DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AgNPs and DEAE-AgNPs
2.2. Characterization
2.3. Modification of Composite Resin Discs
2.4. Isolation of Bacterial Strains
2.5. Antibacterial in Vitro Assay
2.6. Hemolytic Activity for AgNPs and DEAE-Dextran AgNPs
2.7. Mechanical Testing
2.8. Statistical Analysis
3. Results
3.1. The Successful Synthesis of AgNPs and DEAE-Dextran AgNPs
3.2. Zeta Charge
3.3. Particle Morphology
3.4. EDS Analysis
3.5. FTIR Analysis
3.6. Antibacterial Activity of AgNPs and DEAE-Dextran AgNPs
3.7. Hemolytic Assay of DEAE-Dextran AgNPs
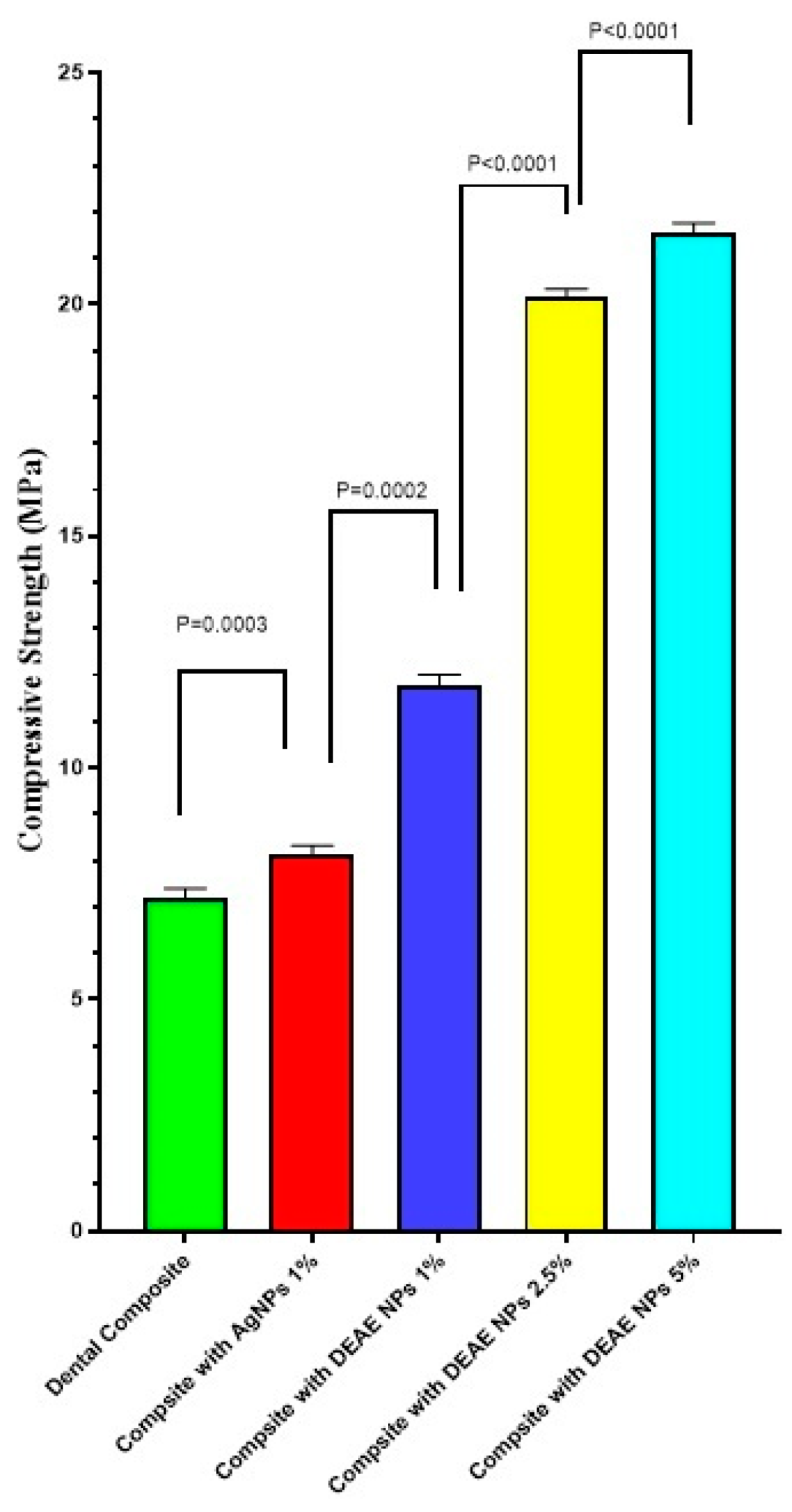
3.8. Compressive Strength Measurement Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primer 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.K.; Saxena, P.; Pant, V.A.; Pant, A.B. Release and Toxicity of Dental Resin Composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An Overview of Application of Silver Nanoparticles for Biomaterials in Dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.B.A.d.; Agnelli, J.A.M.; Kurachi, C.; de Souza, C.W.O. Addition of Silver Nanoparticles to Composite Resin: Effect on Physical and Bactericidal Properties In Vitro. Braz. Dent. J. 2014, 25, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and Their Biomedical Applications—A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Bertassoni, L.E. Nanotechnology in Dental Sciences: Moving towards a Finer Way of Doing Dentistry. Materials 2010, 3, 1674–1691. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology-Based Restorative Materials for Dental Caries Management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Neelakantan P, S. Nanotechnology In Dentistry—What Does The Future Hold In Store? Dentistry 2014, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Garoushi, S.; Säilynoja, E.; Vallittu, P.K.; Lassila, L. The Effect of Adding a New Monomer “Phene” on the Polymerization Shrinkage Reduction of a Dental Resin Composite. Dent. Mater. 2019, 35, 627–635. [Google Scholar] [CrossRef]
- Cho, K.; Yasir, M.; Jung, M.; Willcox, M.D.P.; Stenzel, M.H.; Rajan, G.; Farrar, P.; Prusty, B.G. Hybrid Engineered Dental Composites by Multiscale Reinforcements with Chitosan-Integrated Halloysite Nanotubes and S-Glass Fibers. Compos. Part B Eng. 2020, 202, 108448. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel Nanoparticle-Reinforced Metal Matrix Composites with Enhanced Mechanical Properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Nanotechnology-Based Materials as Emerging Trends for Dental Applications. Rev. Adv. Mater. Sci. 2021, 60, 173–189. [Google Scholar] [CrossRef]
- Mehdi, K.; Rehman, W.; Abid, O.; Fazil, S.; Sajid, M.; Rab, A.; Farooq, M.; Haq, S.; Menaa, F. Green Synthesis of Silver Nanoparticles using Ajuga parviflora Benth and Digera muricata Leaf extract: Their Characterization and Antimicrobial Activity. Rev. Chim. 2020, 71, 50–57. [Google Scholar] [CrossRef]
