PA-12-Zirconia-Alumina-Cenospheres 3D Printed Composites: Accelerated Ageing and Role of the Sterilisation Process for Physicochemical Properties
Abstract
:1. Introduction
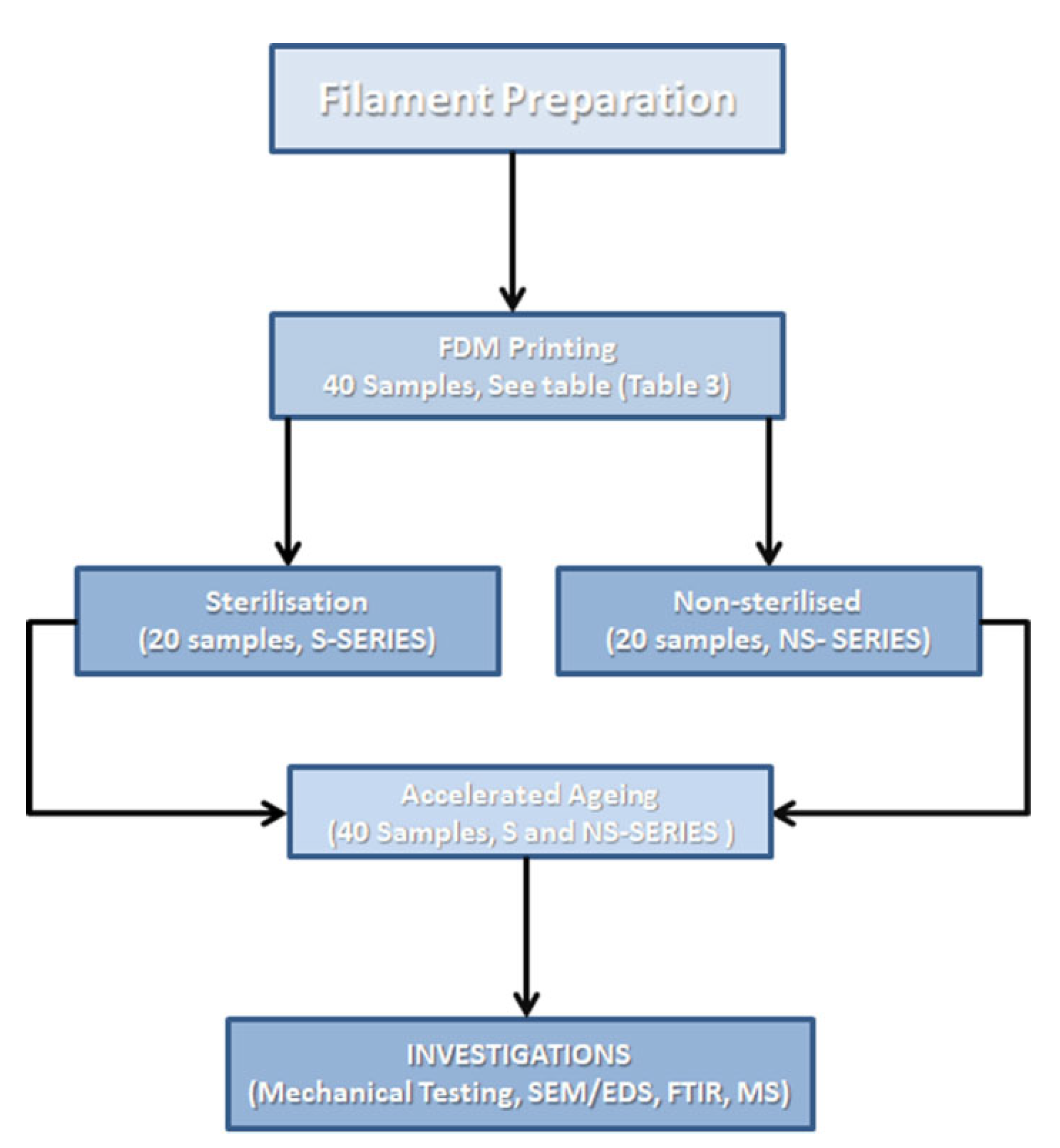
2. Materials and Methods
2.1. Materials and Samples
2.1.1. Ceramic Filler Modification
2.1.2. Filament Preparation
2.1.3. Sample Preparation
2.2. Sterilization
2.3. Artificial Ageing Protocol
- simulated body solution (SBF)—artificial saliva (according to EN ISO 10993-15:2000, chemical composition, Table 4),
- Q10—2,
- T—97 °C,
- TREF—37 °C,
- RTE—365 days.
