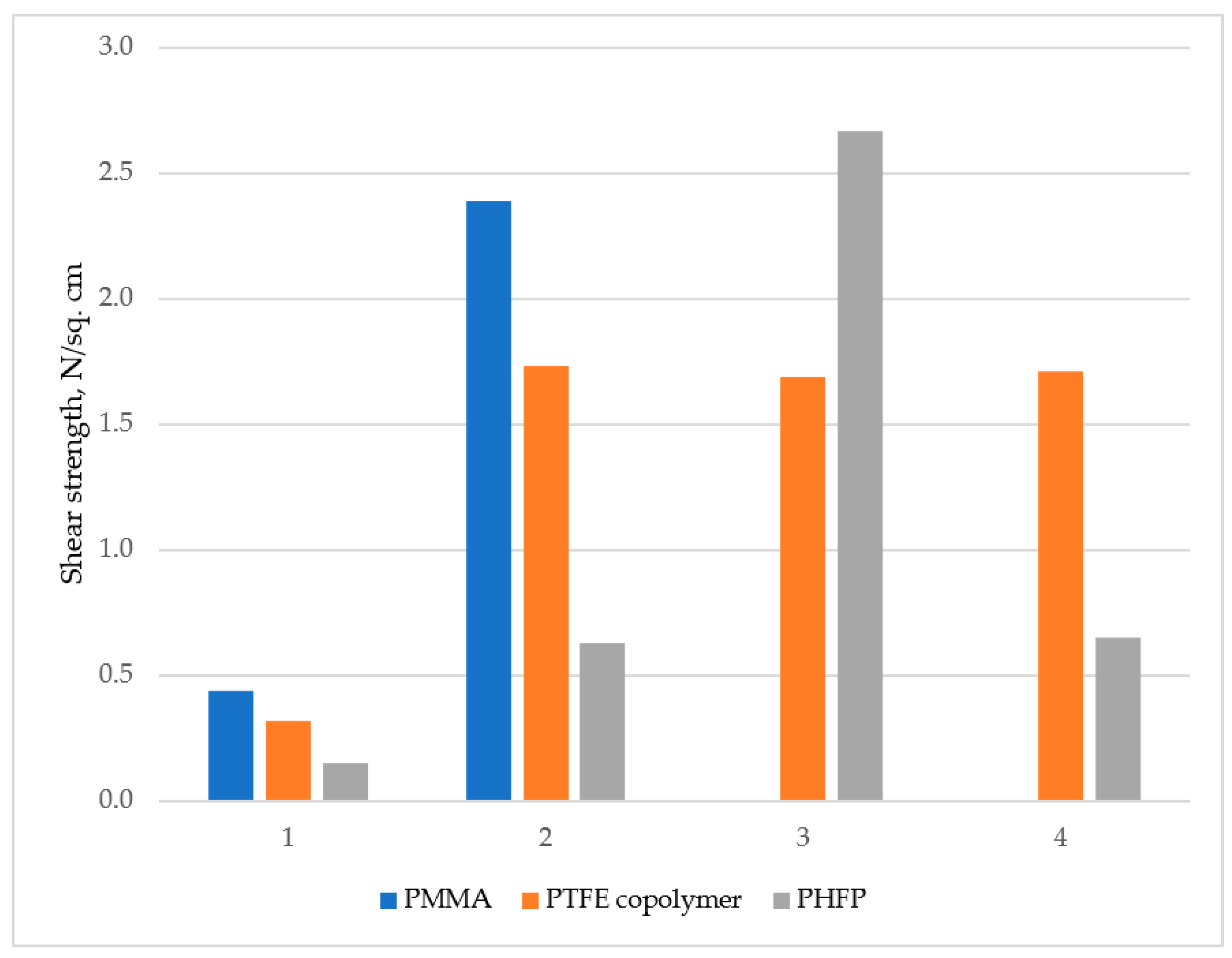
3.5. Polymer Films
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Q.; Xu, J.; Sun, G. Design of High Strength and Lightweight Construction Composites Using Advanced Porous and Tough Cementitious Materials. J. Adv. Concr. Technol. 2021, 19, 240–247. [Google Scholar] [CrossRef]
- Grammatikos, S.; Tsampas, S.; Petterson, J.; Luping, T.; Löfgren, I. Recycling and Re-Purposing Decommisioned Construction Polymer Composites for Construction Applications. In Proceedings of the ECCM 2018—18th European Conference on Composite Materials, Athens, Greece, 24–28 June 2020. [Google Scholar]
- Li, W.; Han, S.; Han, X.; Yao, Y. Experimental and Numerical Analysis of Mechanical Properties of Geocell Reinforced Reclaimed Construction Waste Composite Base Layer. Constr. Build. Mater. 2021, 304, 124587. [Google Scholar] [CrossRef]
- Lechner, T.; Schmitt, V.; Fischer, O.; Kempf, J. Modulbauweisen Im Verbundbrückenbau: Rückblick Und Aktuelle Tendenzen Beim Bau von Brücken Mit Kleinen Und Mittleren Spannweiten. Stahlbau 2021, 90, 116–127. [Google Scholar] [CrossRef]
- Liu, Q.M.; Huang, S.Z.; He, A.J. Application Requirements and Challenges of CMC-SiC Composites on Aero-Engine. Cailiao Gongcheng/J. Mater. Eng. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Jaya Krishna, K.; Jayakumar, V.; Bharathiraja, G. Mechanical Analysis of Medical Waste Reinforced Polymer Composite. Mater. Today Proc. 2020, 22, 473–476. [Google Scholar] [CrossRef]
- Park, S.-B.; Sung, M.H.; Uyama, H.; Han, D.K. Poly(Glutamic Acid): Production, Composites, and Medical Applications of the next-Generation Biopolymer. Prog. Polym. Sci. 2021, 113, 101341. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Wu, J. Processing and Characterization of Natural Rubber/Stearic Acid-Tetra-Needle-like Zinc Oxide Whiskers Medical Antibacterial Composites. J. Polym. Res. 2018, 25, 48. [Google Scholar] [CrossRef]
- Shen, W.; Qhobosheane, R. Durability of Medical Composite Systems. In Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–382. [Google Scholar] [CrossRef]
