Properties and Fabrication of Waterborne Polyurethane Superhydrophobic Conductive Composites with Coupling Agent-Modified Fillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of the WPU Dispersions with Modified Fillers
2.3. Preparation of the Coatings
2.4. Characterization and Test
2.4.1. Electrical Conductivity
2.4.2. Hydrophobicity Test
2.4.3. Adhesion Test
2.4.4. Anticorrosion Property Test
3. Results and Discussion
3.1. Electrical Conductivity
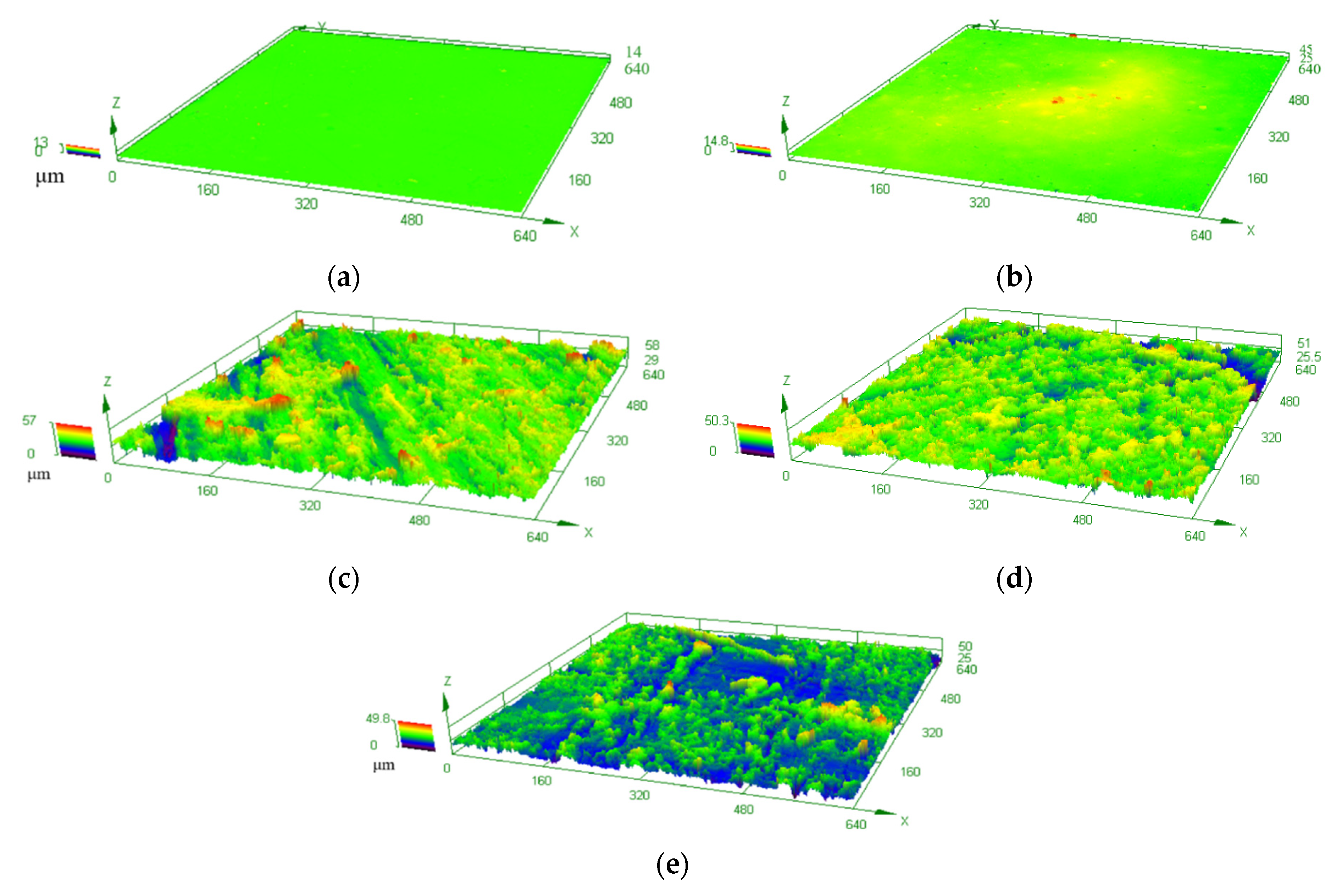
3.2. Hydrophobicity
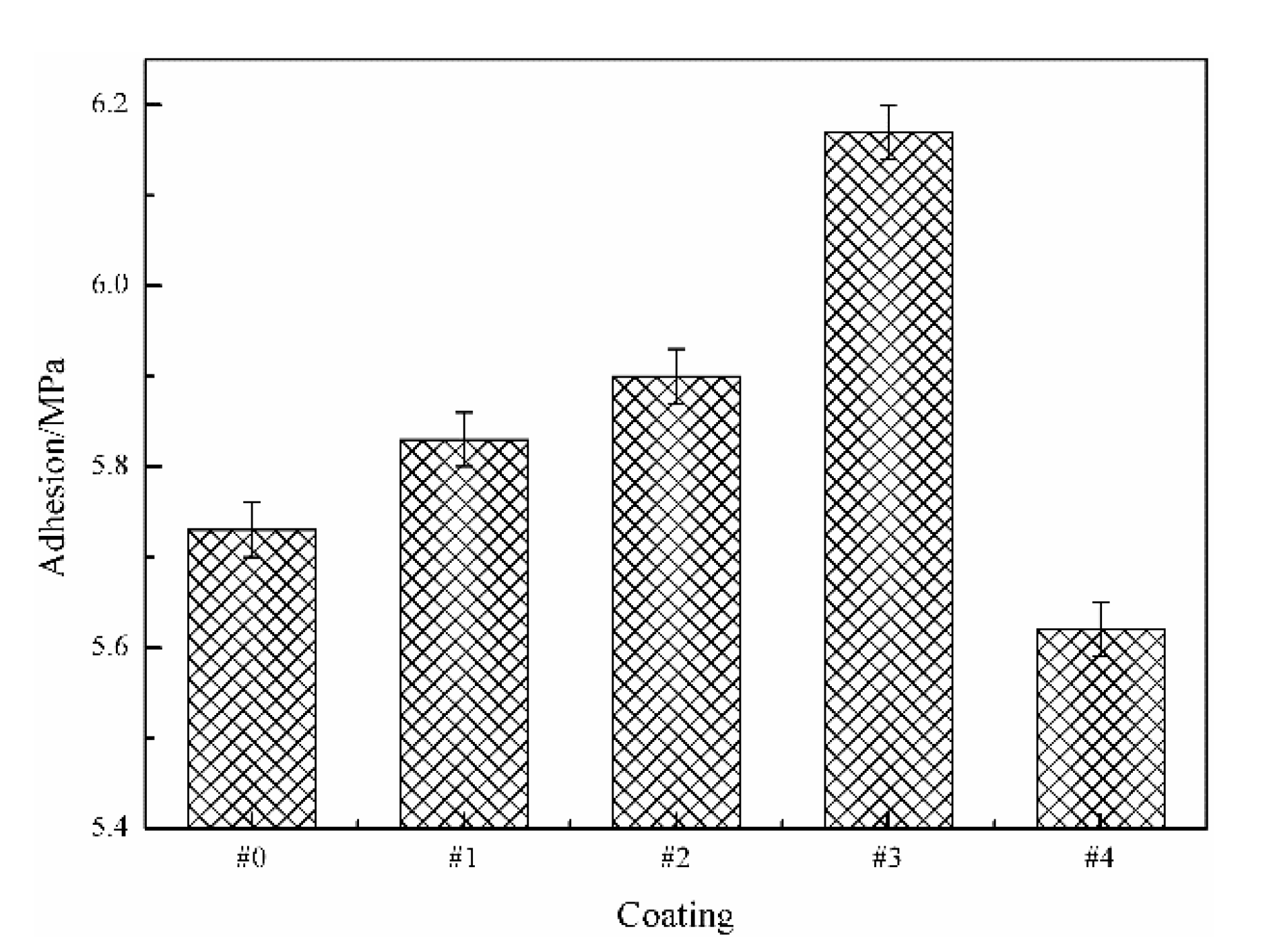
3.3. Adhesion to the Steel Substrate
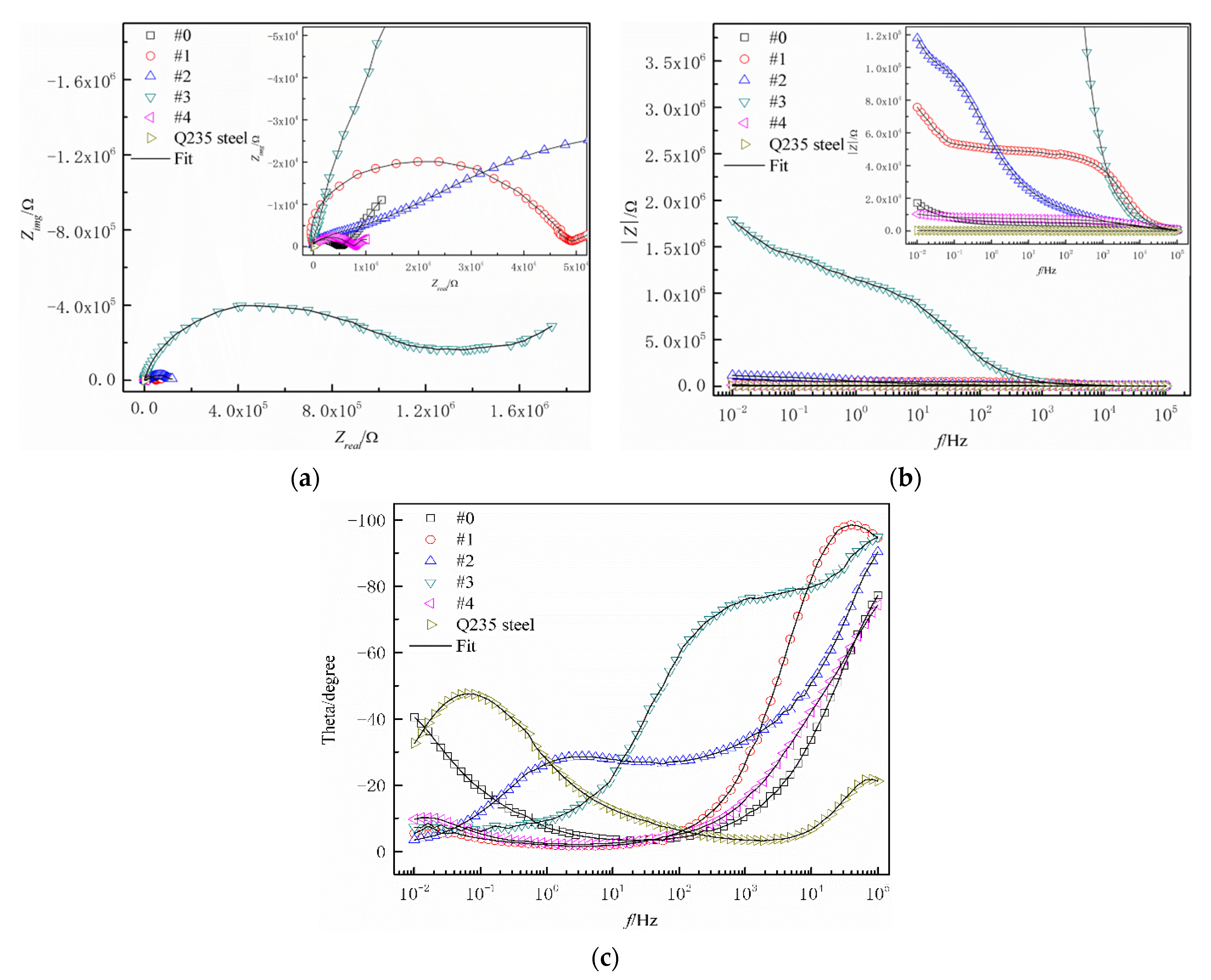
3.4. Anticorrosive Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Zhou, Y.; Dou, Z.; Ding, L.; Dong, S.; Liu, N.; Qin, Z. Composite nanofibers by coating polypyrrole on the surface of polyaniline nanofibers formed in presence of phenylenediamine as electrode materials in neutral electrolyte. Electrochim. Acta 2017, 243, 228–238. [Google Scholar] [CrossRef]
- Li, J.; Ge, S.; Wang, J.; Du, H.; Song, K.; Fei, Z.; Shao, Q.; Guo, Z. Water-based rust converter and its polymer composites for surface anticorrosion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 334–342. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Sun, Q.; Shi, B. Enhanced mechanical property, chemical resistance and abrasion durability of waterborne polyurethane based coating by incorporating highly dispersed polyacrylic acid modified graphene oxide. Prog. Org. Coat. 2022, 170, 106949. [Google Scholar] [CrossRef]
- Cai, J.; Murugadoss, V.; Jiang, J.; Gao, X.; Lin, Z.; Huang, M.; Guo, J.; Alsareii, S.; Algadi, H.; Kathiresan, M. Waterborne polyurethane and its nanocomposites: A mini-review for anti-corrosion coating, flame retardancy, and biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 641–650. [Google Scholar] [CrossRef]
- Deng, H.; Xie, F.; Shi, H.; Li, Y.; Liu, S.; Zhang, C. UV Resistance, Anticorrosion and High Toughness Bio-based Waterborne Polyurethane enabled by a Sorbitan Monooleate. Chem. Eng. J. 2022, 446, 137124. [Google Scholar] [CrossRef]
- You, K.; Park, S.; Lee, C.; Kim, J.; Park, G.; Kim, W. Preparation and characterization of conductive carbon nanotube-polyurethane foam composites. J. Mater. Sci. 2011, 46, 6850–6855. [Google Scholar] [CrossRef]
- Hajializadeh, S.; Barikani, M.; Bellah, S. Synthesis and characterization of multiwall carbon nanotube/waterborne polyurethane nanocomposites. Polym. Int. 2017, 66, 1074–1083. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Qiang, F.; Hua, L.; Gong, L.; Zhao, L.; Li, S.; Tang, L. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbons/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Sethi, J.; Sarlin, E.; Meysami, S.; Suihkonen, R.; Kumar, A.; Honkanen, M.; Keinänen, P.; Grobert, N.; Vuorinen, J. The effect of multi-wall carbon nanotube morphology on electrical and mechanical properties of polyurethane nanocomposites. Compos. Part A-Appl. Sci. Manuf. 2017, 102, 305–313. [Google Scholar] [CrossRef]
- Koti Reddy, C.; Shailaja, D. Improving hydrophobicity of polyurethane by PTFE incorporation. J. Appl. Polym. Sci. 2015, 132, 42779. [Google Scholar] [CrossRef]
- Yu, Z.; Du, X.; Zhu, P.; Zhao, T.; Sun, R.; Chen, J.; Wang, N.; Li, W. Surface modified hollow glass microspheres-epoxy composites with enhanced thermal insulation and reduced dielectric constant. Mater. Today Commun. 2022, 32, 104046. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.; Dudley, K.; Lawrence, R. Novel carbon nanotube-polystyrene foam composites for electromagnetic interference shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, W.; Cheng, H.; Fu, S. Pressure-proof superhydrophobic films from flexible carbon nanotube/polymer coatings. J. Phys. Chem. C 2010, 114, 15607–15611. [Google Scholar] [CrossRef]
