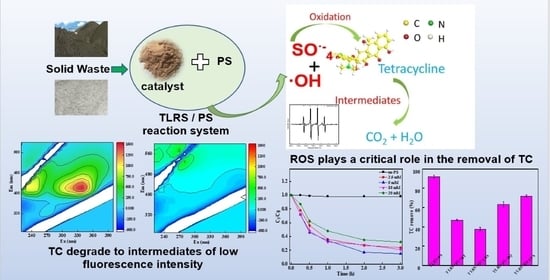
Activation of Persulfate for Degrading Tetracycline Using the Leaching Residues of the Lead-Zinc Flotation Tailing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
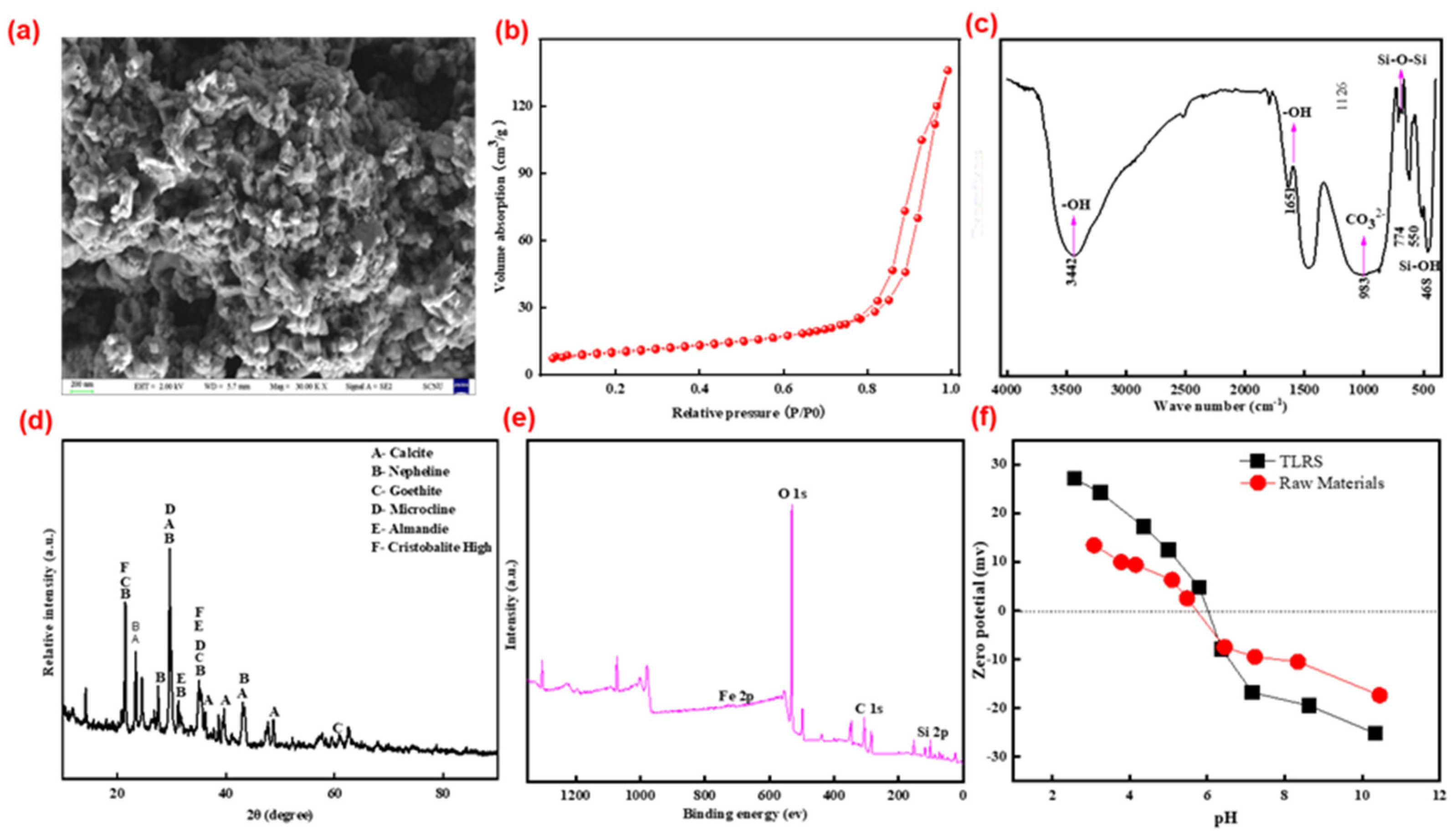
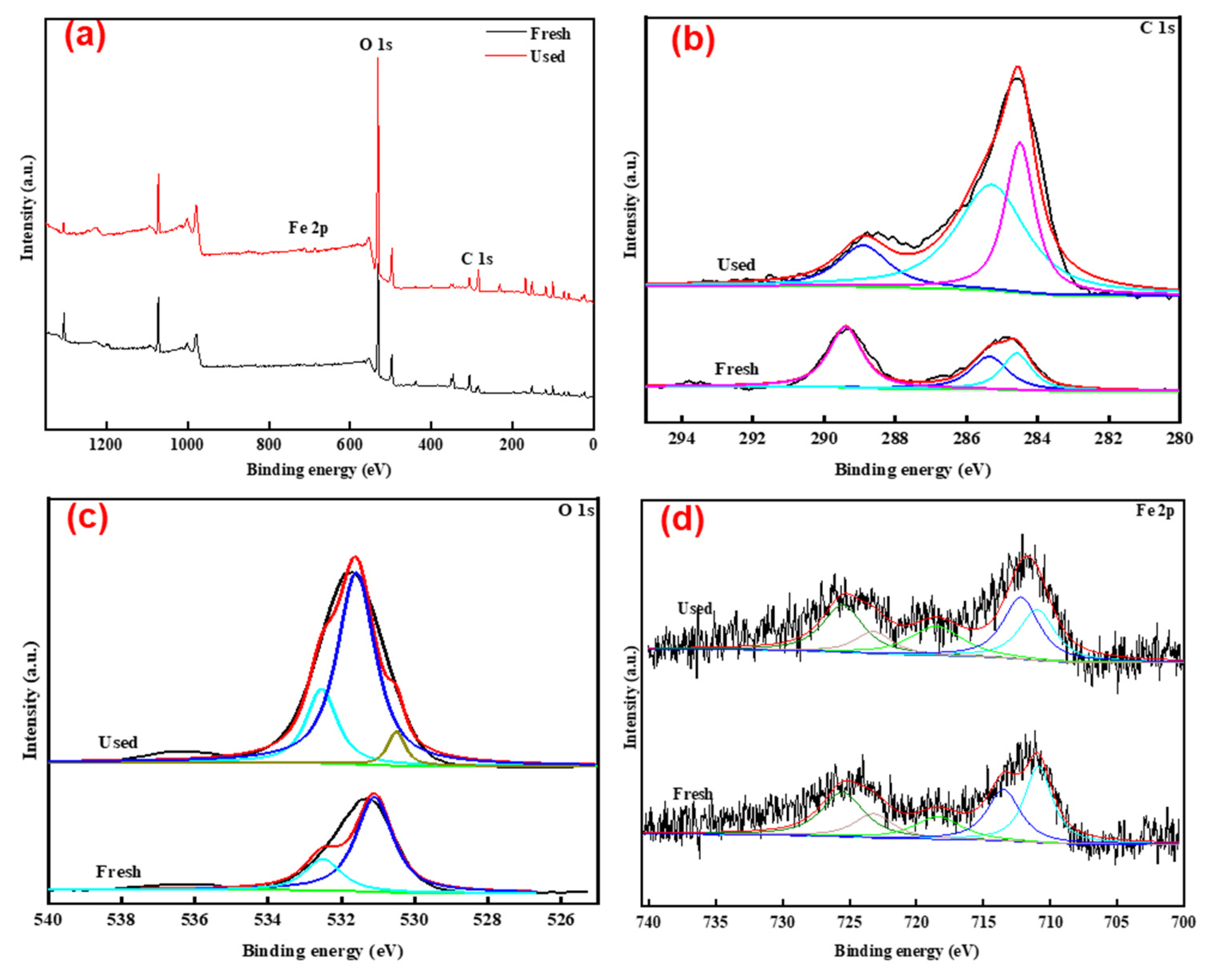
3.1. Characterization of TLRS
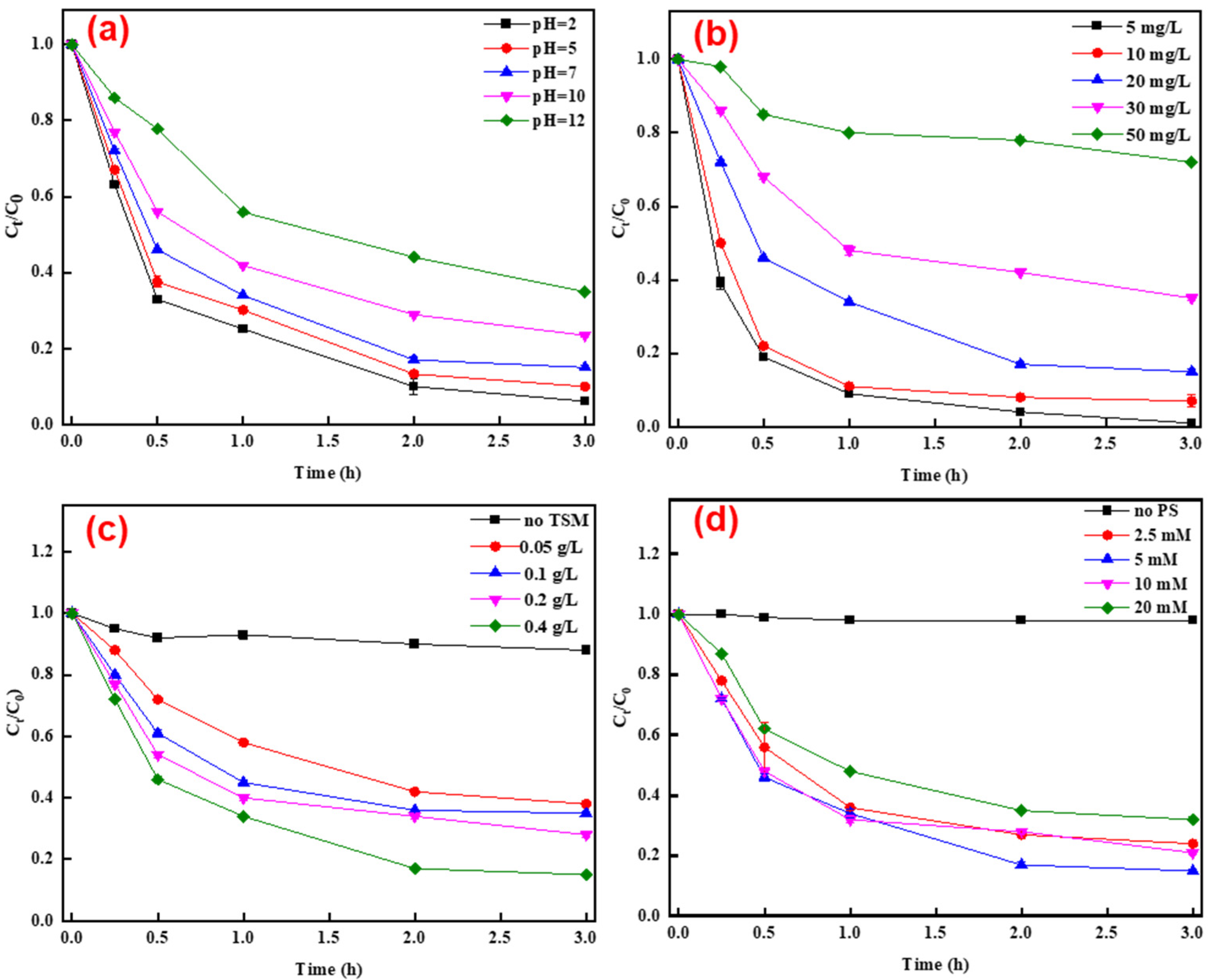
3.2. Degradation of TC
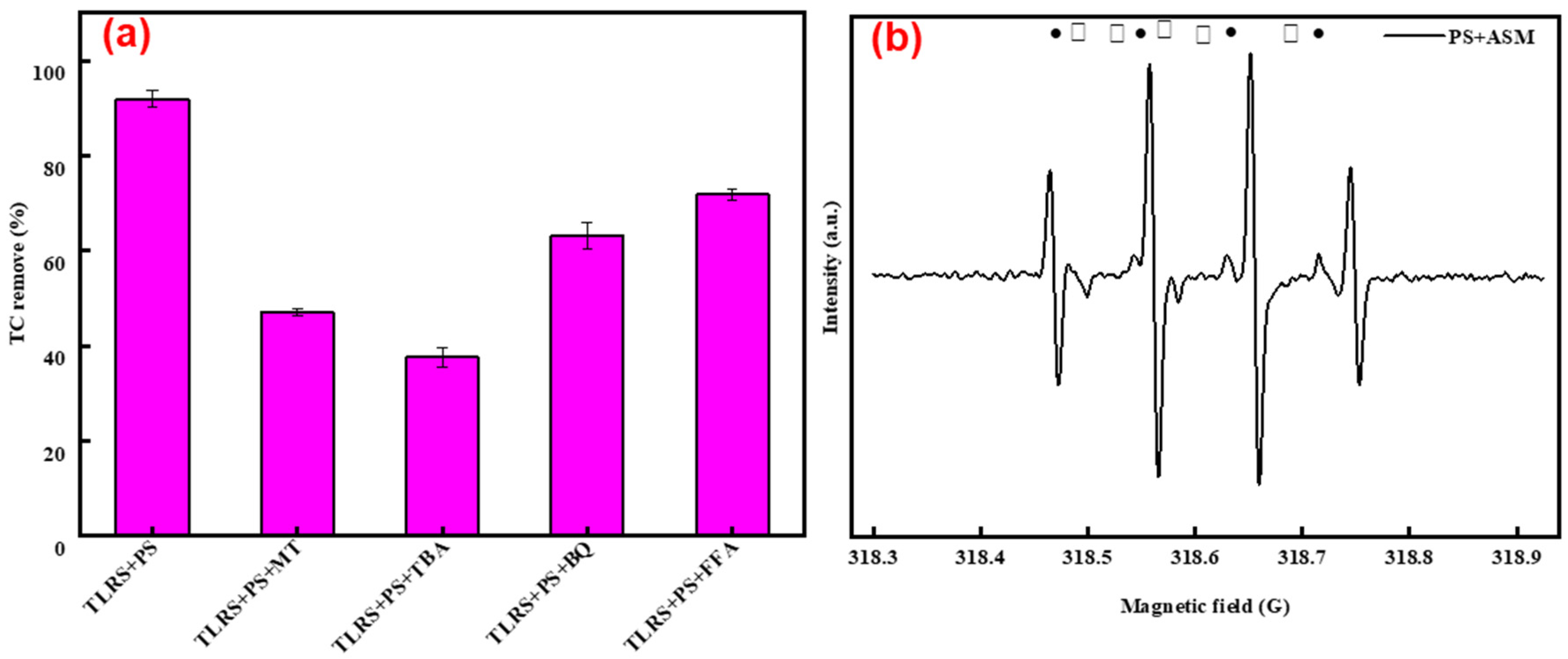
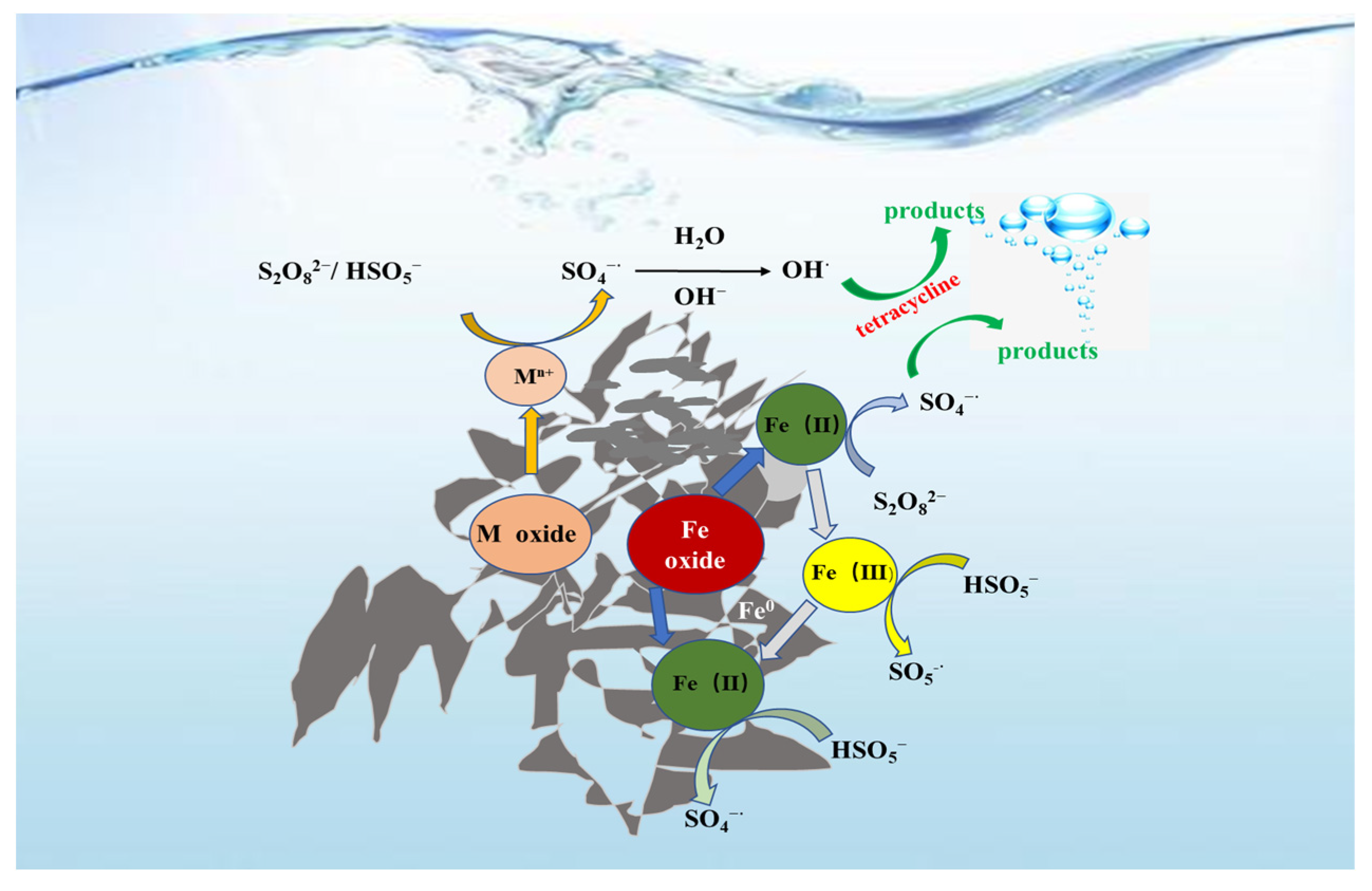
3.3. Reaction Mechanism
3.4. Reusability Tests and Application in Real Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben, Y.; Hu, M.; Zhong, F.; Du, E.; Li, Y.; Zhang, H.; Andrews, C.B.; Zheng, C. Human daily dietary intakes of antibiotic residues: Dominant sources and health risks. Environ. Res. 2022, 212, 113387. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Yuan, T.; Yu, T.; Chen, Y.; Kong, H.; Lin, C.; Shen, Z.; Tian, Y.; Tong, S.; et al. Exposure to antibiotics and precocious puberty in children: A school-based cross-sectional study in China. Environ. Res. 2022, 212, 113365. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, Y.; Hua, S.; Xu, G.; Xu, Z.; Yan, C. Coupling band structure and oxidation-reduction potential to expound photodegradation performance difference of biochar-derived dissolved black carbon for organic pollutants under light irradiation. Sci. Total Environ. 2022, 820, 153300. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Kong, D.; Li, Q.; Zhou, Y.; Jiang, X.; Wang, Z.; Parales, R.E.; Ruan, Z. Metabolomics reveals the mechanism of tetracycline biodegradation by a Sphingobacterium mizutaii S121. Environ. Pollut. 2022, 305, 119299. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Han, Y.; Wang, X.; Liu, S.; Zhang, L.; Sun, Y.; Jiang, B. Development of polyacrylonitrile/perovskite catalytic membrane with abundant channel-assisted reaction sites for organic pollutant removal. Chem. Eng. J. 2022, 437, 135163. [Google Scholar] [CrossRef]
- Han, Y.; Sun, S.; Zhang, B.; Du, J.; Duan, X. Activation of peroxymonosulfate by natural pyrite for efficient degradation of V(IV)-citrate complex in groundwater. J. Colloid Interface Sci. 2022, 617, 683–693. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, W.; Zhang, H.; Gao, P.; Jin, L.; Pan, Y.; Pan, Z. Synthesis of Layered Double Hydroxides with Phosphate Tailings and Its Effect on Flame Retardancy of Epoxy Resin. Polymers 2022, 14, 2516. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Li, C.; Tang, X.; Sun, Y. Removal of tetracycline via the synergistic effect of biochar adsorption and enhanced activation of persulfate. Chem. Eng. J. 2020, 382, 122916. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.; Yang, Y. Leaching of heavy metals from lead-zinc mine tailings and the subsequent migration and transformation characteristics in paddy soil. Chemosphere 2022, 291, 132792. [Google Scholar] [CrossRef]