2.4. Artificial Ageing Process
2.5. Mechanical Tests
2.6. FTIR—Fourier Transform Infrared
2.7. MS—Mass Spectrometry
2.8. SEM/EDS—Scanning Electron Microscopy/Energy Dispersive Spectroscopy
2.9. Saturation Test
3. Results and Discussion
3.1. Results
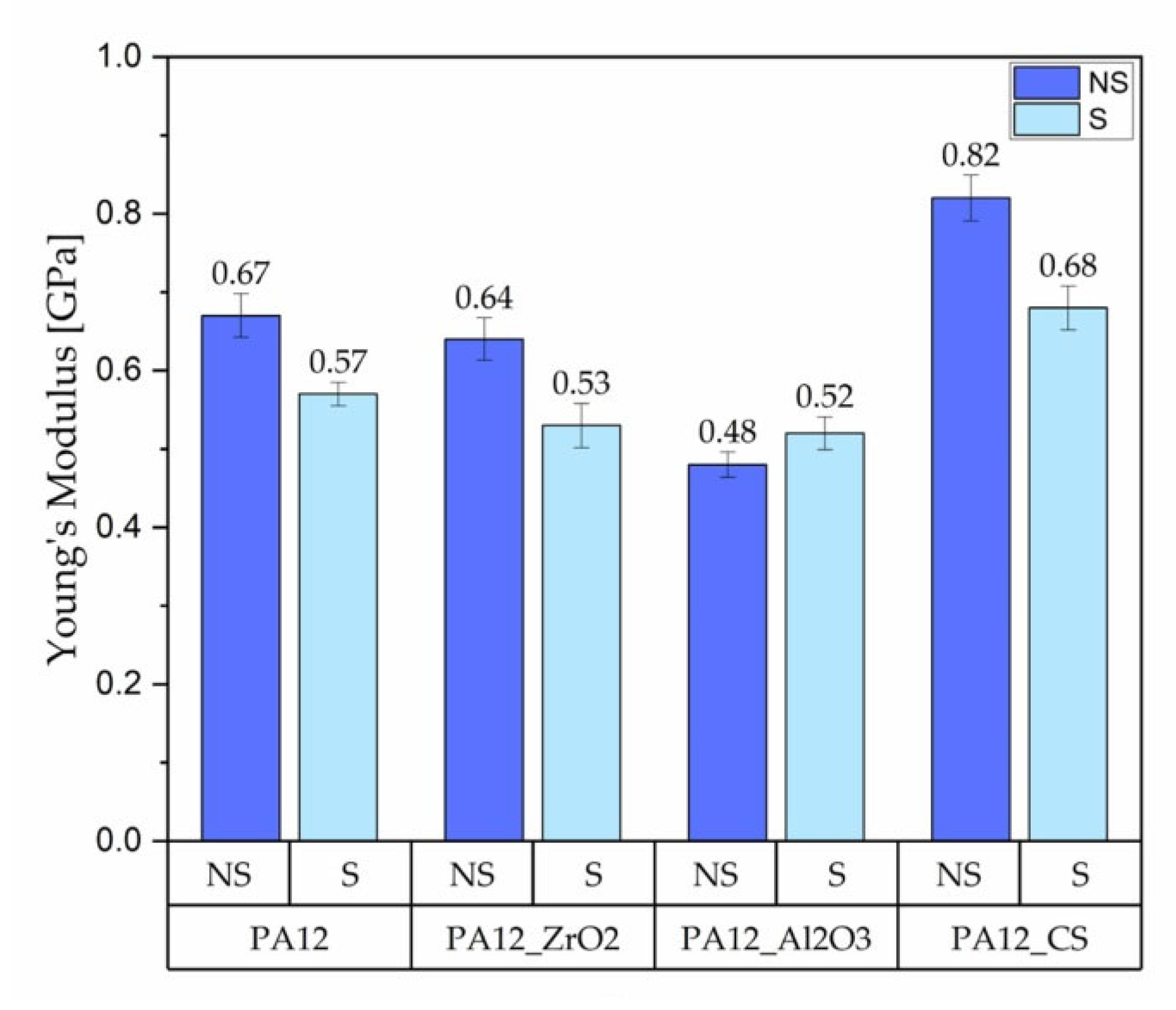
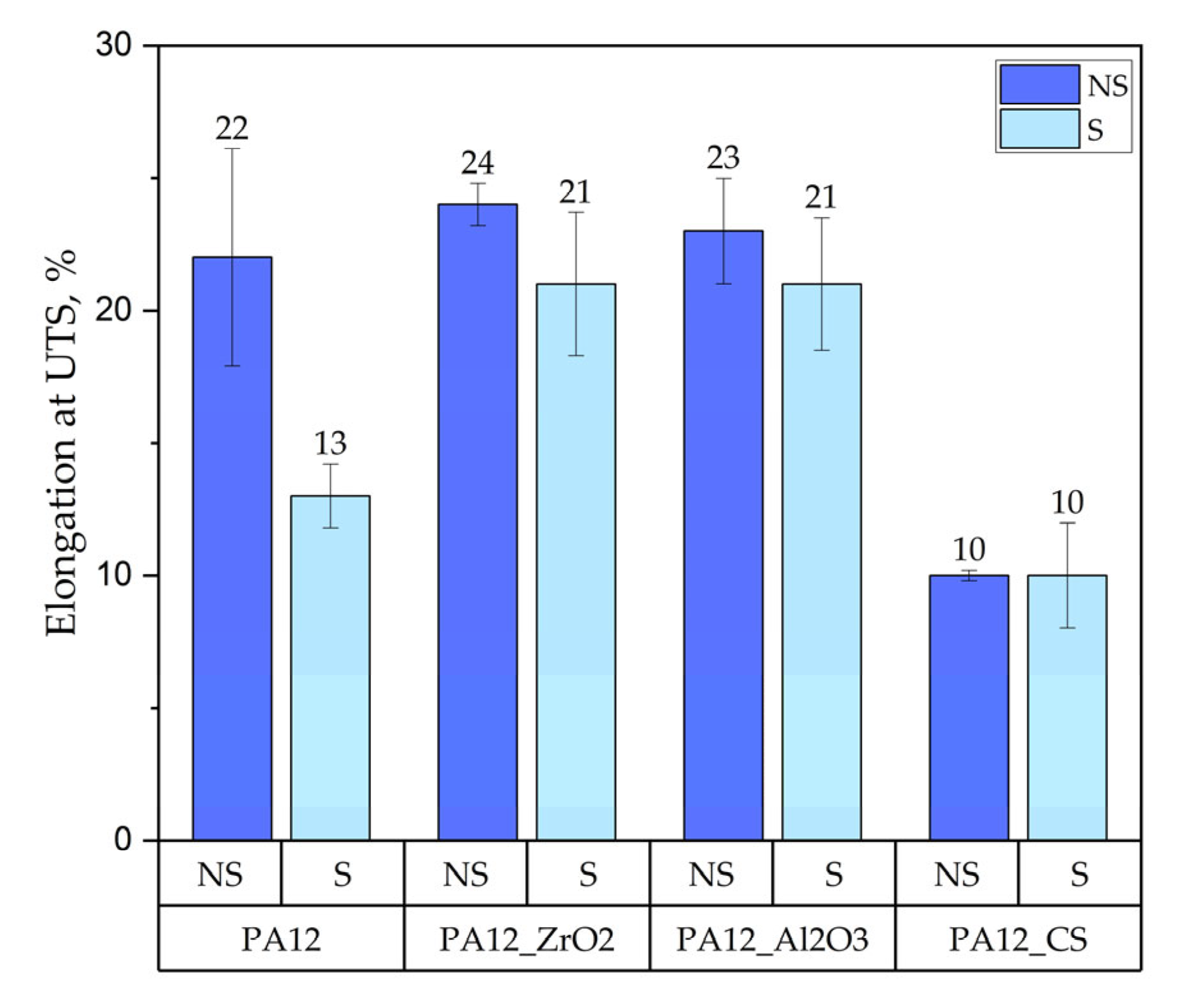