- Wang, F.; Feng, L.; Huang, Y.; Shen, W.; Ma, H. Effect of the gradient distribution of multiwalled carbon nanotubes on the bond strength and corrosion resistance of waterborne polyurethane conductive nanocomposite coatings. Prog. Org. Coat. 2020, 140, 105507. [Google Scholar] [CrossRef]
- Wang, F.; Feng, L.; Lu, M. Mechanical properties of multi-walled carbon nanotube/waterborne polyurethane conductive coatings prepared by electrostatic spraying. Polymers 2019, 11, 714. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Feng, L.; Liu, X.; Luo, H.; Liu, Z.; Tong, P.; Zhang, W. Multiwall carbon nanotubes-reinforced epoxy hybrid coatings with high electrical conductivity and corrosion resistance prepared via electrostatic spraying. Prog. Org. Coat. 2016, 90, 139–146. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.; Dudley, K.; Lawrence, R. The fabrication and electrical properties of carbon nanofiber-polystyrene composites. Nanotechnology 2004, 15, 1545–1548. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, W.; Zhang, L.; Yao, J.; Xu, W.; Wang, X.; Wu, Y. Fabrication of superhydrophobic and conductive surface based on carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2013, 423, 69–76. [Google Scholar] [CrossRef]
- Bayer, I.; Steele, A.; Loth, E. Superhydrophobic and electroconductive carbon nanotube-fluorinated acrylic copolymer nanocomposites from emulsions. Chem. Eng. J. 2013, 221, 522–530. [Google Scholar] [CrossRef]