- Lei, C.; Chen, T.; Yan, B.; Xiao, X.-M. Reaction characteristics and kinetics of gallium in chlorination roasting of copper tailings using calcium chloride. Rare Met. 2022, 41, 1063–1070. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Wang, X.-L.; Xiao, X.-M. Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J. Clean. Prod. 2018, 181, 408–415. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X.-M. Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation. J. Clean. Prod. 2017, 158, 73–80. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Javed, S.; Baig, R.U. Optimization of Alkaline Activator on the Strength Properties of Geopolymer Concrete. Polymers 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X.-M. Preparation and adsorption characteristics for heavy metals of active silicon adsorbent from leaching residue of lead-zinc tailings. Environ. Sci. Pollut. Res. 2018, 25, 21233–21242. [Google Scholar] [CrossRef]
- Tao, L.; Wen, X.; Li, H.; Huang, C.; Jiang, Y.; Liu, D.; Sun, B. Influence of manure fertilization on soil phosphorous retention and clay mineral transformation: Evidence from a 16-year long-term fertilization experiment. Appl. Clay Sci. 2021, 204, 106021. [Google Scholar] [CrossRef]
- Bezlepkina, K.A.; Milenin, S.A.; Vasilenko, N.G.; Muzafarov, A.M. Ring-Opening Polymerization (ROP) and Catalytic Rearrangement as a Way to Obtain Siloxane Mono- and Telechelics, as Well as Well-Organized Branching Centers: History and Prospects. Polymers 2022, 14, 2408. [Google Scholar] [CrossRef]
- Anbia, M.; Aghadoukht, F. Functionalization of silicon nanowires by iron oxide and copper for degradation of phenol. Res. Chem. Intermed. 2019, 45, 1973–1984. [Google Scholar] [CrossRef]
- Tran, H.V.; Hoang, N.T.; Le, T.D.; Tran, L.T.; Dang, H.T.M. Graphene Oxide/Fe3O4/Chitosan−Coated Nonwoven Polyester Fabric Extracted from Disposable Face Mask for Enhanced Efficiency of Organic Dye Adsorption. Adsorpt. Sci. Technol. 2022, 2022, 8055615. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, N.; Shi, Y.; Zhang, L. Pyrite enables persulfate activation for efficient atrazine degradation. Chemosphere 2020, 244, 125568. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic polyacrylamide-assisted construction of thin 2D-2D WO3/g-C3N4 Step-scheme heterojunction for enhanced tetracycline degradation under visible light irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhu, W.; Zhao, J.; Liu, H.; Zhang, X.; Zhang, H.; Zhu, H.; Peng, Y.; Wang, B. Nitrogen-doped graphene oxide aerogel anchored with spinel CoFe2O4 nanoparticles for rapid degradation of tetracycline. Sep. Purif. Technol. 2020, 241, 116690. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Q.; Zhang, C.; Wang, R.; Deng, R.; Luo, H.; Li, T.; Li, J.; Chen, S.; Liu, C. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chem. Eng. J. 2020, 391, 123532. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Zhang, G.; Yang, F.; Yuan, G.-E. Natural bornite as an efficient and cost-effective persulfate activator for degradation of tetracycline: Performance and mechanism. Chem. Eng. J. 2020, 381, 122717. [Google Scholar] [CrossRef]
- Liu, H.; Qu, J.; Zhang, T.; Ren, M.; Zhang, Z.; Cheng, F.; He, D.; Zhang, Y.-N. Insights into degradation pathways and toxicity changes during electro-catalytic degradation of tetracycline hydrochloride. Environ. Pollut. 2020, 258, 113702. [Google Scholar] [CrossRef]
- Qu, Z.; Dong, G.; Zhu, S.; Yu, Y.; Huo, M.; Xu, K.; Liu, M. Recycling of groundwater treatment sludge to prepare nano-rod erdite particles for tetracycline adsorption. J. Clean. Prod. 2020, 257, 120462. [Google Scholar] [CrossRef]
- Chen, T.; Yan, Z.-A.; Xu, D.; Wang, M.; Huang, J.; Yan, B.; Xiao, X.; Ning, X. Current situation and forecast of environmental risks of a typical lead-zinc sulfide tailings impoundment based on its geochemical characteristics. J. Environ. Sci. 2020, 93, 120–128. [Google Scholar] [CrossRef]
