Effects of Yam (Dioscorea rotundata) Mucilage on the Physical, Rheological and Stability Characteristics of Ice Cream
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Extraction of Yam Mucilage
2.2. Formulation and Preparation of Vanilla Flavored Ice Cream
2.3. Physicochemical Properties of Vanilla-Flavored Ice Cream
2.4. Rheological Behavior of Vanilla Flavored Ice Cream
2.5. Physical Stability of Vanilla Flavored Ice Cream
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Vanilla Flavored Ice Cream
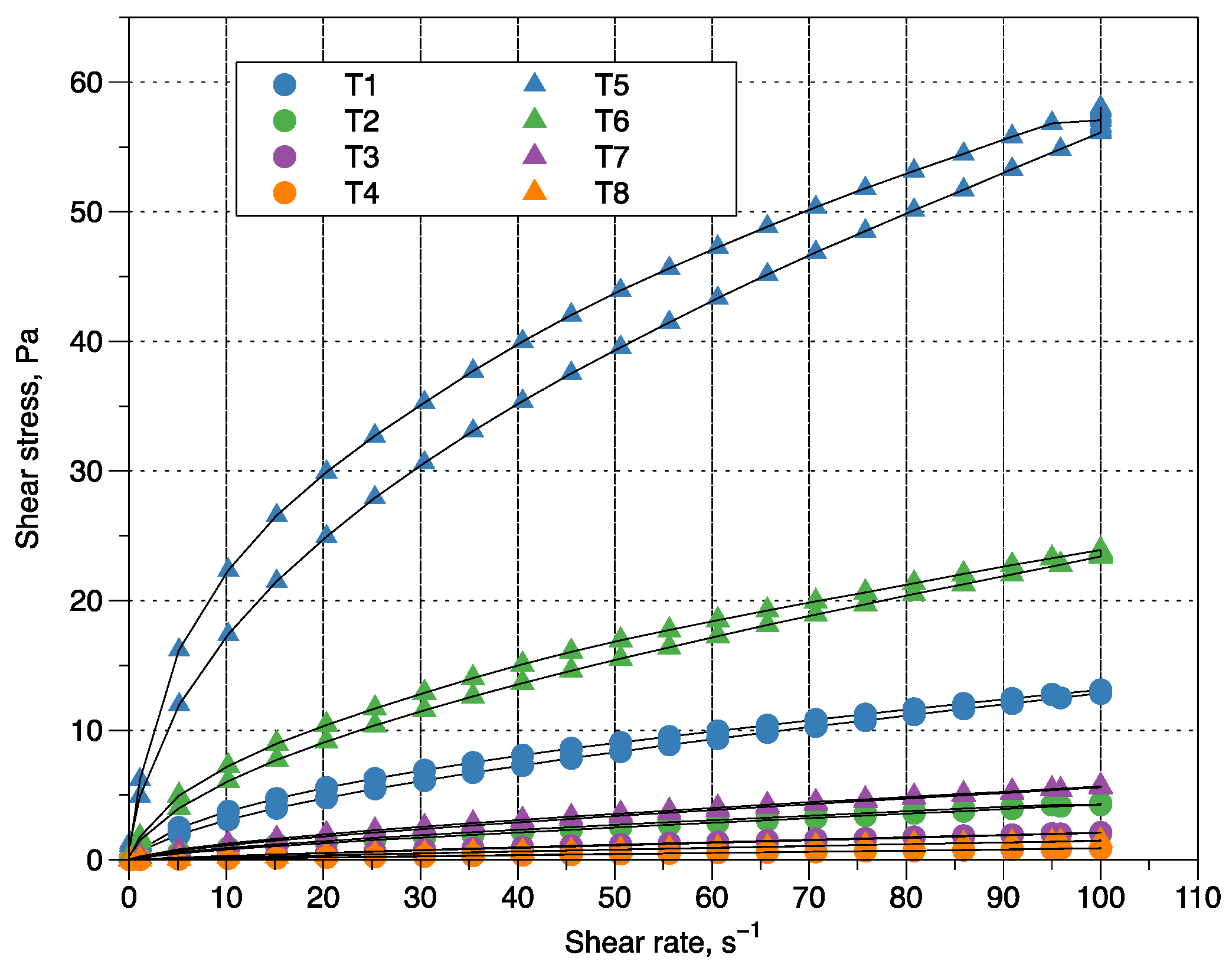
3.2. Rheological Behavior
3.3. Physical Stability of Ice Cream
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiol, C.; Prado, D.; Romero, C.; Laburu, N.; Mora, M.; Alava, J.I. Introduction of a new family of ice creams. Int. J. Gastron. Food Sci. 2017, 7, 5–10. [Google Scholar] [CrossRef]
- Balthazar, C.; Silva, H.; Vieira, A.; Neto, R.; Cappato, L.; Coimbra, P.; Freitas, M. Assessing the effects of different prebiotic dietary oligosaccharides in sheep milk ice cream. Food Res. Int. 2017, 91, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Bahram-Parvar, M. A review of modern instrumental techniques for measurements of ice cream characteristics. Food Chem. 2015, 188, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Fisk, I.; Bohn, T. Ice Cream as a Vehicle for incorporating health-promoting ingredients: Conceptualization and overview of quality and storage stability. Compr. Rev. Food Sci. Food Saf. 2014, 13, 627–655. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of milk protein-polysaccharide interactions on the stability of ice cream mix model systems. Food Hydrocoll. 2015, 45, 327–336. [Google Scholar] [CrossRef]
- Goff, H.; Hartel, R. Ice Cream; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Koocheki, A.; Taherian, A.; Bostan, A. Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Res. Int. 2013, 50, 446–456. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Ren, Z.; Särkkä-Tirkkonen, M.; Zhang, Y.; Zhao, D.; Liu, X. Effects of concentrations, temperature, pH and co-solutes on the rheological properties of mucilage from Dioscorea opposita Thunb. and its antioxidant activity. Food Chem. 2021, 360, 130022. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Yu, Y.; She, M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Yazdi, F.T.; Shahidi, F.; Hesarinejad, M.A.; Mortazavi, S.A.; Mohebbi, M. Plantago major seed mucilage: Optimization of extraction and some physicochemical and rheological aspects. Carbohydr. Polym. 2017, 155, 68–77. [Google Scholar] [CrossRef]