- Zaitsev, N.K.; Melnikov, P.V.; Alferov, V.A.; Kopytin, A.V.; German, K.E. Stable Optical Oxygen Sensing Material Based on Perfluorinated Polymer and Fluorinated Platinum(II) and Palladium(II) Porphyrins. Procedia Eng. 2016, 168, 309–312. [Google Scholar] [CrossRef]
- Naumova, A.O.; Melnikov, P.V.; Dolganova, E.V.; Yashtulov, N.A.; Zaitsev, N.K. Shifts in the PKa Value of Acid–Base Indicators Caused by Immobilization on Solid Substrates via Water-Soluble Polycationic Polymers: A Case Study of Congo Red. Fine Chem. Technol. 2020, 15, 59–70. [Google Scholar] [CrossRef]
- Kano, Y.; Katsumata, T.; Komuro, S.; Aizawa, H. Development of Fluorescence Thermo-Sensor Composite with Cr Doped YAlCl3 and Silicone Resin. ECS Electrochem. Lett. 2014, 3, B9. [Google Scholar] [CrossRef]
- Saito, H.; Shimogawa, S.; Tanaka, S.; Shioda, S. Estimating Parameters of Multiple Heterogeneous Target Objects Using Composite Sensor Nodes. IEEE Trans. Mob. Comput. 2012, 11, 125–138. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Kummerlöwe, C.; Clemens, F. Effect of the Elastomer Matrix on Thermoplastic Elastomer-Based Strain Sensor Fiber Composites. Sensors 2020, 20, 2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, B.; Zhang, J.; Liu, G.; Zhang, T.; Qiu, F.; Zhao, M. Humidity Sensors of Composite Material of Nanocrystal BaTiO3 and Polymer (R)NM+X-. J. Mater. Sci. Lett. 1999, 18, 1603–1605. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials 2021, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.V.; Aleksandrovskaya, A.Y.; Safonov, A.V.; Popova, N.M.; Spitsin, B.V.; Naumova, A.O.; Zaitsev, N.K. Tuning the Wetting Angle of Fluorinated Polymer with Modified Nanodiamonds: Towards New Type of Biosensors. Mendeleev Commun. 2020, 30, 453–455. [Google Scholar] [CrossRef]
- Optical Analyzer for Continuous Monitoring of Dissolved Oxygen in Aviation Fuel and Other Non-Aqueous Media. Available online: https://www.ije.ir/article_88193.html (accessed on 28 June 2022).