- Chen, P.; Sun, F.; Wang, W.; Tan, F.; Wang, X.; Qiao, X. Facile one-pot fabrication of ZnO2 particles for the efficient Fenton-like degradation of tetracycline. J. Alloys Compd. 2020, 834, 155220. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Lin, Y.-C.; Ho, S.-H.; Zhou, Y.; Ren, N.-Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Zhu, G.; Zhou, H.; Yuan, A. Reduced graphene oxide/nickel nanocomposites: Facile synthesis, magnetic and catalytic properties. J. Mater. Chem. 2012, 22, 3471–3477. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
- Di, J.; Jamakanga, R.; Chen, Q.; Li, J.; Gai, X.; Li, Y.; Yang, R.; Ma, Q. Degradation of Rhodamine B by activation of peroxymonosulfate using Co3O4-rice husk ash composites. Sci. Total Environ. 2021, 784, 147258. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tang, J.; Zhang, Z.; Wang, L.; Liu, Q.; Liu, X. Magnetic ball-milled FeS@biochar as persulfate activator for degradation of tetracycline. Chem. Eng. J. 2021, 404, 126997. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-D.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Guan, R.; Yuan, X.; Wu, Z.; Wang, H.; Jiang, L.; Zhang, J.; Li, Y.; Zeng, G.; Mo, D. Accelerated tetracycline degradation by persulfate activated with heterogeneous magnetic NixFe3−xO4 catalysts. Chem. Eng. J. 2018, 350, 573–584. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Z.; Qiu, L.; Chen, Z.; Xiao, X.; Mo, X.; Cui, L. Recycling of Fenton sludge containing Ni as an efficient catalyst for tetracycline degradation through peroxymonosulfate activation. J. Clean. Prod. 2020, 268, 122174. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Zuh, A.A.; Jia, Y.; Ding, F.; Cheng, H.; Wang, Q. Photo-Fenton reaction for the degradation of tetracycline hydrochloride using a FeWO4/BiOCl nanocomposite. J. Alloys Compd. 2022, 903, 163889. [Google Scholar] [CrossRef]
- Sun, R.; Yang, J.; Huang, R.; Wang, C. Controlled carbonization of microplastics loaded nano zero-valent iron for catalytic degradation of tetracycline. Chemosphere 2022, 303, 135123. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Liu, Y.; Fang, T.; Zhang, L.; Xing, B. Insight into the significant contribution of intrinsic defects of carbon-based materials for the efficient removal of tetracycline antibiotics. Chem. Eng. J. 2022, 435, 134822. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Yang, E.; Li, H.; Wu, S.; Yang, W.; Wang, H. Magnetic Fe3O4–N-doped carbon sphere composite for tetracycline degradation by enhancing catalytic activity for peroxymonosulfate: A dominant non-radical mechanism. Chemosphere 2021, 263, 128011. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, W.; You, L.; Li, J.; Chen, J.; Zhou, K. Simultaneous reduction of Cr(VI) and degradation of tetracycline hydrochloride by a novel iron-modified rectorite composite through heterogeneous photo-Fenton processes. Chem. Eng. J. 2020, 393, 124758. [Google Scholar] [CrossRef]