- Huang, C.; Lai, P.; Chen, I.; Liu, Y.; Wang, C. Effects of mucilage on the thermal and pasting properties of yam, taro, and sweet potato starches. LWT-Food Sci. Technol. 2010, 43, 849–855. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.; Yazdi, T.; Koocheki, A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int. J. Biol. Macromol. 2014, 66, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tavares, S.; Pereira, J.; Guerreiro, M.; Pimenta, C.; Pereira, L.; Missagia, S. Caracterização físico-química da mucilagem de inhame liofilizada. Ciência e Agrotec. 2011, 35, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Lai, L. Characterization of the mucilage extracted from the edible fronds of bird’s nest fern (Asplenium australasicum) with enzymatic. Food Hydrocoll. 2016, 53, 84–92. [Google Scholar] [CrossRef]
- Campos, B.; Ruivo, T.; da Silva, M.; Madrona, G.; Bergamasco, R. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Andrade, L.; Nunes, C.; Pereira, J. Relationship between the chemical components of taro rhizome mucilage and its emulsifying property. Food Chem. 2015, 178, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Feizi, R.; Goh, K.K.T.; Mutukumira, A.N. Effect of chia seed mucilage as stabiliser in ice cream. Int. Dairy J. 2021, 120, 105087. [Google Scholar] [CrossRef]
- Fu, Y.C.; Huang, P.Y.; Chu, C.J. Use of continuous bubble separation process for separating and recovering starch and mucilage from yam (Dioscorea pseudojaponica yamamoto). LWT-Food Sc. Technol. 2005, 38, 735–744. [Google Scholar] [CrossRef]
- BahramParvar, M.; Goff, H.D. Basil seed gum as a novel stabilizer for structure formation and reduction of ice recrystallization in ice cream. Dairy Sci. Technol. 2013, 93, 273–285. [Google Scholar] [CrossRef] [Green Version]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Capitani, M.; Corzo-Rios, L.; Chel-Guerrero, L.; Betancur-Ancona, D.; Nolasco, S.; Tomás, M. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J. Food Eng. 2015, 149, 70–77. [Google Scholar] [CrossRef]
- Chien, M.; Ling, N.; Aniza, Y. Modelling of rheological behaviour of soursop juice concentrates using shear rate temperature concentration superposition. J. Food Eng. 2013, 118, 380–386. [Google Scholar]
- Augusto, P.; Ibarz, A.; Cristianini, M. Effect of high pressure homogenization (HPH) on the rheological properties of tomato juice: Creep and recovery behaviours. Food Res. Int. 2013, 54, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Daw, E.; Hartel, R. Fat destabilization and melt-down of ice creams with increased protein content. Int. Dairy J. 2015, 43, 33–41. [Google Scholar] [CrossRef]
- Da Silva, P.; De Fátima Bezerra, M.; Dos Santos, K.; Correia, R. Potentially probiotic ice cream from goat’s milk: Characterization and cell viability during processing, storage and simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2015, 62, 452–457. [Google Scholar] [CrossRef]
- Marshall, R.; Goff, H.D. Ice Cream; Publishers KAP: New York, NY, USA, 2003; p. 371. [Google Scholar]
- Kurt, A.; Atalar, I. Effects of quince seed on the rheological, structural and sensory characteristics of ice cream. Food Hydrocoll. 2018, 82, 186–195. [Google Scholar] [CrossRef]