3.1.1. Mechanical Testing
Microhardness
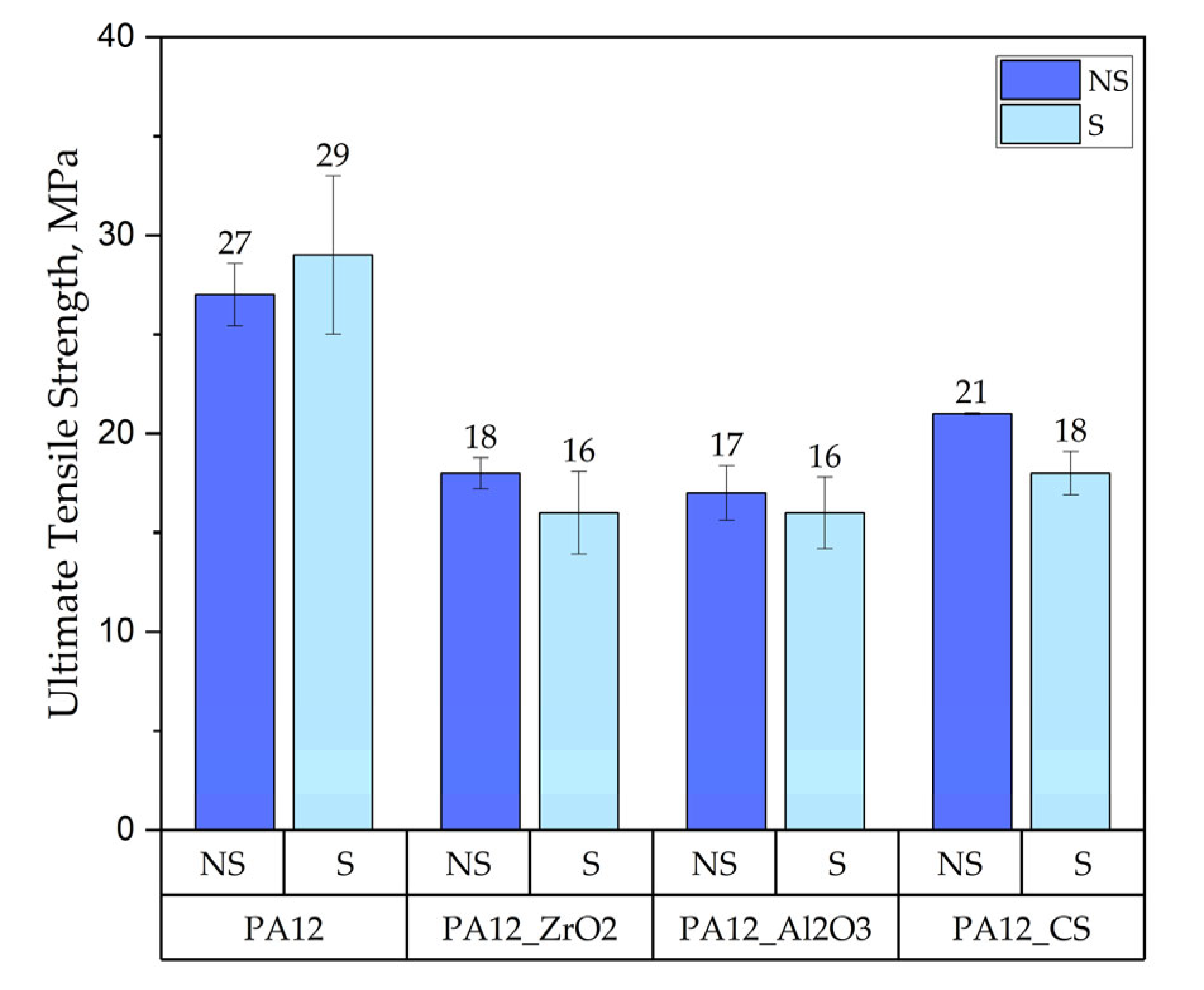
Tensile Test