- Zhu, K.; Xu, H.; Chen, C.; Ren, X.; Alsaedi, A.; Hayat, T. Encapsulation of Fe0-dominated Fe3O4/Fe0/Fe3C nanoparticles into carbonized polydopamine nanospheres for catalytic degradation of tetracycline via persulfate activation. Chem. Eng. J. 2019, 372, 304–311. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Jia, Y.; Lian, X.; Zhang, Y.; Feng, F.; Liu, Q.; Xi, B.; Jiang, Y. Heterogeneous catalytic degradation of 2,4-dinitrotoluene by the combined persulfate and hydrogen peroxide activated by the as-synthesized Fe-Mn binary oxides. Chem. Eng. J. 2019, 374, 776–786. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wen, X.; Jiang, S.; Chen, T. Activation of Persulfate for Degrading Tetracycline Using the Leaching Residues of the Lead-Zinc Flotation Tailing. Polymers 2022, 14, 2959. https://doi.org/10.3390/polym14142959
Wang J, Wen X, Jiang S, Chen T. Activation of Persulfate for Degrading Tetracycline Using the Leaching Residues of the Lead-Zinc Flotation Tailing. Polymers. 2022; 14(14):2959. https://doi.org/10.3390/polym14142959
Chicago/Turabian StyleWang, Jun, Xiaocui Wen, Shaojun Jiang, and Tao Chen. 2022. "Activation of Persulfate for Degrading Tetracycline Using the Leaching Residues of the Lead-Zinc Flotation Tailing" Polymers 14, no. 14: 2959. https://doi.org/10.3390/polym14142959