- Novák, L.; Fojtl, L.; Kadlečková, M.; Maňas, L.; Smolková, I.; Musilová, L.; Minařík, A.; Mráček, A.; Sedláček, T.; Smolka, P. Surface Modification of Metallic Inserts for Enhancing Adhesion at the Metal–Polymer Interface. Polymers 2021, 13, 4015. [Google Scholar] [CrossRef]
- Kafkopoulos, G.; Padberg, C.J.; Duvigneau, J.; Vancso, G.J. Adhesion Engineering in Polymer-Metal Comolded Joints with Biomimetic Polydopamine. ACS Appl. Mater. Interfaces 2021, 13, 19244–19253. [Google Scholar] [CrossRef]
- Suriani, M.J.; Ruzaidi, C.M.; Wan Nik, W.B.; Zulkifli, F.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A.; Abubakar, A.; Musapha, R.; Mohd Radzi, F.S. Effect of Fibre Contents toward Manufacturing Defects and Interfacial Adhesion of Arenga Pinnata Fibre Reinforced Fibreglass/Kevlar Hybrid Composite in Boat Construction. J. Phys. Conf. Ser. 2021, 1960, 12022. [Google Scholar] [CrossRef]
- Plichta, T.; Sirjovova, V.; Zvonek, M.; Kalinka, G.; Cech, V. The Adhesion of Plasma Nanocoatings Controls the Shear Properties of Gf/Polyester Composite. Polymers 2021, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Pei, L.; Xu, J.; Cao, S.; Wang, Y.; Gong, X. Magnetically Tunable Adhesion of Composite Pads with Magnetorheological Polymer Gel Cores. Compos. Sci. Technol. 2020, 192, 108115. [Google Scholar] [CrossRef]
- Tian, H.; Yao, Y.; Wang, C.; Jv, R.; Ge, X.; Xiang, A. Essential Work of Fracture Analysis for Surface Modified Carbon Fiber/Polypropylene Composites with Different Interfacial Adhesion. Polym. Compos. 2020, 41, 3541–3551. [Google Scholar] [CrossRef]
- You, X.; Chen, Y.; Huang, Y.; Wang, C.; Zhou, G.; He, W.; Wang, S.; Tang, Y.; Zhang, W.; Li, Z.; et al. Surface Coarsening of Carbon Fiber/Cyanate Ester Composite for Adhesion Improvement of Electroless Copper Plating as Conductive Patterns. Mater. Chem. Phys. 2020, 255, 123597. [Google Scholar] [CrossRef]
- Shakhmurzova, K.T.; Kurdanova, Z.I.; Zhansitov, A.A.; Baikaziev, A.E.; Khashirova, S.Y. Possible Ways to Improve Interphase Adhesion between Fiberglass and Polyphenylenesulfide. Key Eng. Mater. 2021, 899, 440–450. [Google Scholar] [CrossRef]
- Tal, N.Y.; Timor, Y.; Dodiuk, H.; Kenig, S. Polymer/Nanoparticle Interface in Polymer Nanocomposites: A Critical Review. Rev. Adhes. Adhes. 2021, 9, 368–400. [Google Scholar] [CrossRef]
