Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
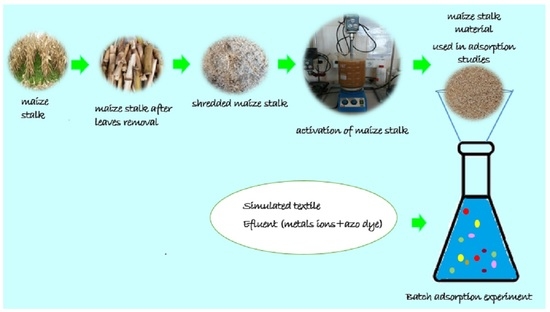
2.3. Procedure for Obtained Shredded Maize Stalk Acid Treatment of MS
2.4. Metal Ions Used in Adsorption Studies
2.5. Validation Parameters of AAS Method
2.6. Preparation of Simulated Textile Matrices for Adsorption Studies
2.7. Kinetic of Adsorption Experiments
2.8. Batch Adsorption Experiments
2.9. Adsorption Experiments
2.10. Procedures for MX+ from Tannery Wastewater onto MS
2.11. Procedures for Desorption Experiments
2.12. Characterization of Solid phases by TG and SEM
3. Results and Discussion
3.1. Effect of Contact Time
3.2. Effect of Initial Concentration
3.3. Kinetic Studies
3.4. Adsorption Studies
3.5. Applications of Maize Stalk in Tannery Wastewater Treatment
3.6. Characterization of MS before and after Adsorption
3.6.1. Thermal Analysis
3.6.2. SEM Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desta, M.B. Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J. Thermodyn. 2013, 64–71, 375830. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, M.W.; Gonçalves, S.; de Oliveira, E.C.; Rubert, S.; de Castilhos Ghisi, N. Textile effluent toxicity trend: A scientometric review. J. Cleaner Prod. 2022, 366, 132756. [Google Scholar] [CrossRef]
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.; Đolić, M. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in) organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Othmani, A.; Magdouli, S.; Senthil Kumar, P.; Kapoor, A.; Chellam, P.V.; Gökkuş, Ö. Agricultural waste materials for adsorptive removal of phenols, chromium (VI) and cadmium (II) from wastewater: A review. Environ. Res. 2022, 204, 111916. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Soans, J.C.; Rachitha, R.; Shilpalekha, M.; Gowda, S.G.S.; Juvvi, P.; Chakka, A.K. Green technology approach for heavy metal adsorption by agricultural and food industry solid wastes as bio-adsorbents: A review. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Imran-Shaukat, M.; Wahi, R.; Ngaini, Z. The application of agricultural wastes for heavy metals adsorption: A meta-analysis of recent studies. Bioresour. Technol. Rep. 2022, 17, 100902. [Google Scholar] [CrossRef]
- Vijayaraghavan, J.; Thivya, J. Removal of Ni (II) Ions from Wastewater by Raw and Modified Plant Wastes as Adsorbents: A Review. Iran. J. Chem. Chem. Eng. 2022, 41, 174–206. [Google Scholar]
- Bayuo, J.; Rwiza, M.; Mtei, K. A comprehensive review on the decontamination of lead (II) from water and wastewater by low-cost biosorbents. RSC Adv. 2022, 12, 11233–11254. [Google Scholar] [CrossRef]
- Figueira, P.; Henriques, B.; Teixeira, F.; Afonso, N.; Pinto, J.; Tavares, D.; Vale, C.; Pereira, E. Potentialities of agro-based wastes to remove Cd, Hg, Pb, and As from contaminated waters. Water Air Soil Pollut. 2022, 233, 1–17. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, P.; Kumar, P.; Rajoriya, S.; Pathak, P.; Singh, S.R.; Kumar, S.; Singh, L. Comprehensive technological assessment for different treatment methods of leather tannery wastewater. Environ. Sci. Pollut. Res. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Madeła, M.; Skuza, M. Towards a Circular Economy: Analysis of the Use of Biowaste as Biosorbent for the Removal of Heavy Metals. Energies 2021, 14, 5427. [Google Scholar] [CrossRef]
- Santhosh, C.; Daneshvar, E.; Tripathi, K.M.; Baltrėnas, P.; Kim, T.; Baltrėnaitė, E.; Bhatnagar, A. Synthesis and characterization of magnetic biochar adsorbents for the removal of Cr (VI) and Acid orange 7 dye from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 32874–32887. [Google Scholar] [CrossRef]
- Vafakhah, S.; Bahrololoom, M.; Bazarganlari, R.; Saeedikhani, M. Removal of copper ions from electroplating effluent solutions with native corn cob and corn stalk and chemically modified corn stalk. J. Environ. Chem. Eng. 2014, 2, 356–361. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D. Enhancing biosorption characteristics of marine green algae (Ulva lactuca) for heavy metals removal by alkaline treatment. J. Bioprocess. Biotech. 2014, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Periodic Table of the Elements Characterization. Available online: https://chemglobe.org/ptoe/_/29.php (accessed on 1 July 2022).