- Talaeemashhadi, S.; Sansotera, M.; Gambarotti, C.; Famulari, A.; Bianchi, C.; Guarda, P.; Navarrini, W. Functionalization of multi-walled carbon nanotubes with perfluoropolyether peroxide to produce superhydrophobic properties. Carbon 2013, 59, 150–159. [Google Scholar] [CrossRef]
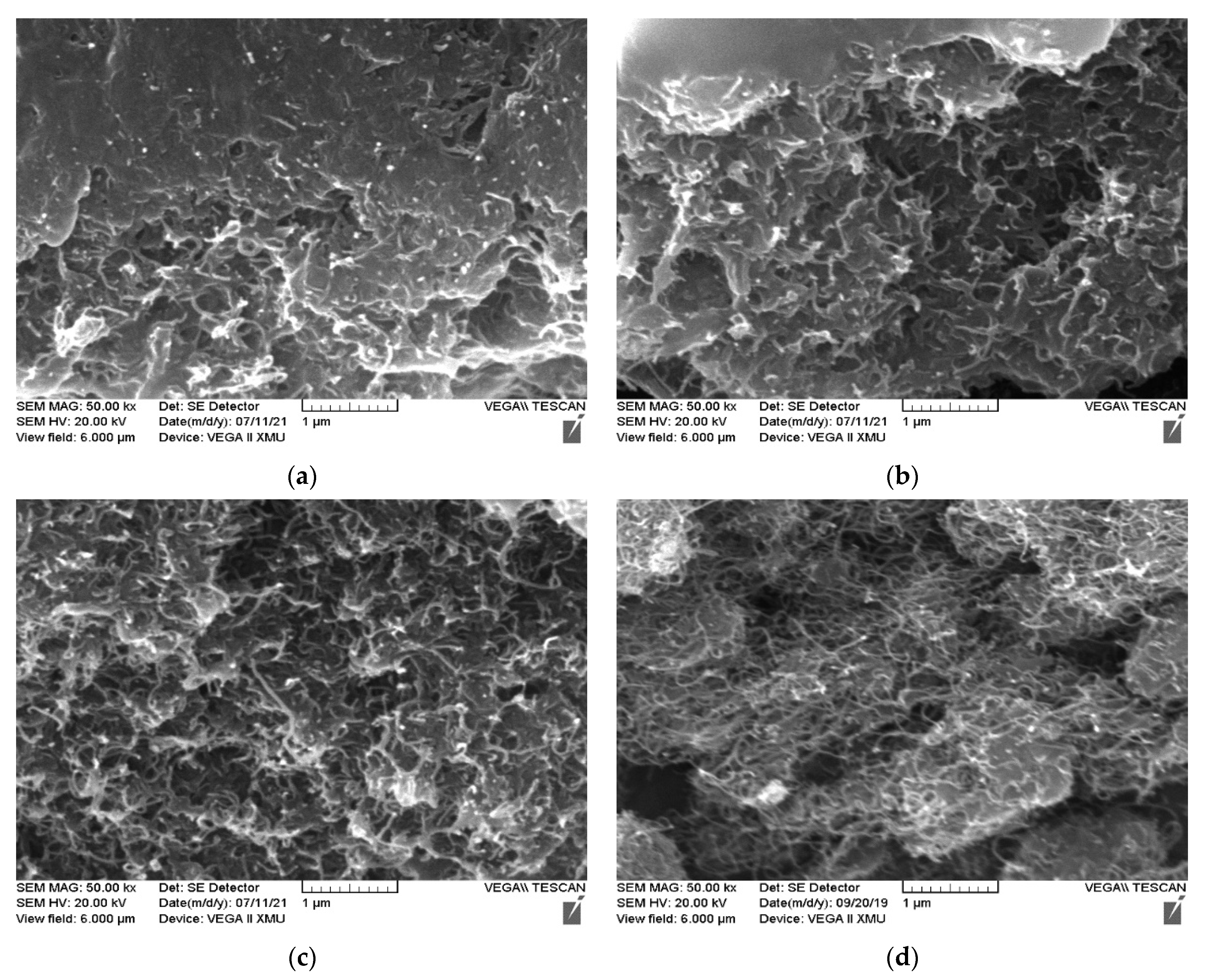

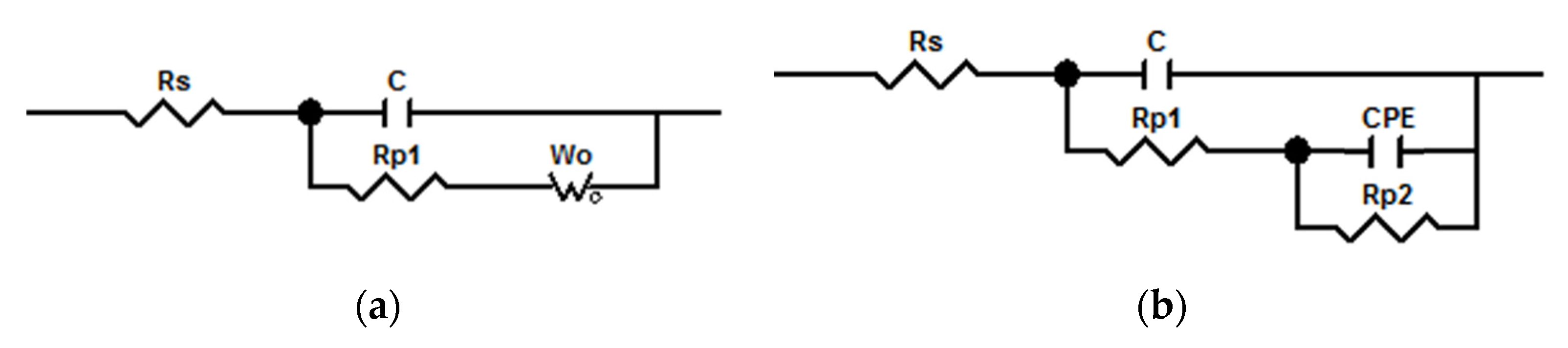

| Dispersions | #1 | #2 | #3 | #4 |
|---|---|---|---|---|
| WPU/g | 10 | 10 | 7 | 7 |
| PTFE/g | 1.5 | 2 | 1.5 | 2 |
| MWCNTs/g | 0.05 | 0.1 | 0.1 | 0.15 |
| KH-550/g | 0.031 | 0.042 | 0.032 | 0.043 |
| Process Parameters | Spray Voltage (kV) | Compressed Air Pressure (MPa) | Feedwell Diameter (mm) | Liquid Flow Rate (mL·min−1) | Spray Distance (mm) | Spray Time (min) |
|---|---|---|---|---|---|---|
| 50~60 | 0.6~0.7 | 1 | 2 | 100~120 | 1~2 |
| Composites | #1 | #2 | #3 | #4 |
|---|---|---|---|---|
| Surface resistances (Ω) | (7.2 ± 0.5) × 106 | (4.2 ± 0.5) × 106 | (1.8 ± 0.5) × 106 | (1.1 ± 0.5) × 106 |
| Volume resistivities (Ω·cm) | (6.1 ± 0.5) × 104 | (3.5 ± 0.5) × 104 | (1.5 ± 0.5) × 104 | (9.3 ± 0.5) × 103 |
| Hardness (μm) | 85.0 ± 4 | 84.5 ± 4 | 85.0 ± 4 | 84.5 ± 4 |
| Coatings | #0 | #1 | #2 | #3 | #4 |
|---|---|---|---|---|---|
| Average surface roughness (μm) | 0.137 | 0.160 | 3.113 | 3.339 | 3.674 |