- Peng, J.; Lin, J.; Chen, Z.; Wei, M.; Fu, Y.; Lu, S.; Yu, D.; Zhao, W. Enhanced Antimicrobial Activities of Silver-Nanoparticle-Decorated Reduced Graphene Nanocomposites against Oral Pathogens. Mater. Sci. Eng. C 2017, 71, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; da Cruz, A.D.; Poiate, E.; Poiate, I.A.V.P. Silver Nanoparticles in Dental Biomaterials. Int. J. Biomater. 2015, 2015, 485275. [Google Scholar] [CrossRef] [Green Version]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and Biological Assessment of Silver Nanoparticles Immobilized Halloysite Nanotubes-Based Resin Composite for Dental Applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced Antimicrobial Activity of Naturally Derived Bioactive Molecule Chitosan Conjugated Silver Nanoparticle against Dental Implant Pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Nganga, S.; Travan, A.; Marsich, E.; Donati, I.; Söderling, E.; Moritz, N.; Paoletti, S.; Vallittu, P.K. In Vitro Antimicrobial Properties of Silver–Polysaccharide Coatings on Porous Fiber-Reinforced Composites for Bone Implants. J. Mater. Sci. Mater. Med. 2013, 24, 2775–2785. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Lo, E.C.M.; Tang, J.; Li, Q.; So, L.Y.; Chu, C.H. Synthesis and Characterization of Fluoridated Silver Nanoparticles and Their Potential as a Non-Staining Anti-Caries Agent. Int. J. Nanomed. 2020, 15, 3207–3215. [Google Scholar] [CrossRef]
- Chávez-Andrade, G.M.; Tanomaru-Filho, M.; Basso Bernardi, M.I.; de Toledo Leonardo, R.; Faria, G.; Guerreiro-Tanomaru, J.M. Antimicrobial and Biofilm Anti-Adhesion Activities of Silver Nanoparticles and Farnesol against Endodontic Microorganisms for Possible Application in Root Canal Treatment. Arch. Oral Biol. 2019, 107, 104481. [Google Scholar] [CrossRef]
- Mikac, L.; Jurkin, T.; Štefanić, G.; Ivanda, M.; Gotić, M. Synthesis of Silver Nanoparticles in the Presence of Diethylaminoethyl-Dextran Hydrochloride Polymer and Their SERS Activity. J. Nanopart. Res. 2017, 19, 299. [Google Scholar] [CrossRef]
- Schenborn, E.T.; Goiffon, V. DEAE-Dextran Transfection of Mammalian Cultured Cells. In Transcription Factor Protocols; Tymms, M.J., Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2000; pp. 147–153. [Google Scholar] [CrossRef]
- Sondi, I.; Siiman, O.; Koester, S.; Matijević, E. Preparation of Aminodextran−CdS Nanoparticle Complexes and Biologically Active Antibody−Aminodextran−CdS Nanoparticle Conjugates. Langmuir 2000, 16, 3107–3118. [Google Scholar] [CrossRef]
- Morrow, B.J.; Matijević, E.; Goia, D.V. Preparation and Stabilization of Monodisperse Colloidal Gold by Reduction with Aminodextran. J. Colloid Interface Sci. 2009, 335, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial Properties of Composite Resins Incorporating Silver and Zinc Oxide Nanoparticles on Streptococcus Mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Dias, H.B.; Bernardi, M.I.B.; Bauab, T.M.; Hernandes, A.C.; de Souza Rastelli, A.N. Titanium Dioxide and Modified Titanium Dioxide by Silver Nanoparticles as an Anti Biofilm Filler Content for Composite Resins. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, e36–e46. [Google Scholar] [CrossRef]
- Li, X.; Shang, L.; Brandt, B.W.; Buijs, M.J.; Roffel, S.; van Loveren, C.; Crielaard, W.; Gibbs, S.; Deng, D.M. Saliva-Derived Microcosm Biofilms Grown on Different Oral Surfaces in Vitro. Npj Biofilms Microbiomes 2021, 7, 74. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Effect of Salivary Pellicle on Antibacterial Activity of Novel Antibacterial Dental Adhesives Using a Dental Plaque Microcosm Biofilm Model. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Thom, D.C.; Davies, J.E.; Santerre, J.P.; Friedman, S. The Hemolytic and Cytotoxic Properties of a Zeolite-Containing Root Filling Material in Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 101–108. [Google Scholar] [CrossRef]