- Posada, L.R.; Sepulveda, J.U.; Restrepo, D.A. Selection and evaluation of a stabilizer composed of rubbers on quality properties in mixtures for hard ice cream. Vitae 2012, 19, 166–177. [Google Scholar]
- Kavaz Yüksel, A.; Yüksel, M. Determination of certain microbiological quality characteristics of ice cream, detection of salmonella by conventional and immunomagnetic separation methods and antibiotic susceptibility of Salmonella spp. Isolates. J. Saf. 2015, 35, 385–394. [Google Scholar]
- He, W.; Zhao, W.; Yang, R. Effects of wheat gluten modified by deamidation-heating with three different acids on the microstructure of model oil-in-water emulsion and rheological–physical property of ice cream. Food Hydrocoll. 2019, 87, 679–690. [Google Scholar] [CrossRef]
- Akalin, A.S.; Kesenkas, H.; Dinkci, N.; Unal, G.; Kınık, O. Enrichment of probiotic ice cream with different dietary fibers: Structural characteristics and culture viability. J. Dairy Sci. 2018, 101, 37–46. [Google Scholar] [CrossRef]
- Góral, M.; Kozłowicz, K.; Pankiewicz, U.; Góral, D.; Kluza, F.; Wójtowicz, A. Impact of stabilizers on the freezing process, and physicochemical and organoleptic properties of coconut milk-based ice cream. LWT-Food Sci. Technol. 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Kaleda, A.; Tsanev, R.; Klesment, T.; Vilu, R.; Laos, K. Ice cream structure modification by ice-binding proteins. Food Chem. 2018, 246, 164–171. [Google Scholar] [CrossRef]
- Dertli, E.; Toker, O.; Durak, M.; Yilmaz, M.; Tatlısu, N.; Sagdic, O.; Cankurt, H. Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: Rheological, molecular, microstructural and sensory characterization. Carbohydr. Polym. 2016, 136, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Javidi, F.; Razavi, S.; Behrouzian, F.; Alghooneh, A. The influence of basil seed gum, guar gum and their blend on the rheological, physical and sensory properties of low fat ice cream. Food Hydrocoll. 2016, 52, 625–633. [Google Scholar] [CrossRef]
- Kurt, A.; Cengiz, A.; Kahyaoglu, T. The effect of gum tragacanth on the rheological properties of salep based ice cream mix. Carbohydr. Polym. 2016, 143, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.; Pintor, A.; Fiszman, S. How hydrocolloids affect the temporal oral perception of ice cream. Food hydrocoll. 2014, 36, 220–228. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Salahi, M.; Rad, T. Effects of basil seed gum, Cress seed gum and Quince seed gum on the physical, textural and rheological properties of whipped cream. Int. J. Biol. Macromol. 2017, 98, 820–828. [Google Scholar] [CrossRef]
- Sherafati, M.; Kalbasi-Ashtari, A.; Ali-Mousavi, S.M. Effects of low and high acyl gellan gums on engineering properties of carrot juice. J. Food Process Eng. 2013, 36, 418–427. [Google Scholar] [CrossRef]