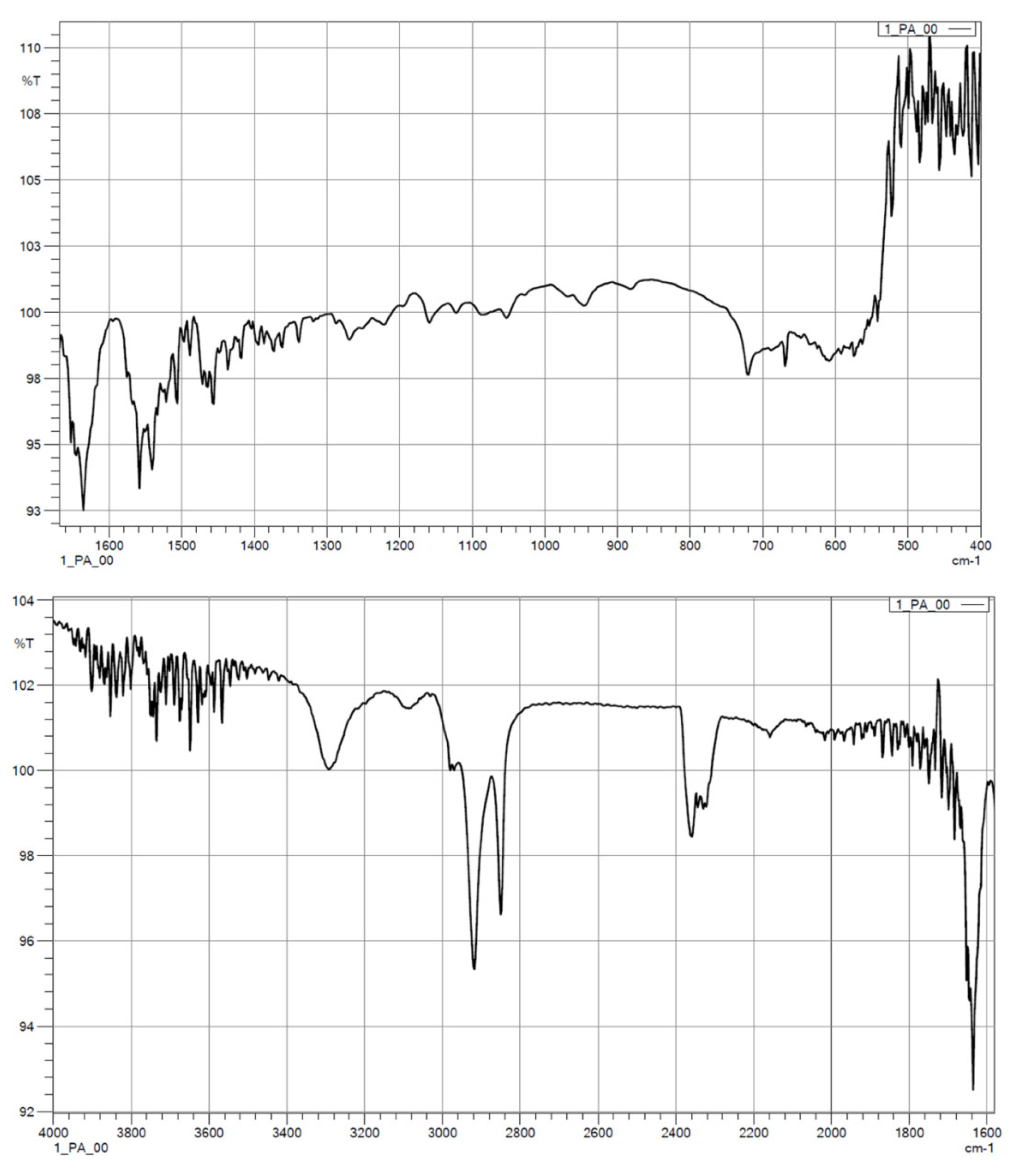
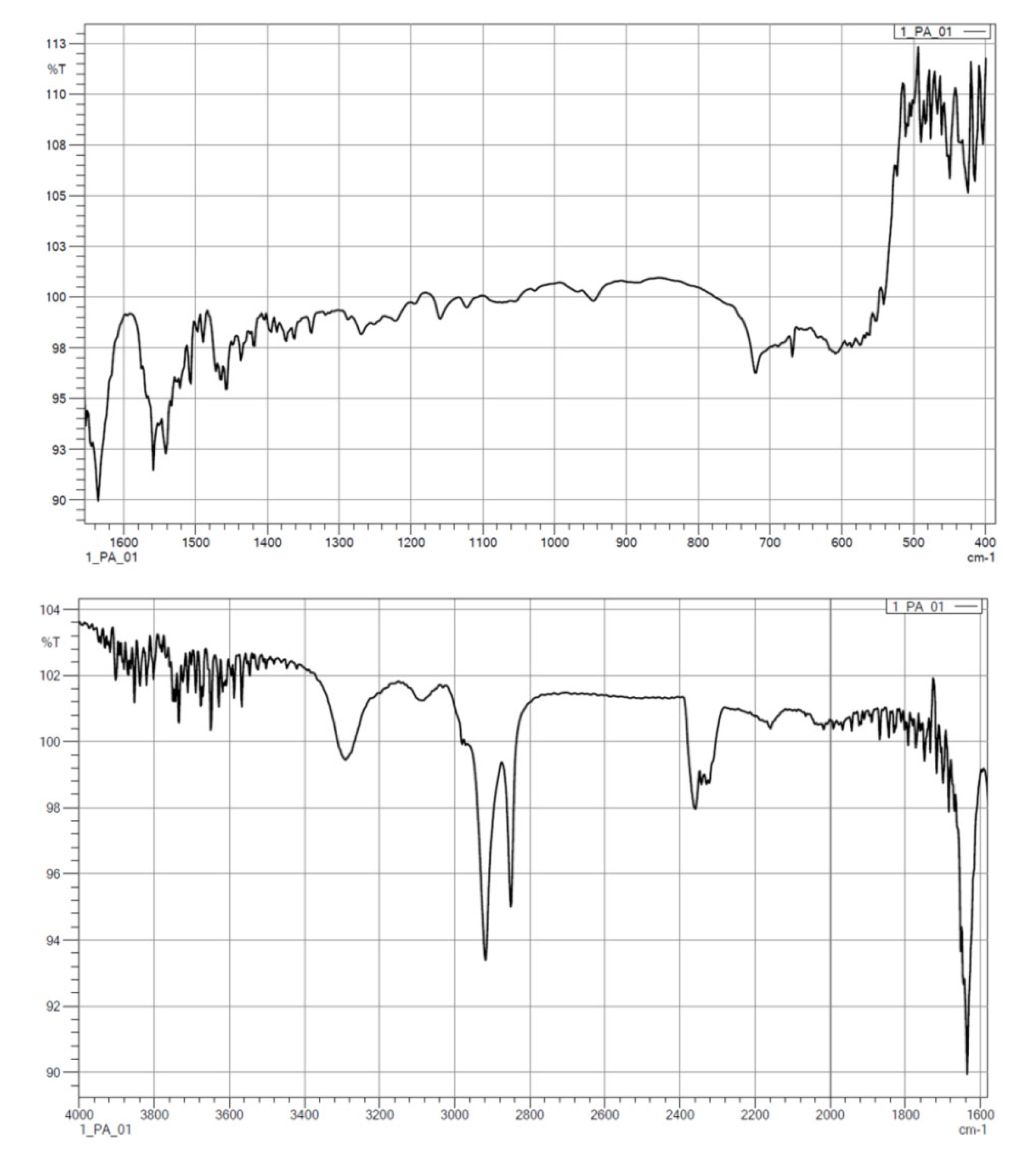