- Gravis, D.; Rigolé, G.; Yengui, M.; Knapp, W.; Coulon, J.F.; Poncin-Epaillard, F. Enhancement of Metal Adhesion, Owing to the Plasma Texturing of PEEK. Plasma Processes Polym. 2021, 18, 2100009. [Google Scholar] [CrossRef]
- Preda, N.; Costas, A.; Lilli, M.; Sbardella, F.; Scheffler, C.; Tirillò, J.; Sarasini, F. Functionalization of Basalt Fibers with ZnO Nanostructures by Electroless Deposition for Improving the Interfacial Adhesion of Basalt Fibers/Epoxy Resin Composites. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106488. [Google Scholar] [CrossRef]
- Liu, W.; Hu, C.; Zhang, W.; Liu, Z.; Shu, J.; Gu, J. Modification of Birch Wood Surface with Silane Coupling Agents for Adhesion Improvement of UV-Curable Ink. Prog. Org. Coat. 2020, 148, 105833. [Google Scholar] [CrossRef]
- Kwak, B.M.; Kwon, Y. Enhanced Mechanical Properties of Porous Silicone Composites Prepared by Silane Surface Functionalization of Hollow Silica Particles. Mol. Cryst. Liq. Cryst. 2021, 734, 89–99. [Google Scholar] [CrossRef]
- Pei, M.; Huo, L.; Zhao, X.; Chen, S.; Li, J.; Peng, Z.; Zhang, K.; Zhou, H.; Liu, P. Facile Construction of Stable Hydrophobic Surface via Covalent Self-Assembly of Silane-Terminated Fluorinated Polymer. Appl. Surf. Sci. 2020, 507, 145138. [Google Scholar] [CrossRef]
- Yu, M.; Li, P.; Feng, Y.; Li, Q.; Sun, W.; Quan, M.; Liu, Z.; Sun, J.; Shi, S.; Gong, Y. Positive Effect of Polymeric Silane-Based Water Repellent Agents on the Durability of Superhydrophobic Fabrics. Appl. Surf. Sci. 2018, 450, 492–501. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, S.; Yang, H.; Zhu, Y.; Chen, J. Preparation of Emulsion-Templated Fluorinated Polymers and Their Application in Oil/Water Separation. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1508–1515. [Google Scholar] [CrossRef]
- Kallitsis, K.; Thuau, D.; Brochon, C.; Cloutet, E.; Hadziioannou, G. Tailoring Fluorinated Electroactive Polymers toward Specific Applications. Colloid Polym. Sci. 2021, 299, 457–464. [Google Scholar] [CrossRef]
- Huang, H.; Guo, H.; Feng, Y. Study on UV-Aging Performance of Fluorinated Polymer Coating and Application on Painted Muds. Mater. Res. Express 2021, 8, 15301. [Google Scholar] [CrossRef]