- Tsevdou, M.; Gogou, E.; Dermesonluoglu, E.; Taoukis, P. Modelling the effect of storage temperature on the viscoelastic properties and quality of ice cream. J. Food Eng. 2015, 148, 35–42. [Google Scholar] [CrossRef]
- Adapa, S.; Dingeldein, H.; Schmidt, K.; Herald, T. Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers. J. Dairy Sci. 2000, 83, 2224–2229. [Google Scholar] [CrossRef]
- Moriano, M.; Alamprese, C. Honey, trehalose and erythritol as sucrose-alternative sweeteners for artisanal ice cream. A pilot study. LWT-Food Sci. Technol. 2017, 75, 329–334. [Google Scholar] [CrossRef]
- Trgo, C.; Koxholt, M.; Kessler, H. Effect of freezing point and texture regulating parameters on the initial ice crystal growth in ice cream. J. Dairy Sci. 1999, 82, 460–465. [Google Scholar] [CrossRef]
- Arellano, M.; Benkhelifa, H.; Flick, D.; Alvarez, G. Online ice crystal size measurements during sorbet freezing by means of the focused beam reflectance measurement (FBRM) technology. Influence of operating conditions. J. Food Eng. 2012, 113, 351–359. [Google Scholar] [CrossRef]

| Ingredients | Level 1 | Level 2 | ||
|---|---|---|---|---|
| Amount (g) | Percentage (%) | Amount (g) | Percentage (%) | |
| Milk | 1150.5 | 76.7 | 1149 | 76.6 |
| Milk powder | 118.5 | 7.9 | 118.5 | 7.9 |
| Unsalted butter | 28.5 | 1.9 | 28.5 | 1.9 |
| Sugar | 196.5 | 13.1 | 192 | 12.8 |
| Stabilizer / emulsifier | 6 | 0.4 | 12 | 0.8 |
| Total | 1500 | 100 | 1500 | 100 |
| Physicochemical Characteristics | Concentration (%) | Stabilizers Ratio (Mucilage:CMC) | |||
|---|---|---|---|---|---|
| 20:80 | 50:50 | 80:20 | 100:0 | ||
| pH | 0.4 | 6.45 ± 0.01 bA | 6.44 ± 0.01 bA | 6.44 ± 0.02 aA | 6.47 ± 0.04 aA |
| 0.8 | 6.49 ± 0.02 aA | 6.49 ± 0.02 aA | 6.42 ± 0.02 aB | 6.42 ± 0.03 aB | |
| Total solids (TS), % | 0.4 | 32.09 ± 0.21 bC | 32.65 ± 0.52 aB | 33.57 ± 0.27 aA | 32.91 ± 0.18 aB |
| 0.8 | 32.60 ± 0.11 aA | 32.39 ± 0.09 aA | 32.20 ± 0.04 bA | 32.07 ± 0.06 bA | |
| AT (% lactic acid) | 0.4 | 0.036 ± 0.001 aA | 0.036 ± 0.003 aA | 0.084 ± 0.011 aA | 0.048 ± 0.011 aA |
| 0.8 | 0.037 ± 0.001 aA | 0.035 ± 0.001 aA | 0.028 ± 0.008 bA | 0.037 ± 0.002 bA | |
| Ashes, % | 0.4 | 1.01 ± 0.02 bA | 0.98 ± 0.05 aA | 1.04 ± 0.03 aA | 0.98 ± 0.07 aA |
| 0.8 | 1.12 ± 0.06 aA | 1.05 ± 0.05 aAB | 0.97 ± 0.04 aB | 0.96 ± 0.06 aB | |
| Fat, % | 0.4 | 7.70 ± 0.26 aA | 6.77 ± 0.16 aA | 7.47 ± 0.15 aA | 7.20 ± 0.26 aA |
| 0.8 | 7.23 ± 0.16 aA | 7.03 ± 0.15 aA | 7.17 ± 0.21 aA | 7.50 ± 0.10 aA | |
| Protein, % | 0.4 | 3.42 ± 0.20 aA | 3.46 ± 0.18 aA | 3.26 ± 0.19aA | 3.70 ± 0.28 aA |
| 0.8 | 2.68 ± 0.30 bB | 3.64 ± 0.11 aA | 3.68 ± 0.41aA | 3.96 ± 0.34 aA | |
| Rheological Parameters | Concentration (%) | Ratio (Mucilage:CMC) | |||
|---|---|---|---|---|---|
| 20:80 | 50:50 | 80:20 | 100: 0 | ||