- Marin, N.M. Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers 2022, 14, 2139. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Batrinescu, G.; Stanculescu, I.; Constantin, L.A.; Cristea, I.; Ionescu, I.; Catrina, G.A. Experimental Model for Cu (II) and Fe (III) Sorption from Synthetic Solutions Based on Maize Stalk. Rev. Chim. 2020, 71, 366–367. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Marin, N.M.; Pascu, L.F.; Stanculescu, I.; Iordache, O.; Jianu, D.; Petrescu, L.; Badea, I.A. Maize stalk as natural ion exchanger for hazardous pollutants. Rev. Chim. 2017, 68, 1726–1731. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Application of amberlite IRA 402 resin adsorption and laccase treatment for acid blue 113 removal from aqueous media. Polymers 2021, 13, 3991. [Google Scholar] [CrossRef]
- Shvartseva, O.; Skripkina, T.; Gaskova, O.; Podgorbunskikh, E. Modification of Natural Peat for Removal of Copper Ions from Aqueous Solutions. Water 2022, 14, 2114. [Google Scholar] [CrossRef]
- Shan, S.; Sun, X.F.; Xie, Y.; Li, W.; Ji, T. High-Performance Hydrogel Adsorbent Based on Cellulose, Hemicellulose, and Lignin for Copper(II) Ion Removal. Polymers 2021, 13, 3063. [Google Scholar] [CrossRef] [PubMed]
- Călinescu, O.; Marin, N.M.; Ioniță, D.; Pascu, L.F.; Tudorache, A.; Surpățeanue, G.; Badea, I.A.; Aboul-Enein, H.Y. Selective removal of sulfate ion from different drinking waters. Environ. Nanotechnol. Monit. Manag. 2016, 6, 164–168. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent advances in applications of hybrid graphene materials for metals removal from wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.; Ashour, M. Dried brown seaweed’s phytoremediation potential for methylene blue dye removal from aquatic environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef]
- Kokate, S.; Parasuraman, K.; Prakash, H. Adsorptive removal of lead ion from water using banana stem scutcher generated in fiber extraction process. Res. Eng. 2022, 14, 100439. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of lead on cucumber peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Duru, C.; Duru, I.; Ogbonna, C.; Enedoh, M.; Emele, P. Adsorption of copper ions from aqueous solution onto natural and pretreated maize husk: Adsorption efficiency and kinetic studies. J. Niger. Soc. Chem. Eng. 2019, 44. [Google Scholar]
- Pandey, R.; Ansari, N.G.; Prasad, R.L.; Murthy, R.C. Removal of Cd(II) ions from simulated wastewater by HCl modified Cucumis sativus peel: Equilibrium and kinetic study. Air Soil Water Res. 2014, 7, ASWR-S16488. [Google Scholar] [CrossRef]