- Shkinev, P.; Evdokimova, A.; Drozdov, F.V.; Gervits, L.L.; Muzafarov, A.M. Non-Accumulative in the Environment Facile Hydrophobic Coatings Based on Branched Siloxanes with Perfluoroalkyl Substituents. J. Organomet. Chem. 2021, 948, 121910. [Google Scholar] [CrossRef]
- Belov, N.A.; Zharov, A.A.; Shashkin, A.V.; Shaikh, M.Q.; Raetzke, K.; Yampolskii, Y.P. Gas Transport and Free Volume in Hexafluoropropylene Polymers. J. Membr. Sci. 2011, 383, 70–77. [Google Scholar] [CrossRef]
- Tasaki, T.; Guo, Y.; Machida, H.; Akasaka, S.; Fujimori, A. Nano-Dispersion of Fluorinated Phosphonate-Modified Nanodiamond in Crystalline Fluoropolymer Matrix to Achieve a Transparent Polymer/Nanofiller Hybrid. Polym. Compos. 2019, 40, E842–E855. [Google Scholar] [CrossRef]
- Baby, M.; Periya, V.K.; Soundiraraju, B.; Balachandran, N.; Cheriyan, S.; Sankaranarayanan, S.K.; Maniyeri, S.C. Bio-Mimicking Hybrid Polymer Architectures as Adhesion Promoters for Low and High Surface Energy Substrates. J. Ind. Eng. Chem. 2021, 100, 351–363. [Google Scholar] [CrossRef]
- Geim, A.K.; Dubonos, S.V.; Grigorieva, I.V.; Novoselov, K.S.; Zhukov, A.A.; Shapoval, S.Y. Microfabricated Adhesive Mimicking Gecko Foot-Hair. Nat. Mater. 2003, 2, 461–463. [Google Scholar] [CrossRef]
- Ryabkov, E.D.; Antropov, A.P.; Zaitsev, N.K.; Yashtulov, N.A. Methods of Measuring the Depth of Nanoholes in Metallic Aluminum in the Process of High-Voltage Anodizing. Mosc. Univ. Chem. Bull. 2021, 76, 388–393. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fernandes, F.A.O.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Chiu, L.H.; Chen, C.C.; Yang, C.F. Improvement of Corrosion Properties in an Aluminum-Sprayed AZ31 Magnesium Alloy by a Post-Hot Pressing and Anodizing Treatment. Surf. Coat. Technol. 2005, 191, 181–187. [Google Scholar] [CrossRef]
- Salman, S.A.; Okido, M. Anodization of Magnesium (Mg) Alloys to Improve Corrosion Resistance; Woodhead Publishing Limited: Sawston, UK, 2013; ISBN 9780857094377. [Google Scholar]
- Zharov, A.A.; Guzyaeva, I.A. Kinetics and Mechanism of Thermal Polymerization of Hexafluoropropylene under High Pressures. Russ. Chem. Bull. 2010, 59, 1225–1231. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Krapivko, A.L.; Cherkaev, G.V.; Gervits, L.L.; Yashtulov, N.A.; Kalinina, A.A.; Muzafarov, A.M. Multifunctional Hydrophobic Coatings Based on Siloxane Polymers with Branched Perfluoroalkyl Substituents: Fast, Simple and Ecologically Safe Synthesis in Active Media. J. Organomet. Chem. 2020, 921, 121398. [Google Scholar] [CrossRef]
