Corrosion Resistance and Thermal Conductivity Enhancement of Reduced Graphene Oxide–BaSO4–Epoxy Composites
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Method for rGO–B–Epoxy Composites
2.3. Characterization
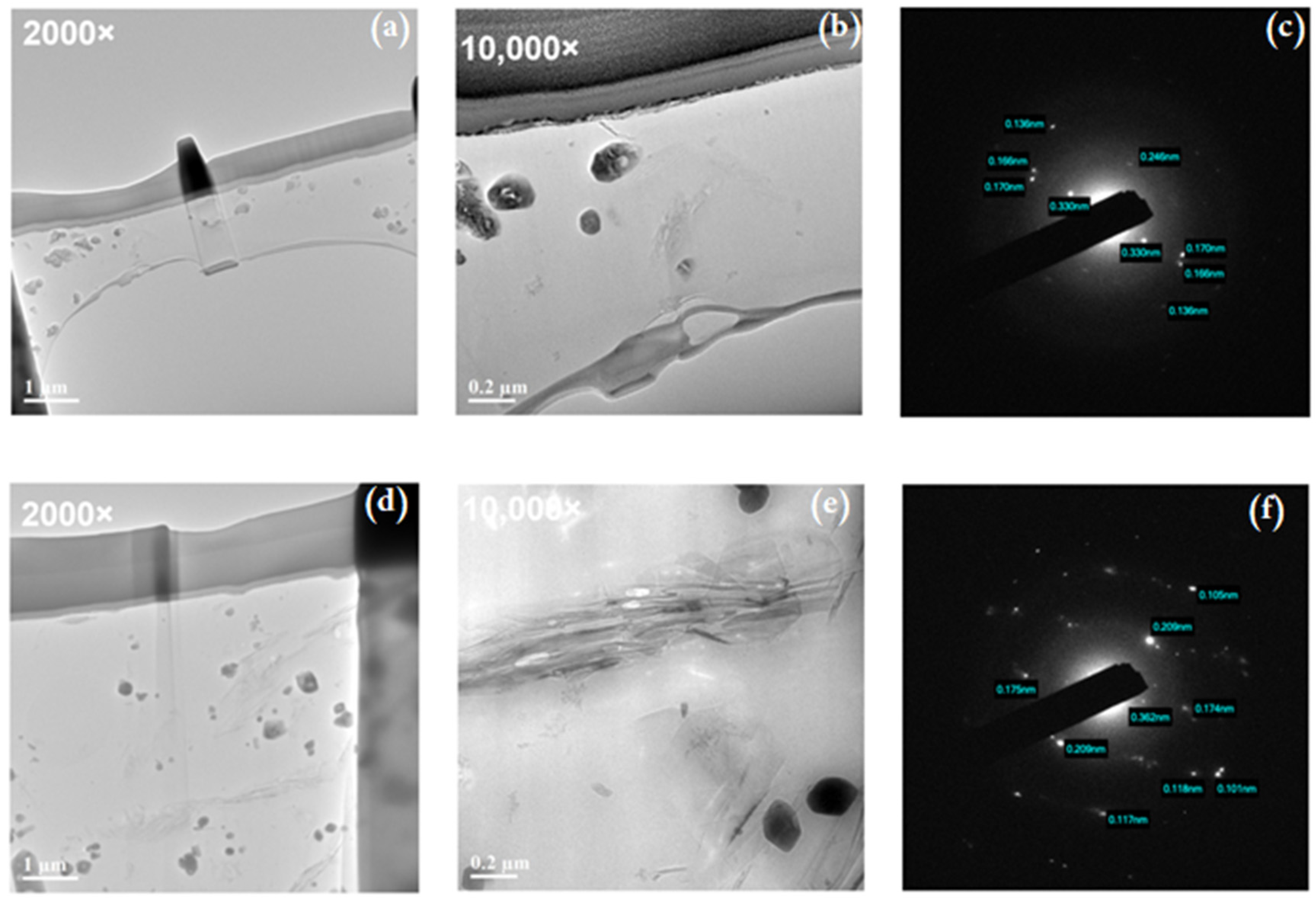
2.3.1. The Surface Characterization with SEM and TEM
2.3.2. Raman, FT-IR, X-ray Photon Spectroscopy (XPS), and Atomic Force Microscopy (AFM) for rGO Analysis
2.3.3. Corrosion Resistance
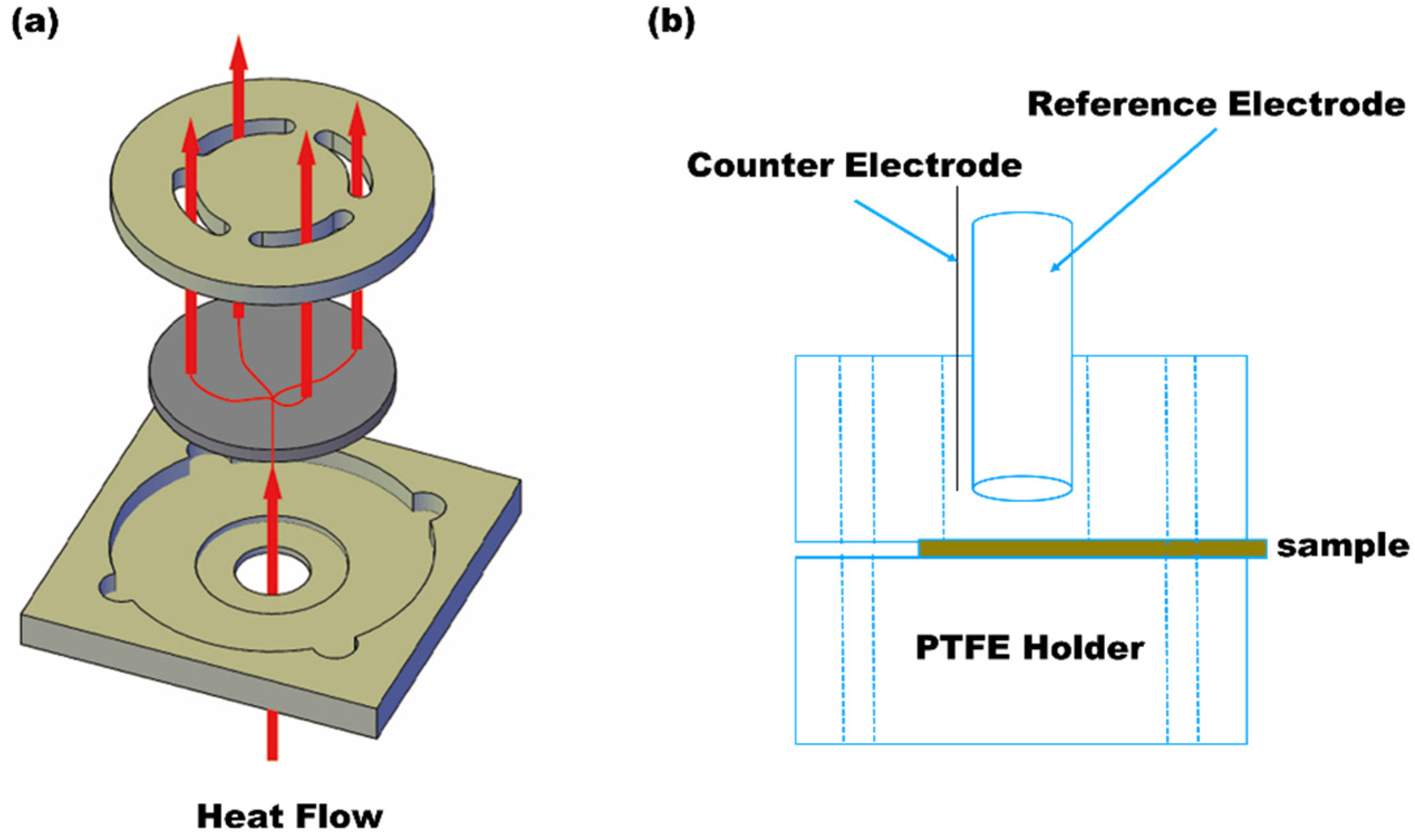
2.3.4. Thermal Analysis (Laser Flash Method) and Thermogravimetric Analyzer (TGA)
3. Results and Discussion
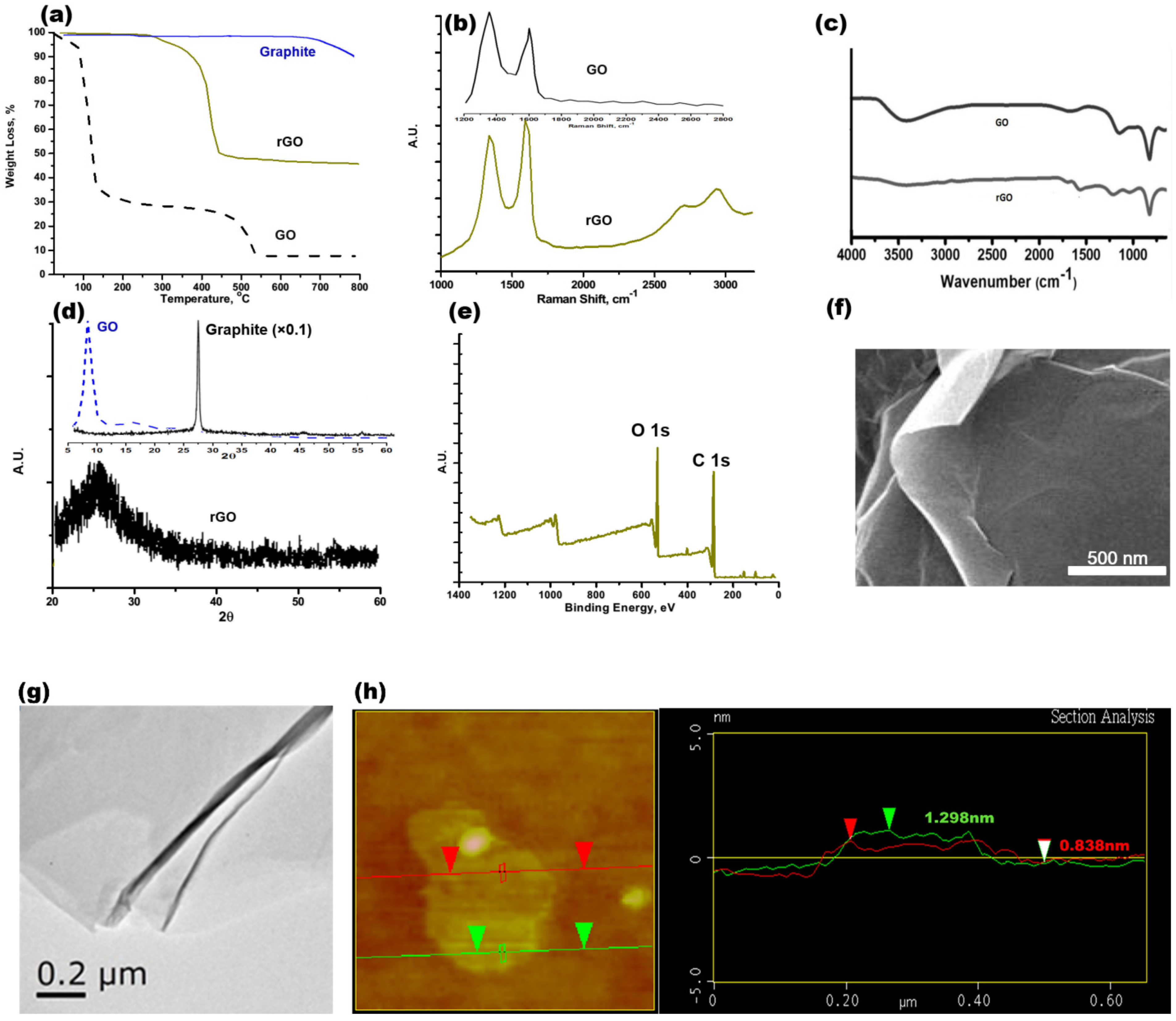
3.1. rGO Synthesis and Characterization
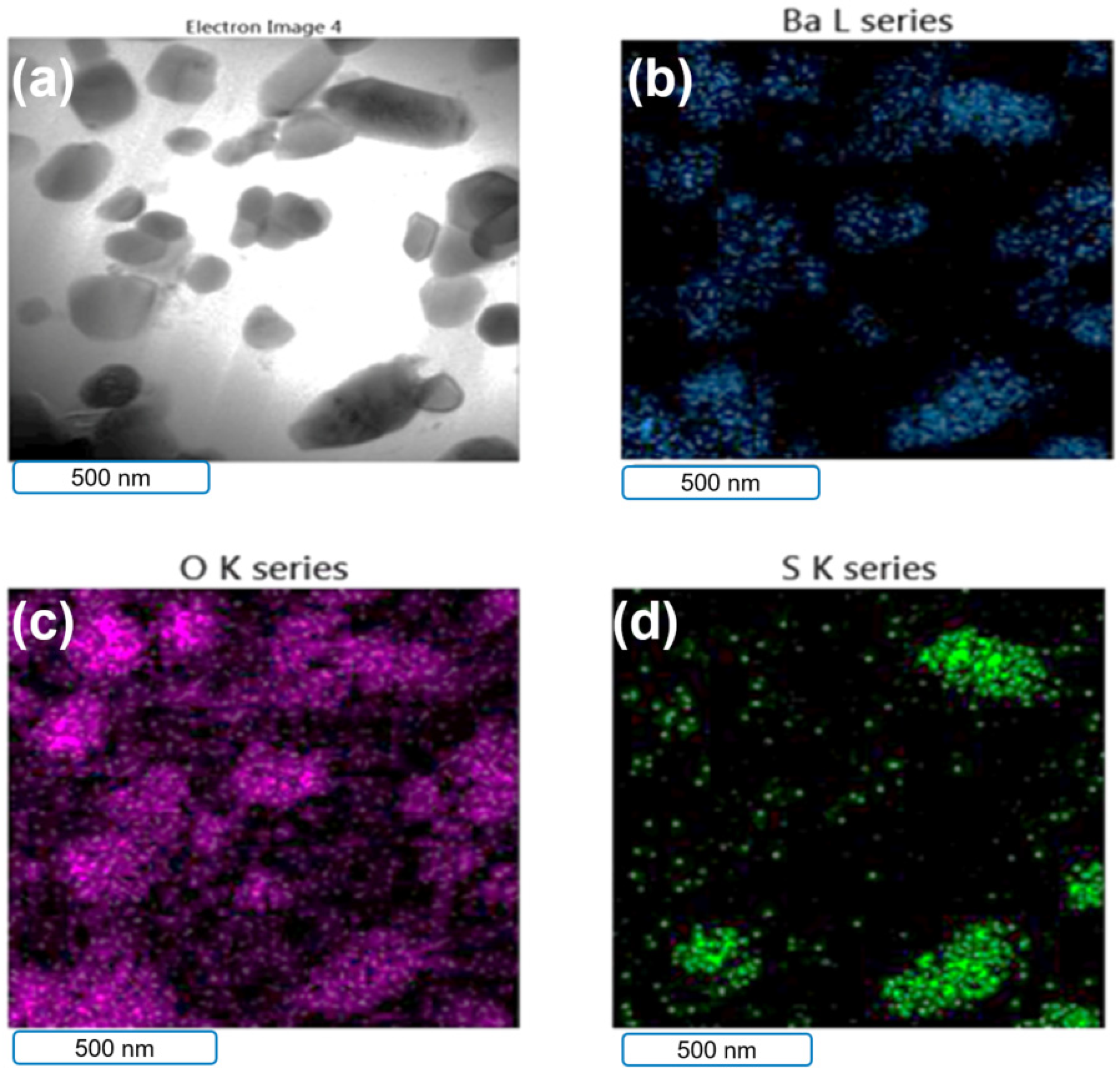
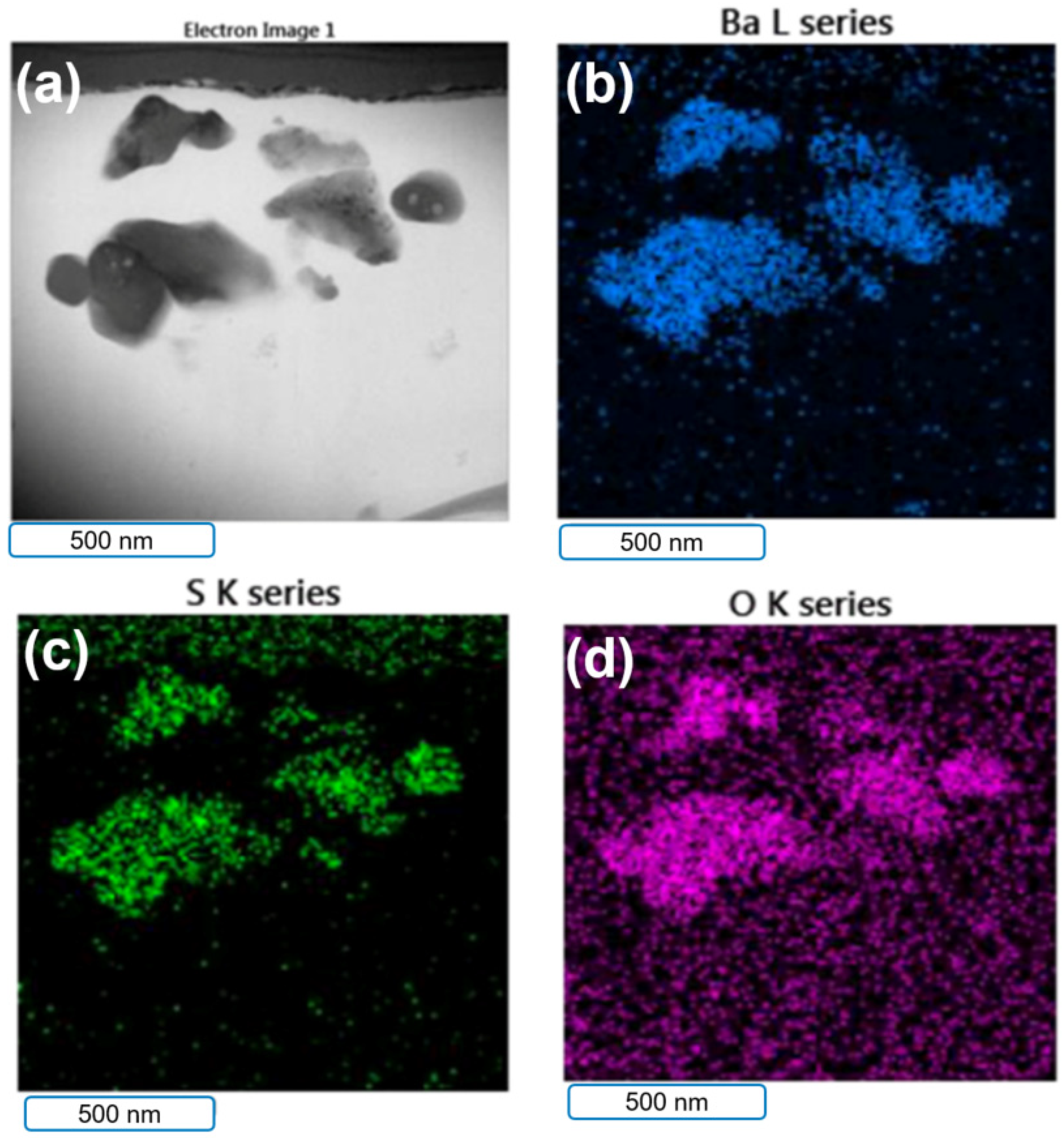