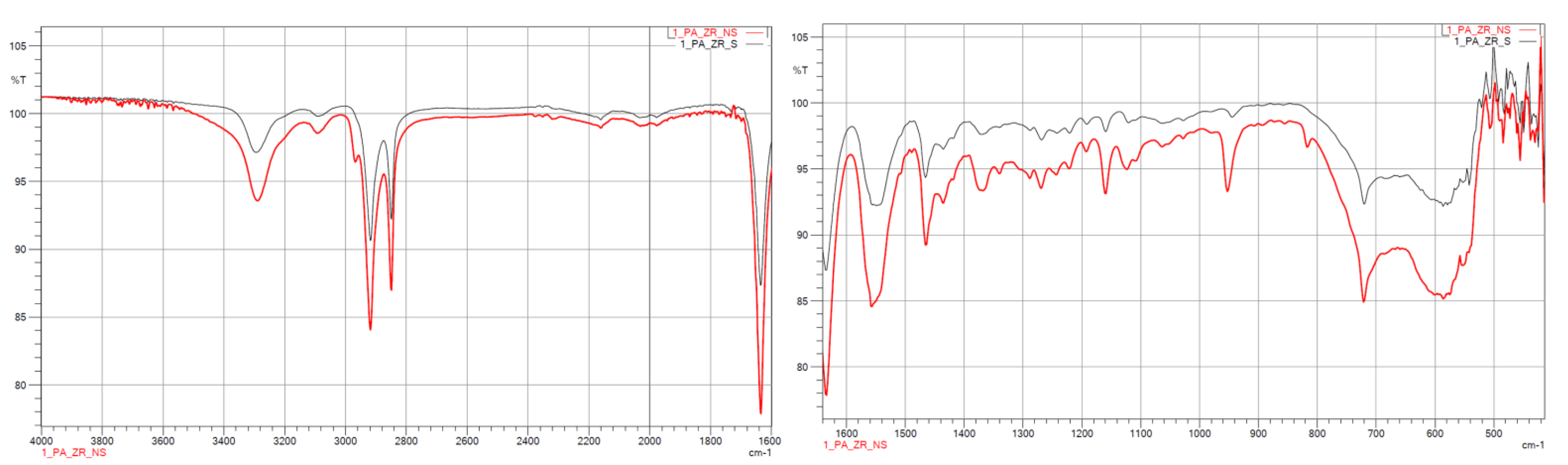
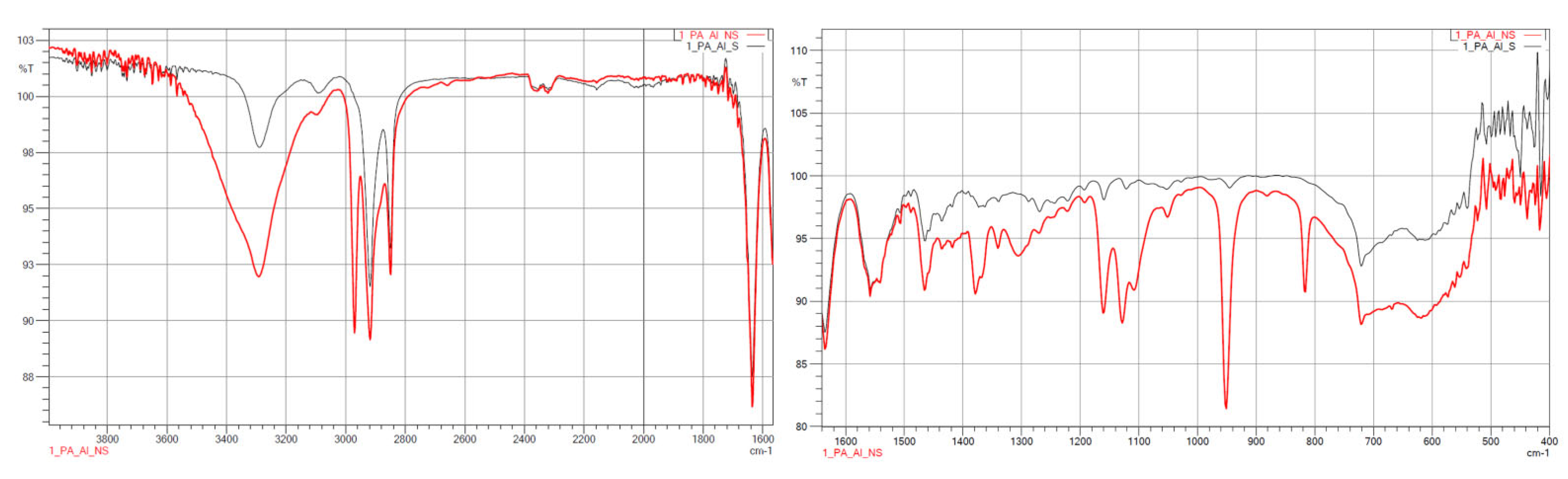
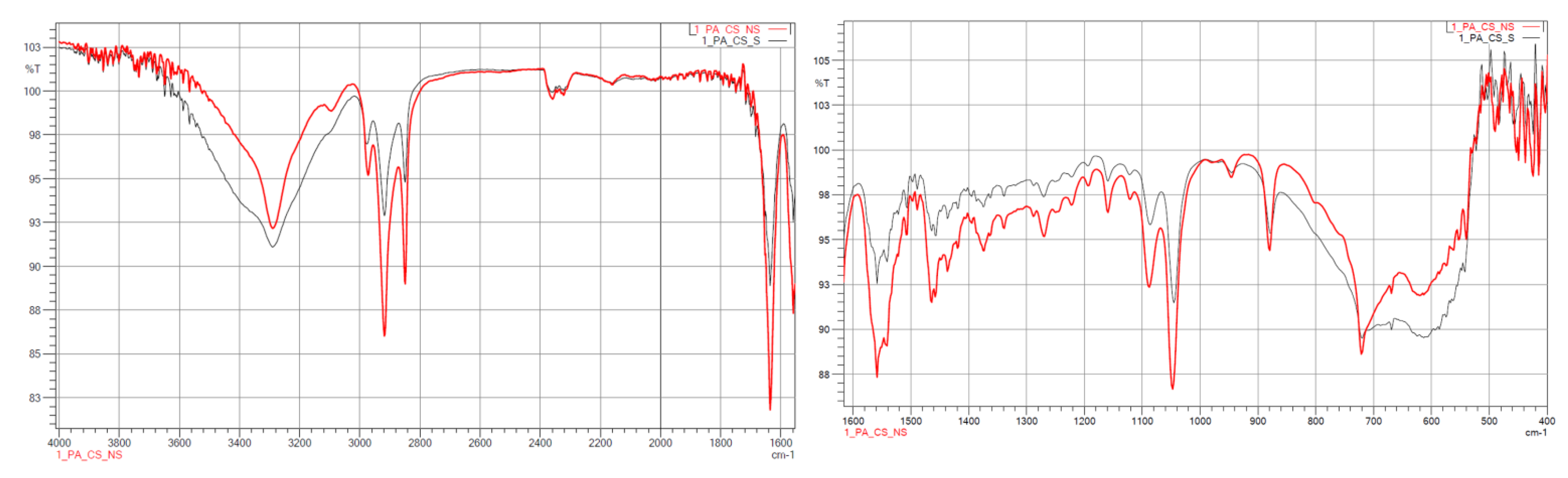