- Makowski, T. Hydrophobization of Cotton Fabric with Silanes with Different Substituents. Cellulose 2020, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Asoh, H.; Haraguchi, S.; Ono, S. Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters. Materials 2015, 8, 1350–1368. [Google Scholar] [CrossRef]
- Antropov, A.P.; Zaytsev, N.K.; Ryabkov, Y.D.; Yashtulov, N.A.; Mudrakova, P.N. Manufacturing of Nanopillar (Ultra-Dispersed) Catalytically Active Materials through Chemical Engineering. Fine Chem. Technol. 2021, 16, 105–112. [Google Scholar] [CrossRef]



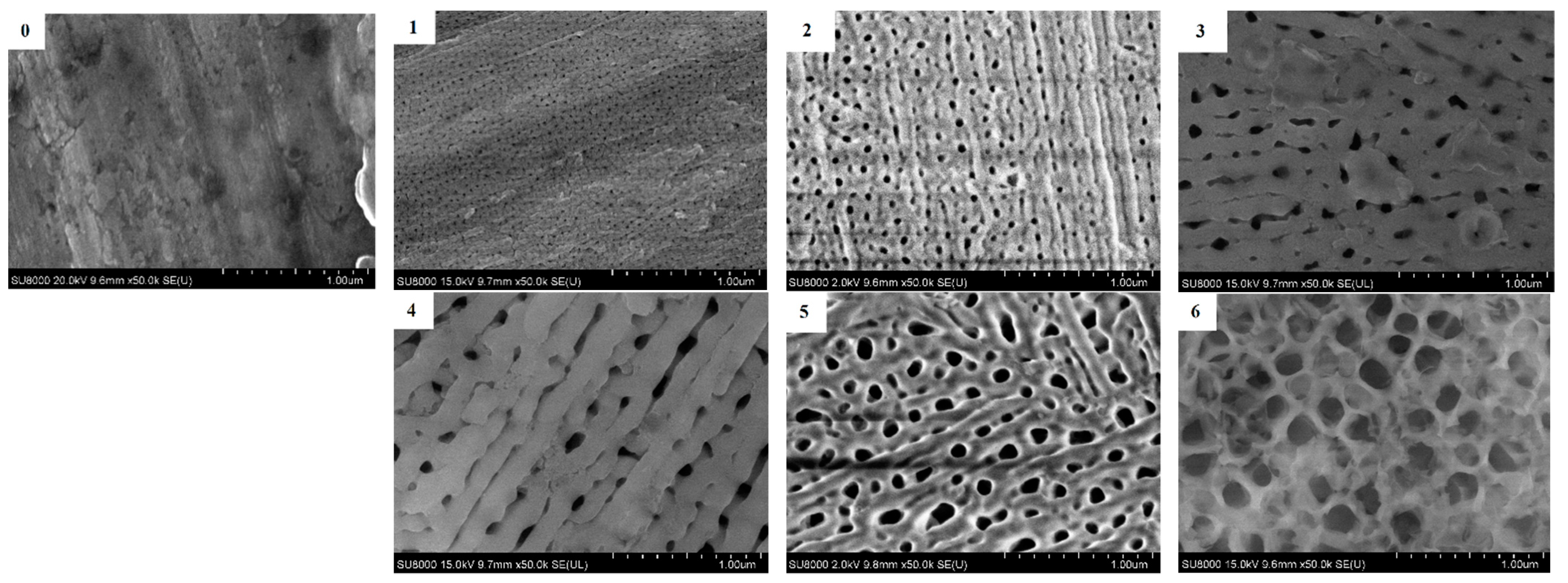
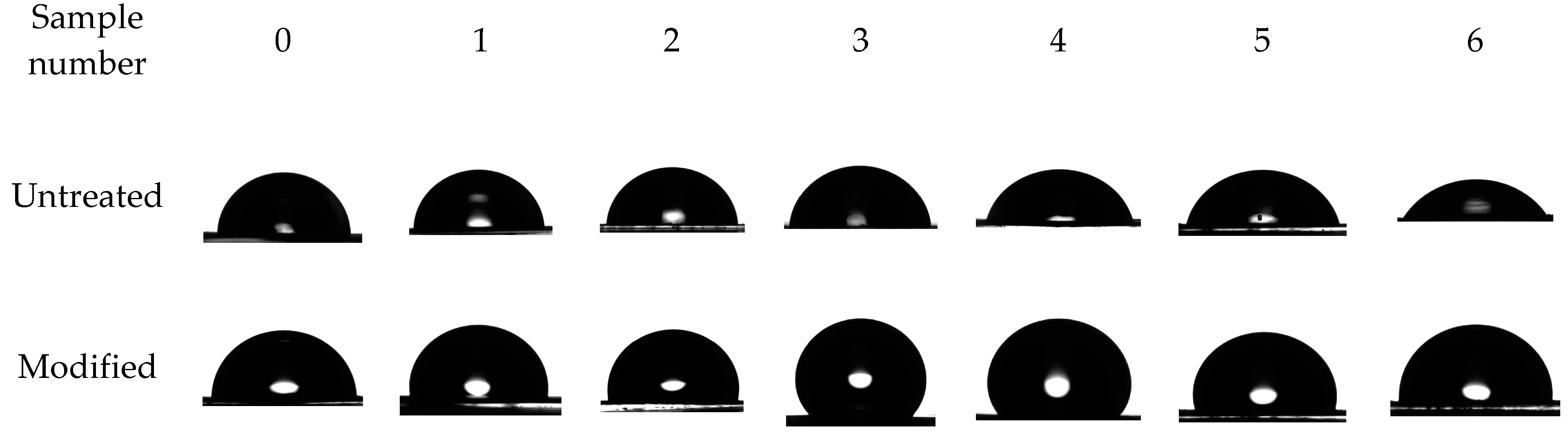

| № | Voltage, V | Background Electrolyte Composition | Time, Min |
|---|---|---|---|
| 1 | 10 | 0.3 M sulfuric acid | 15 |
| 2 | 60 | 0.3 M oxalic acid | 15 |
| 3 | 60 | 0.3 M phosphoric acid | 15 |
| 4 | 80 | 0.3 M phosphoric acid | 15 |
| 5 | 100 | 0.3 M phosphoric acid | 15 |
| 6 | 120 | 0.3 M phosphoric acid | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krapivko, A.L.; Ryabkov, Y.D.; Drozdov, F.V.; Yashtulov, N.A.; Zaitsev, N.K.; Muzafarov, A.M. Chemical Structural Coherence Principle on Polymers for Better Adhesion. Polymers 2022, 14, 2829. https://doi.org/10.3390/polym14142829
Krapivko AL, Ryabkov YD, Drozdov FV, Yashtulov NA, Zaitsev NK, Muzafarov AM. Chemical Structural Coherence Principle on Polymers for Better Adhesion. Polymers. 2022; 14(14):2829. https://doi.org/10.3390/polym14142829
Chicago/Turabian StyleKrapivko, Alena L., Yegor D. Ryabkov, Fedor V. Drozdov, Nikolay A. Yashtulov, Nikolay K. Zaitsev, and Aziz M. Muzafarov. 2022. "Chemical Structural Coherence Principle on Polymers for Better Adhesion" Polymers 14, no. 14: 2829. https://doi.org/10.3390/polym14142829
APA StyleKrapivko, A. L., Ryabkov, Y. D., Drozdov, F. V., Yashtulov, N. A., Zaitsev, N. K., & Muzafarov, A. M. (2022). Chemical Structural Coherence Principle on Polymers for Better Adhesion. Polymers, 14(14), 2829. https://doi.org/10.3390/polym14142829