- Jayanthi, N.; Vinod, V. Comparative Evaluation of Compressive Strength and Flexural Strength of Conventional Core Materials with Nanohybrid Composite Resin Core Material an in Vitro Study. J. Indian Prosthodont. Soc. 2013, 13, 281–289. [Google Scholar] [CrossRef]
- Can, H.K.; Kavlak, S.; ParviziKhosroshahi, S.; Güner, A. Preparation, Characterization and Dynamical Mechanical Properties of Dextran-Coated Iron Oxide Nanoparticles (DIONPs). Artif. Cells Nanomed. Biotechnol. 2018, 46, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-Biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Bankura, K.P.; Maity, D.; Mollick, M.M.R.; Mondal, D.; Bhowmick, B.; Bain, M.K.; Chakraborty, A.; Sarkar, J.; Acharya, K.; Chattopadhyay, D. Synthesis, Characterization and Antimicrobial Activity of Dextran Stabilized Silver Nanoparticles in Aqueous Medium. Carbohydr. Polym. 2012, 89, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- İspirli, H.; Sagdic, O.; Dertli, E. Synthesis of Silver Nanoparticles Prepared with a Dextran-Type Exopolysaccharide from Weissella Cibaria MED17 with Antimicrobial Functions. Prep. Biochem. Biotechnol. 2021, 51, 112–119. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Marangoni, V.S.; de Abreu Bernardi, A.C.; de Souza Rastelli, A.N.; Hernandes, A.C. Synthesis, Characterization and Application of Ag Doped ZnO Nanoparticles in a Composite Resin. Mater. Sci. Eng. C 2019, 96, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Tormena, R.P.I.; Rosa, E.V.; Mota, B.d.O.; Chaker, J.A.; Fagg, C.W.; Freire, D.O.; Martins, P.M.; da Silva, I.C.R.; Sousa, M.H. Evaluation of the antimicrobial activity of silver nanoparticles obtained by microwave-assisted green synthesis using Handroanthus impetiginosus (Mart. ex DC.) Mattos underbark extract. RSC Adv. 2020, 10, 20676–20681. [Google Scholar] [CrossRef]
- Kassaee, M.; Akhavan, A.; Sheikh, N.; Sodagar, A. Antibacterial Effects of a New Dental Acrylic Resin Containing Silver Nanoparticles. J. Appl. Polym. Sci. 2008, 110, 1699–1703. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, S.; Rana, N.F.; Kashif, A.S.; Tanweer, T.; Shafique, I.; Menaa, F. DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites. Polymers 2022, 14, 3143. https://doi.org/10.3390/polym14153143
Azhar S, Rana NF, Kashif AS, Tanweer T, Shafique I, Menaa F. DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites. Polymers. 2022; 14(15):3143. https://doi.org/10.3390/polym14153143
Chicago/Turabian StyleAzhar, Shabia, Nosheen Fatima Rana, Amer Sohail Kashif, Tahreem Tanweer, Iqra Shafique, and Farid Menaa. 2022. "DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites" Polymers 14, no. 15: 3143. https://doi.org/10.3390/polym14153143
APA StyleAzhar, S., Rana, N. F., Kashif, A. S., Tanweer, T., Shafique, I., & Menaa, F. (2022). DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites. Polymers, 14(15), 3143. https://doi.org/10.3390/polym14153143