| Samples | #0 | #1 | #2 | #3 | #4 | Q235 Steel |
|---|---|---|---|---|---|---|
| I/(A·cm−2) | 1.3945 × 10−6 | 3.2091 × 10−7 | 4.8596 × 10−7 | 2.6172 × 10−9 | 1.0848 × 10−6 | 9.3593 × 10−5 |
| EvsSCE/V | −0.6529 | −0.4618 | −0.2950 | −0.0192 | −0.3720 | −0.6808 |
| Corrosion rates/(mm·a−1) | 0.01644 | 0.00378 | 0.00573 | 0.00003 | 0.01279 | 1.10370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Ci, J.; Fan, J. Properties and Fabrication of Waterborne Polyurethane Superhydrophobic Conductive Composites with Coupling Agent-Modified Fillers. Polymers 2022, 14, 3093. https://doi.org/10.3390/polym14153093
Wang F, Ci J, Fan J. Properties and Fabrication of Waterborne Polyurethane Superhydrophobic Conductive Composites with Coupling Agent-Modified Fillers. Polymers. 2022; 14(15):3093. https://doi.org/10.3390/polym14153093
Chicago/Turabian StyleWang, Fangfang, Jihao Ci, and Jiang Fan. 2022. "Properties and Fabrication of Waterborne Polyurethane Superhydrophobic Conductive Composites with Coupling Agent-Modified Fillers" Polymers 14, no. 15: 3093. https://doi.org/10.3390/polym14153093
APA StyleWang, F., Ci, J., & Fan, J. (2022). Properties and Fabrication of Waterborne Polyurethane Superhydrophobic Conductive Composites with Coupling Agent-Modified Fillers. Polymers, 14(15), 3093. https://doi.org/10.3390/polym14153093