- Esfandian, H.; Javadian, H.; Parvini, M.; Khoshandam, B.; Katal, R. Batch and column removal of copper by modified brown algae sargassum bevanom from aqueous solution. Asia-Pac. J. Chem. Eng. 2013, 8, 665–678. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ.-Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Ho, Y.S.; John Wase, D.A.; Forster, C.F. Batch nickel removal from aqueous solution by sphagnum moss peat. Water Res. 1995, 29, 1327–1332. [Google Scholar] [CrossRef]
- Kalantar, Z.; Ghanavati Nasab, S. Modeling and optimizing Cd(II) ions adsorption onto Corn Silk/Zeolite-Y composite from industrial effluents applying response surface methodology: Isotherm, kinetic, and reusability studies. J. Iran. Chem. Soc. 2022. [Google Scholar] [CrossRef]
- Islam, I.U.; Ahmad, M.; Ahmad, M.; Rukh, S.; Ullah, I. Kinetic studies and adsorptive removal of chromium Cr (VI) from contaminated water using green adsorbent prepared from agricultural waste, rice straw. Eur. J. Chem. 2022, 13, 78–90. [Google Scholar] [CrossRef]
- Gao, W.; Roger Razanajatovo, M.; Song, Y.; Zhao, X.; Zhao, Z.; Peng, Q.; Jiao, T.; Liu, X.; Zhang, Q. Efficient heavy metal sequestration from water by Mussel-inspired polystyrene conjugated with polyethyleneimine (PEI). Chem. Eng. J. 2022, 429, 132599. [Google Scholar] [CrossRef]
- Levio-Raiman, M.; Briceño, G.; Schalchli, H.; Bornhardt, C.; Diez, M.C. Alternative treatment for metal ions removal from acid mine drainage using an organic biomixture as a low cost adsorbent. Environ. Technol. Innov. 2021, 24, 101853. [Google Scholar]
- Marin, N.M.; Dinu, L.; Stanculescu, I.; Cristea, N.I.; Ionescu, A.I. Maize stalk material for on-site treatment of highly polluted leachate and mine wastewater. Materials 2021, 14, 956. [Google Scholar] [CrossRef] [PubMed]
- Ház, A.; Jablonský, M.; Šurina, I.; Kačík, F.; Bubeníková, T.; Ďurkovič, J. Chemical composition and thermal behavior of kraft lignins. Forests 2019, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Bu, Q.; Lei, H.; Qian, M.; Yadavalli, G. A thermal behavior and kinetics study of the catalytic pyrolysis of lignin. RSC Adv. 2016, 6, 100700–100707. [Google Scholar] [CrossRef]
- Cortés, A.M.; Bridgwater, A. Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process. Technol. 2015, 138, 184–193. [Google Scholar] [CrossRef] [Green Version]










| Metal | Copper | Lead | Nickel | Iron | Chromium | Cadmium |
|---|---|---|---|---|---|---|
| Atomic number | 29 | 82 | 28 | 26 | 25 | 48 |
| Atomic mass (g/mol) | 63.5 | 207.2 | 58.7 | 55.8 | 51.9 | 121.4 |
| Oxidation states | +2, +1 | +2, +4 | +2, +3 | +3, +2 | +6, +3 | +2 |
| Atomic radius (Å) | 1.57 | 1.81 | 1.62 | 1.72 | 1.85 | 1.71 |
| Ionic radius (Å) | 0.73 (+2) | 1.19 (+2) | 0.69 (+2) | 0.55 (+3) | 0.62 (+3) | 0.95 (+2) |