3.2. rGO–B–Epoxy Composite Surface Morphology
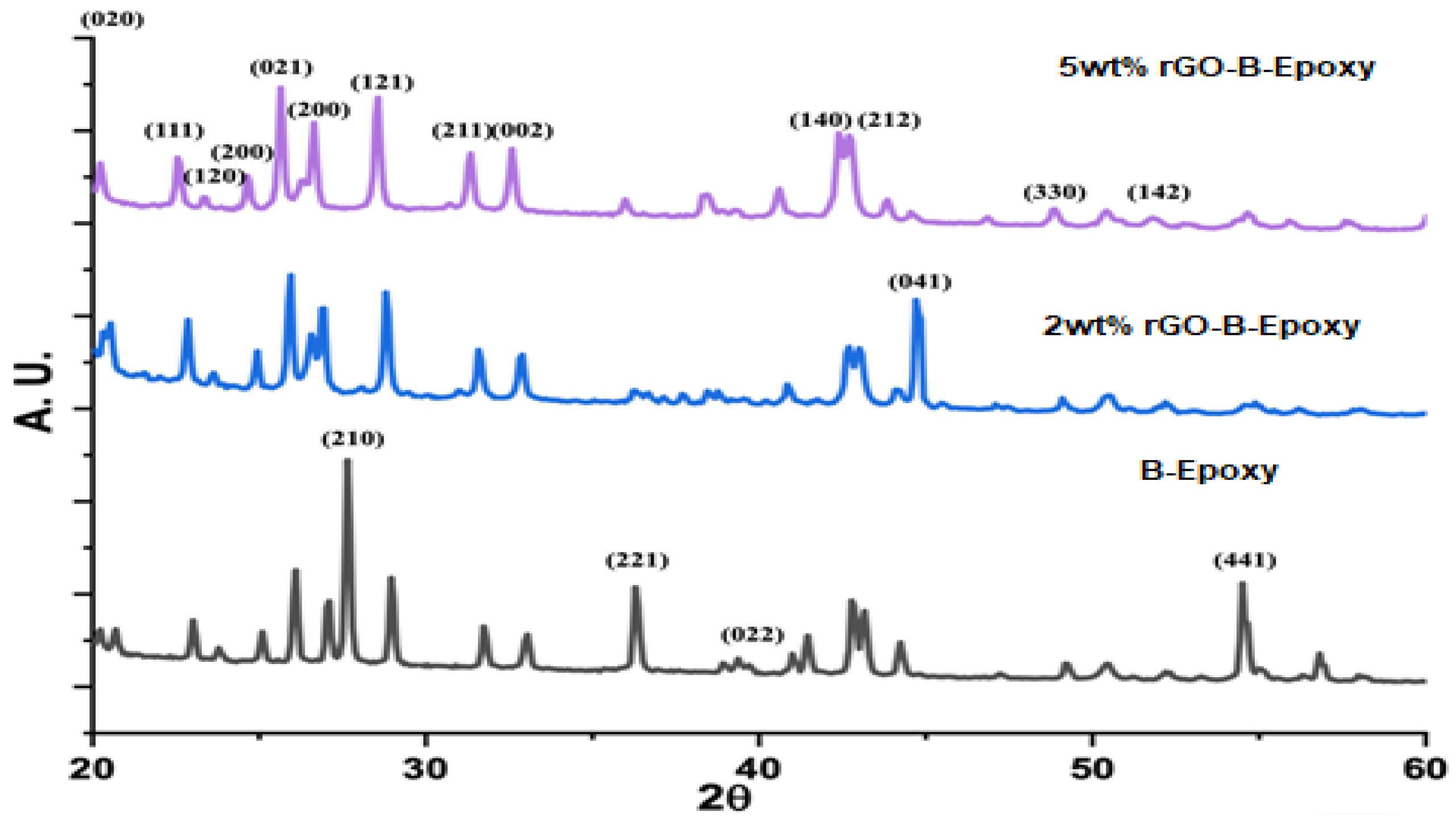
3.3. XRD for rGO–B–Epoxy Composites
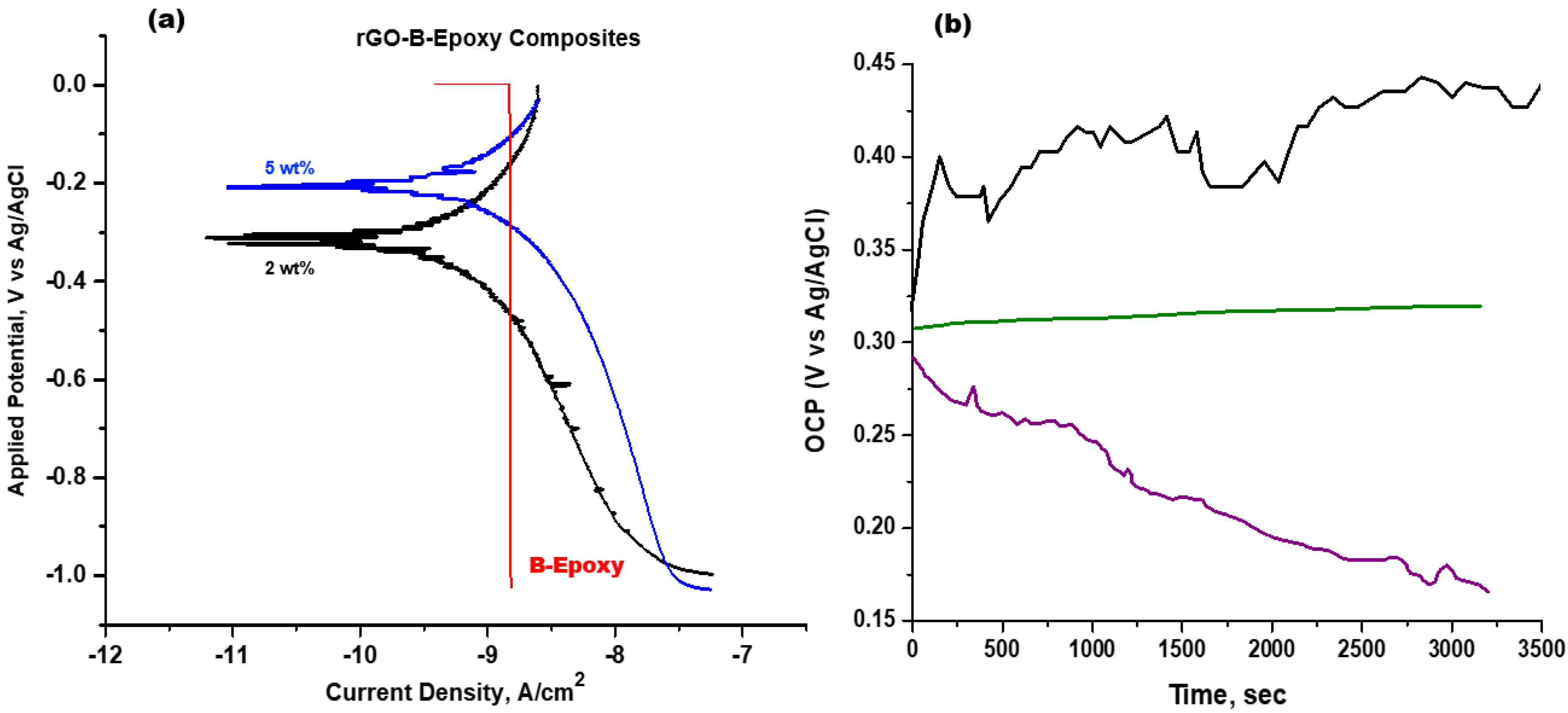
3.4. rGO–B–Epoxy Composites for Corrosion Proofing
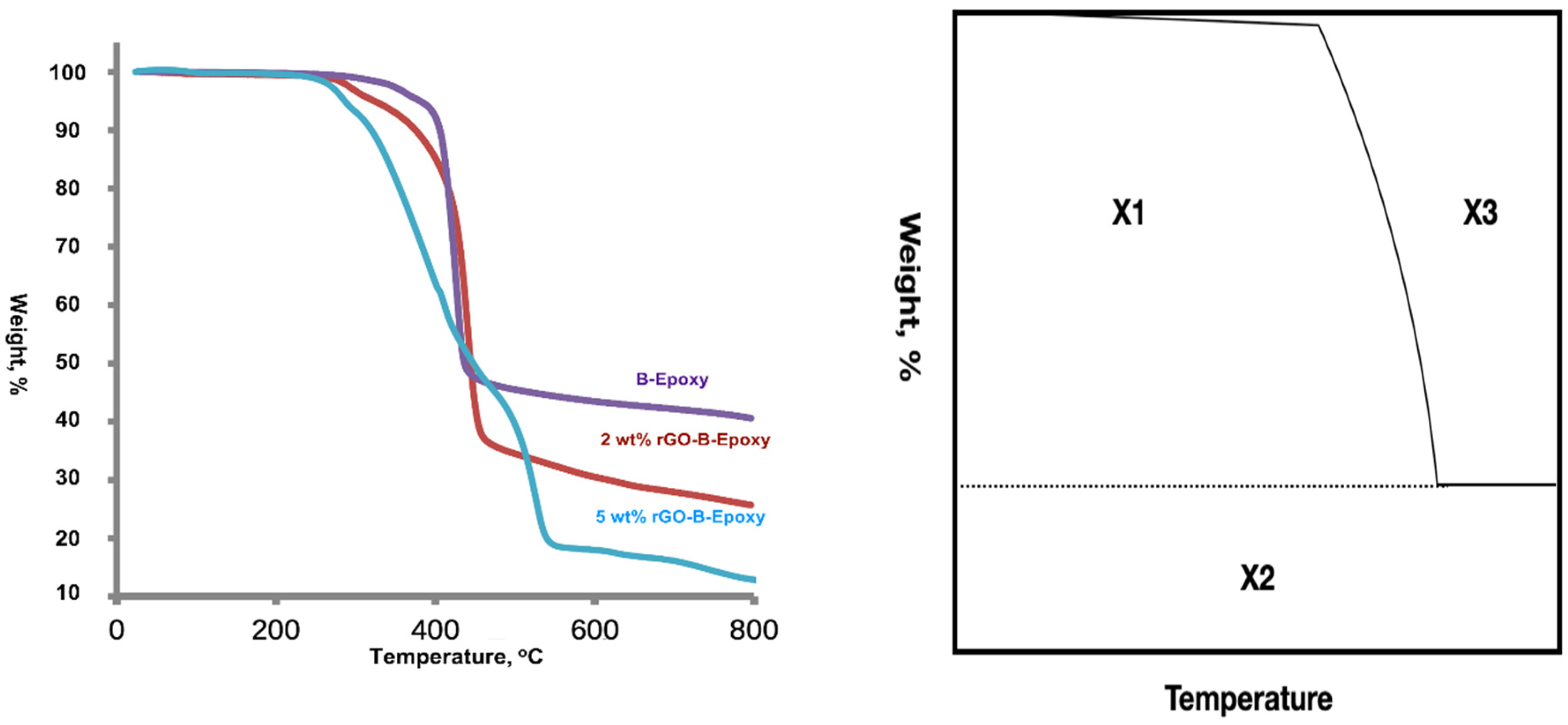
3.5. Thermal Properties of rGO–B–Epoxy Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Wu, L.; Song, Y.; Xia, W.; Guo, K. Effect of molecular chain length on the properties of amine-functionalized graphene oxide nanosheets/epoxy resins nanocomposites. RSC Adv. 2015, 5, 45987. [Google Scholar] [CrossRef]
- Paraskar, P.; Bari, P.; Mishra, S. Influence of amino functionalized graphene oxide on mechanical and thermal properties of epoxy matrix composites. Iran. Polym. J. 2020, 29, 47–55. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Wang, S.-H.; Liu, C.-M.; Chien, M.-Y.; Hsu, C.-C.; Liu, T.-Y. Amino-modified graphene oxide nanoplatelets for photothermal and antibacterial capability. Surf. Coat. Technol. 2020, 385, 125441. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Chen, Y.; Zou, H.; Liang, M. Enhanced mechanical properties of epoxy nanocomposites based on graphite oxide with amine-rich surface. RSC Adv. 2015, 5, 98472. [Google Scholar] [CrossRef]
- Singh, P.K.; Sharma, K. Mechanical and Viscoelastic Properties of in-situ amine functionalized multiple layer graphene/epoxy nanocomposites. Curr. Nanosci. 2018, 14, 252–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Mohanty, A. Investigation of mechanical properties of graphene decorated with graphene quantum dot-reinforced epoxy nanocomposite. J. Appl. Polym. Sci. 2019, 137, 48680. [Google Scholar] [CrossRef]
- Yung, T.-Y.; Sangeetha, T.; Yan, W.-M.; Yang, C.-J.; Chen, P.-T. Non-precious and accessible nanocomposite of iron oxide on PDDA-modified graphene for catalyzing oxygen reduction reaction. J. Power Sources Adv. 2020, 5, 100025. [Google Scholar] [CrossRef]
- Yung, T.-Y.; Huang, L.-Y.; Chan, T.-Y.; Wang, K.-S.; Liu, T.-Y.; Chen, P.-T.; Chao, C.Y.; Liu, L.K. Synthesis and characterizations of Ni-NiO nanoparticles on PDDA-modified graphene for oxygen reduction reaction. Nanoscale Res. Lett. 2014, 9, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Luo, Z.; Vora, P.M.; Mele, E.J.; Johnson, A.T.C.; Kikkawa, J.M. Photoluminescence and band gap modulation in graphene oxide. Appl. Phys. Lett. 2009, 94, 111909. [Google Scholar] [CrossRef]