| n | 0.4 | 0.586 ± 0.044 aD | 0.668 ± 0.031 aC | 0.855 ± 0.017 aB | 0.955 ± 0.022 aA |
| 0.8 | 0.471 ± 0.046 bD | 0.556 ± 0.041 bC | 0.691 ± 0.026 bB | 0.9 ± 0.001 bA | |
| K (Pa·s n) | 0.4 | 0.869 ± 0.19 bA | 0.266 ± 0.031 bA | 0.04 ± 0.004 aA | 0.011 ± 0.001 aA |
| 0.8 | 6.659 ± 1.522 aA | 1.859 ± 0.369 aB | 0.239 ± 0.029 aC | 0.023 ± 0.001 aC | |
| Viscoelastic Parameters | Concentration (%) | Ratio (Mucilage:CMC) | |||
|---|---|---|---|---|---|
| 20:80 | 50:50 | 80:20 | 100:0 | ||
| K’, Pa·sn’ | 0.4 | 1.352 ± 0.002 bAB | 0.755 ± 0.001 bC | 1.255 ± 0.071 aB | 1.589 ± 0.008 aA |
| 0.8 | 2.201 ± 0.046 aA | 1.478 ± 0.051 aBC | 1.234 ± 0.148 aC | 1.629 ± 0.123 aB | |
| K”, Pa·sn” | 0.4 | 0.502 ± 0.078 aA | 0.157 ± 0.000 aA | 0.131 ± 0.001 aA | 0.369 ± 0.021 aA |
| 0.8 | 0.679 ± 0.089 aA | 0.192 ± 0.035 aA | 0.347 ± 0.107 aA | 0.907 ± 0.084 aA | |
| N’ | 0.4 | 2.136 ± 0.014 aA | 2.264 ± 0.071 aA | 2.145 ± 0.021 aA | 2.130 ± 0.071 aA |
| 0.8 | 2.062 ± 0.073 aA | 2.072 ± 0.013 bA | 2.155 ± 0.019 aA | 2.068 ± 0.017 aA | |
| N” | 0.4 | 1.872 ± 0.047 aBC | 2.201 ± 0.07 aA | 2.031 ± 0.002 aAB | 1.799 ± 0.131 bC |
| 0.8 | 1.792 ± 0.025 aB | 2.031 ± 0.014 bA | 1.746 ± 0.091 bB | 2.050 ± 0.008 aA | |
| tan (δ) | 0.4 | 0.45 ± 0.04 aB | 0.42 ± 0.08 aB | 0.56 ± 0.07 aB | 1.08 ± 0.11 aA |
| 0.8 | 0.39 ± 0.01 aB | 0.38 ± 0.01 aB | 0.36 ± 0.01 bB | 0.75 ± 0.06 bA | |
| Variables | Concentration (CE) (%) | Ratio (M:CMC) | |||
|---|---|---|---|---|---|
| 20:80 | 50:50 | 80:20 | 100:0 | ||
| Overrum,% | 0.4 | 60.97 ± 2.40 bC | 57.94 ± 2.48 bC | 64.95 ± 1.14 bB | 72.35 ± 0.98 bA |
| 0.8 | 56.27 ± 1.25 aD | 73.76 ± 1.08 aB | 66.98 ± 0.83 aC | 78.27 ± 1.67 aA | |
| Freezing point, °C | 0.4 | −4.60 ± 0.46 aC | −4.90 ± 0.36 aBA | −5.40 ± 0.47 aCB | −6.10 ± 0.55 aA |
| 0.8 | −2.80 ± 0.04 bB | −3.30 ± 0.61 bB | −5.70 ± 0.33 aA | −6.00 ± 0.49 aA | |
| Melt time, min | 0.4 | 56.67 ± 2.89 aA | 41.67 ± 2.89 bB | 65 ± 5 aA | 61.67 ± 2.5 bA |
| 0.8 | 33.33 ± 5.00 bC | 50.00 ± 5.00 aAB | 53.33 ± 2.89 bB | 70.00 ± 5.00 aA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, E.; Padilla, K.; Salcedo, J.; Arrieta, A.; Andrade-Pizarro, R. Effects of Yam (Dioscorea rotundata) Mucilage on the Physical, Rheological and Stability Characteristics of Ice Cream. Polymers 2022, 14, 3142. https://doi.org/10.3390/polym14153142
Lozano E, Padilla K, Salcedo J, Arrieta A, Andrade-Pizarro R. Effects of Yam (Dioscorea rotundata) Mucilage on the Physical, Rheological and Stability Characteristics of Ice Cream. Polymers. 2022; 14(15):3142. https://doi.org/10.3390/polym14153142
Chicago/Turabian StyleLozano, Ermides, Karen Padilla, Jairo Salcedo, Alvaro Arrieta, and Ricardo Andrade-Pizarro. 2022. "Effects of Yam (Dioscorea rotundata) Mucilage on the Physical, Rheological and Stability Characteristics of Ice Cream" Polymers 14, no. 15: 3142. https://doi.org/10.3390/polym14153142