| MX+ | λ (nm) | Calibration Curves | R2 | LOQ (µg/L) |
|---|---|---|---|---|
| Cu2+ | 232.75 | y = 0.1376x + 0.0012 | 0.9999 | 3.5 |
| Pb2+ | 283.31 | y = 0.0203x − 0.0015 | 0.999 | 4 |
| Ni2+ | 232 | y = 0.0753x − 0.0042 | 0.9998 | 2.7 |
| Fe3+ | 248.33 | y = 0.0772x − 0.0016 | 0.9995 | 6.5 |
| Cr3+ | 358.87 | y = 0.0181x − 0.00009 | 0.9991 | 2.3 |
| Cd2+ | 228.8 | y = 0.4324x − 0.002 | 0.9998 | 3.1 |
| Pseudo-First-Order Model | ||||||
|---|---|---|---|---|---|---|
| Metal Ion | Cu2+ | Pb2+ | Ni2+ | Cd2+ | Cr3+ | Fe3+ |
| Qe (mg/g) | 17.140 | 44.460 | 24.333 | 18.828 | 14.870 | 16.508 |
| k1 (min−1) | 0.021 | 0.014 | 0.024 | 0.017 | 0.019 | 0.022 |
| R2 | 0.9855 | 0.9454 | 0.9769 | 0.9430 | 0.9628 | 0.9333 |
| Pseudo-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|
| Metal Ion | Cu2+ | Pb2+ | Ni2+ | Cd2+ | Cr3+ | Fe3+ |
| Qe (mg/g) | 0.096 | 0.040 | 0.060 | 0.570 | 0.230 | 0.200 |
| k2 (g/mg min) | 0.051 | 0.320 | 0.210 | 0.002 | 0.015 | 0.021 |
| R2 | 0.2915 | 0.7411 | 0.9390 | 0.2000 | 0.2769 | 0.2484 |
| Langmuir Isotherm Model | ||||||
|---|---|---|---|---|---|---|
| Metal Ion | Cu2+ | Pb2+ | Ni2+ | Cd2+ | Cr3+ | Fe3+ |
| R2 | 0.9712 | 0.9828 | 0.9376 | 0.9992 | 0.9998 | 0.9985 |
| Qm (mg/g) | 1.258 | 0.085 | 0.526 | 0.346 | 8.058 | 4.405 |
| b | 0.048 | 0.484 | 0.102 | 0.240 | 0.008 | 0.013 |
| RL | 0.9970 | 0.9890 | 0.9960 | 0.9880 | 0.9992 | 0.9990 |
| Freundlich Isotherm Model | ||||||
|---|---|---|---|---|---|---|
| Metal ion | Cu2+ | Pb2+ | Ni2+ | Cd2+ | Cr3+ | Fe3+ |
| R2 | 0.6065 | 0.9800 | 0.6283 | 0.9962 | 0.944 | 0.7934 |
| Kf (mg/g) | 1.3 | 33.9 | 16.7 | 11.7 | 11.6 | 14.3 |
| 1/n | 2.60 | 0.51 | 0.33 | 0.52 | 0.16 | 0.15 |
| n | 0.40 | 1.95 | 3.00 | 1.93 | 6.16 | 6.78 |
| Thermal Behavior of Maize Stalk | |||
|---|---|---|---|
| Before Adsorption | After Adsorption | ||
| T °C | Weight (%) | T °C | Weight (%) |
| Ti–35 | –* | Ti–35 | 0.03 |
| 35–130 | 5.5 | 35–130 | 4.0 |
| 130–350 | 61.2 | 130–350 | 60.4 |
| 350–520 | 27.4 | 350–540 | 31.9 |
| Ti–1200 | 94.2 ** | Ti–1200 | 96.3 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, N.M. Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+. Polymers 2022, 14, 3141. https://doi.org/10.3390/polym14153141
Marin NM. Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+. Polymers. 2022; 14(15):3141. https://doi.org/10.3390/polym14153141
Chicago/Turabian StyleMarin, Nicoleta Mirela. 2022. "Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+" Polymers 14, no. 15: 3141. https://doi.org/10.3390/polym14153141
APA StyleMarin, N. M. (2022). Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+. Polymers, 14(15), 3141. https://doi.org/10.3390/polym14153141