- Byon, H.R.; Gallant, B.M.; Lee, S.W.; Shao-Horn, Y. Role of oxygen functional groups in carbon nanotube/graphene freestanding electrodes for high performance lithium batteries. Adv. Funct. Mater. 2013, 23, 1037–1045. [Google Scholar] [CrossRef]
- Deng, W.; Ji, X.; Gomez-Mingot, M.; Lu, F.; Chen, Q.; Banks, C.E. Graphene electrochemical super-capacitors: The influence of oxygen functional groups. Chem. Commun. 2012, 48, 2770–2772. [Google Scholar] [CrossRef]
- Yung, T.-Y.; Lu, Y.-C.; Chen, J.-S.; Cheng, Y.-W.; Liu, T.-Y.; Chen, P.-T. Reinforcement of epoxy resin by additives of amine-functionalized graphene nanosheets. Coatings 2021, 11, 35. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; Liu, M.; Motta, N.; Waclawik, E.; Yan, C. Recent advances in fabrication and characterization of graphene-polymer nanocomposites. Graphene 2012, 1, 30–49. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Wei, J.; Vo, T.; Inam, F. Epoxy/graphene nanocomposites—Processing and properties: A review. RSC Adv. 2015, 5, 73510. [Google Scholar] [CrossRef] [Green Version]
- Amirova, L.; Surnova, A.; Balkaev, D.; Musin, D.; Amirov, R.; Dimiev, A.M. Homogeneous liquid phase transfer of graphene oxide into epoxy resins. ACS Appl. Mater. Interfaces 2017, 9, 11909–11917. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. N,N-Dimethylformamide (DMF) usage in epoxy/graphene nanocomposites: Problems associated with reaggregation. Polym. 2017, 9, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. Dichlorobenzene: An effective solvent for epoxy/graphene nanocomposites preparation. R. Soc. Open Sci. 2017, 4, 170778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lu, J.; Chu, Y. Improved thermal conductivity of epoxy resin by graphene-nickel 3-D fillers. Carbon Resour. Convers. 2020, 3, 29–35. [Google Scholar] [CrossRef]
- Kashfipour, M.A.; Mehraj, N.; Zhu, J. A review on the role of interface in mechanical, thermal, and electrical properties of polymer composites. Adv. Compos. Hybrid Mater. 2018, 1, 415–439. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 24414, Barium Sulfate. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Barium-sulfate (accessed on 27 April 2022).
- Yamashita, N.; Yamamoto, I.; Ninagawa, K.; Wada, T.; Yamashita, Y.; Nakao, Y. Investigation of TLD phosphors by optical excitation–luminescence of Eu2+ centers in MgSO4, CaSO4, SrSO4 and BaSO4. Jpn. J. Appl. Phys. 1985, 24, 1174–1180. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N. Prospects for applying BaSO4 powders as pigments for spacecraft thermal control coatings. Acta Astronaut. 2019, 165, 191–194. [Google Scholar] [CrossRef]
- Mikhailov, M.; Yuryev, S.; Lapin, A.; Lovitskiy, A. The effects of heating on BaSO4 powders diffuse reflectance spectra and radiation stability. Dye Pigment. 2019, 163, 420–424. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Byun, S.; Paik, K.W.; Song, S.H. Novel dielectric BN/epoxy Nanocompositea with enhanced heat dissipation performance for electronic packaging. Compos. Part A Appl. Sci. Manuf. 2018, 107, 217–223. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Nair, B.; Pradeep, T. Coalescence of Nanoclusters and formation of submicron crystallites assisted by lactobacillus strains. Crystl. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Thermal and mechanical characterization of an aeronautical graded epoxy resin loaded with hybrid nanoparticles. Nanomaterials 2020, 10, 1388. [Google Scholar] [CrossRef]
| Element | Content (wt%) |
|---|---|
| Mg | 0.499 |
| Si | 0.474 |
| Mn | 0.025 |
| Cu | 0.007 |
| Zn | 0.004 |
| Ti | 0.028 |
| Fe | 0.243 |
| Be | 0.004 |
| Pb | 0.001 |
| Al | 98.50 |
| BaSO4–Epoxy (A* = 0.8200; K* = 1.9385) | 2 wt% Graphene–Epoxy (A* = 0.71389; K* = 1.5831) | 5 wt% Graphene–Epoxy (A* = 0.5250; K* = 1.2836) |
| Thermal Diffusivity, mm2/s | ||
| Standard, 0.179 (±0.002) | Standard, 9.781 (±0.063) In-plane, 51.46 (±3.902) | Standard, 15.83 (±0.071) In-plane, 71.38 (±2.960) |
| Thermal Conductivity | ||
| Standard, 0.121 (±0.001) | Standard, 25.28 (±0.160) In-plane, 133.0 (±10.10) | Standard, 36.60 (±0.170) In-plane, 165.0 (±6.800) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yung, T.-Y.; Lu, W.-F.; Tsai, K.-C.; Chen, J.-S.; Pang, K.-N.; Tzeng, Y.-C.; Cheng, H.-M.; Chen, P.-T. Corrosion Resistance and Thermal Conductivity Enhancement of Reduced Graphene Oxide–BaSO4–Epoxy Composites. Polymers 2022, 14, 3144. https://doi.org/10.3390/polym14153144
Yung T-Y, Lu W-F, Tsai K-C, Chen J-S, Pang K-N, Tzeng Y-C, Cheng H-M, Chen P-T. Corrosion Resistance and Thermal Conductivity Enhancement of Reduced Graphene Oxide–BaSO4–Epoxy Composites. Polymers. 2022; 14(15):3144. https://doi.org/10.3390/polym14153144
Chicago/Turabian StyleYung, Tung-Yuan, Wen-Fang Lu, Kun-Chao Tsai, Jeng-Shiung Chen, Kwan-Nang Pang, Yu-Chih Tzeng, Hsin-Ming Cheng, and Po-Tuan Chen. 2022. "Corrosion Resistance and Thermal Conductivity Enhancement of Reduced Graphene Oxide–BaSO4–Epoxy Composites" Polymers 14, no. 15: 3144. https://doi.org/10.3390/polym14153144
APA StyleYung, T.-Y., Lu, W.-F., Tsai, K.-C., Chen, J.-S., Pang, K.-N., Tzeng, Y.-C., Cheng, H.-M., & Chen, P.-T. (2022). Corrosion Resistance and Thermal Conductivity Enhancement of Reduced Graphene Oxide–BaSO4–Epoxy Composites. Polymers, 14(15), 3144. https://doi.org/10.3390/polym14153144