3.1.2. FTIR
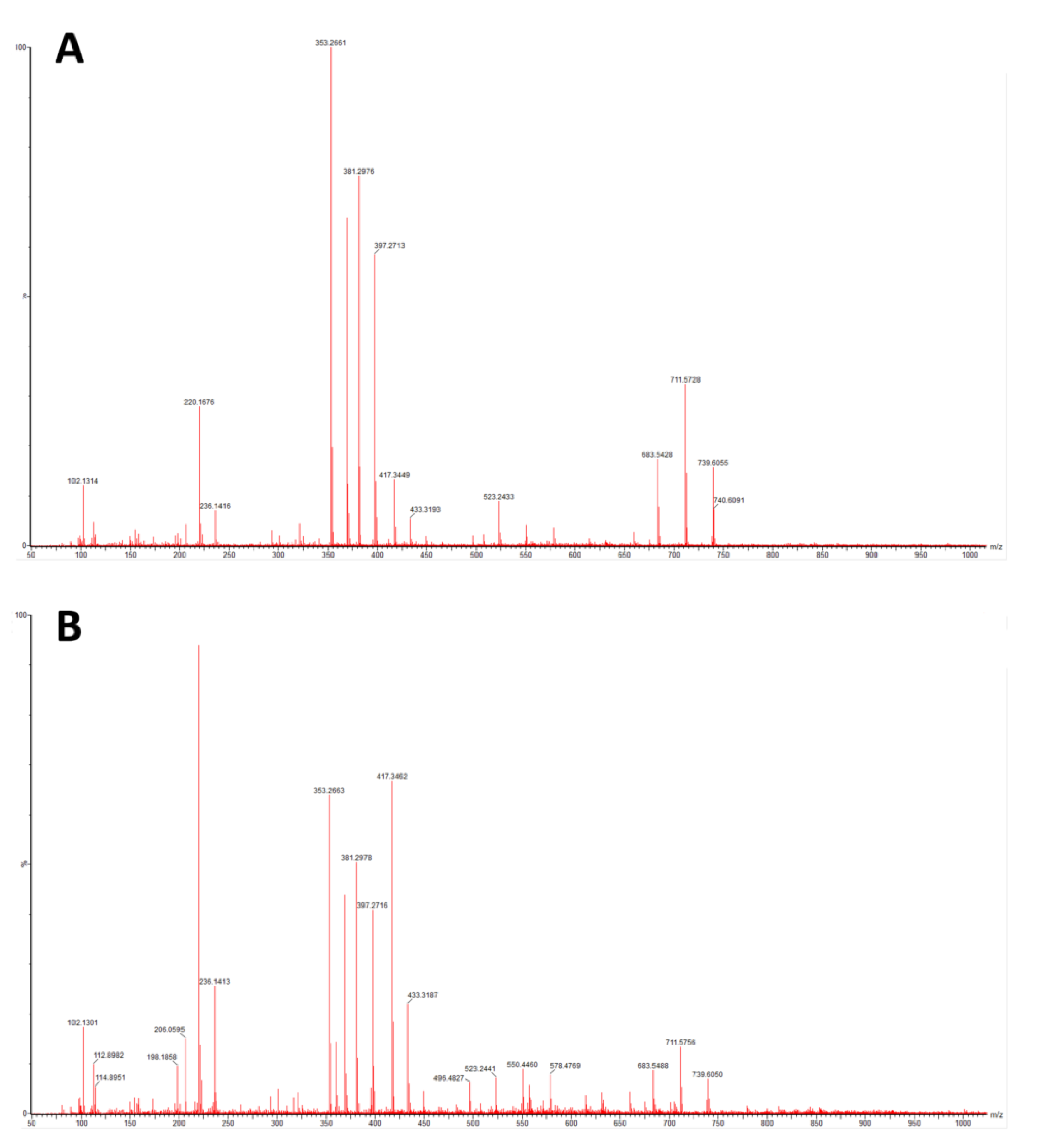
3.1.3. MS
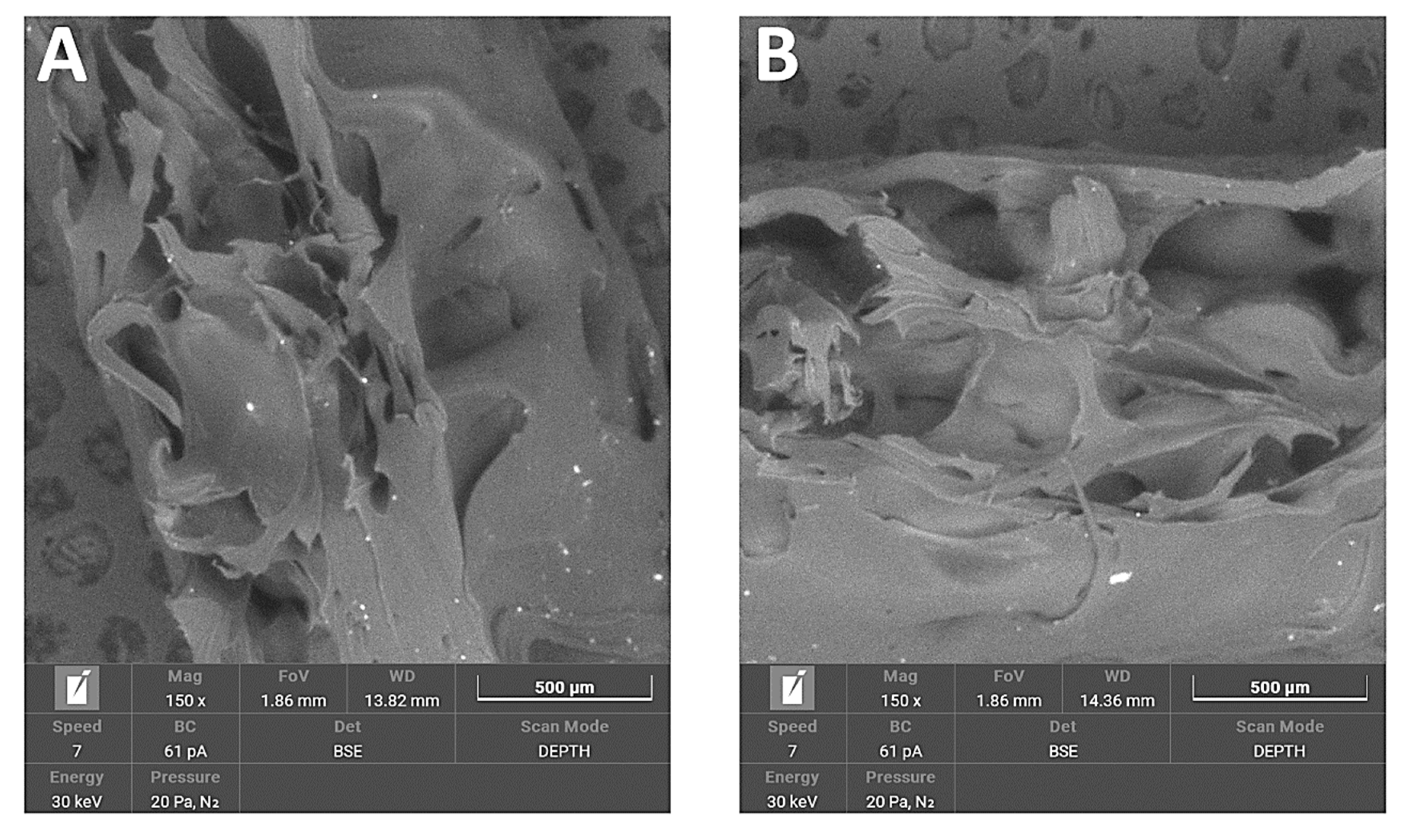
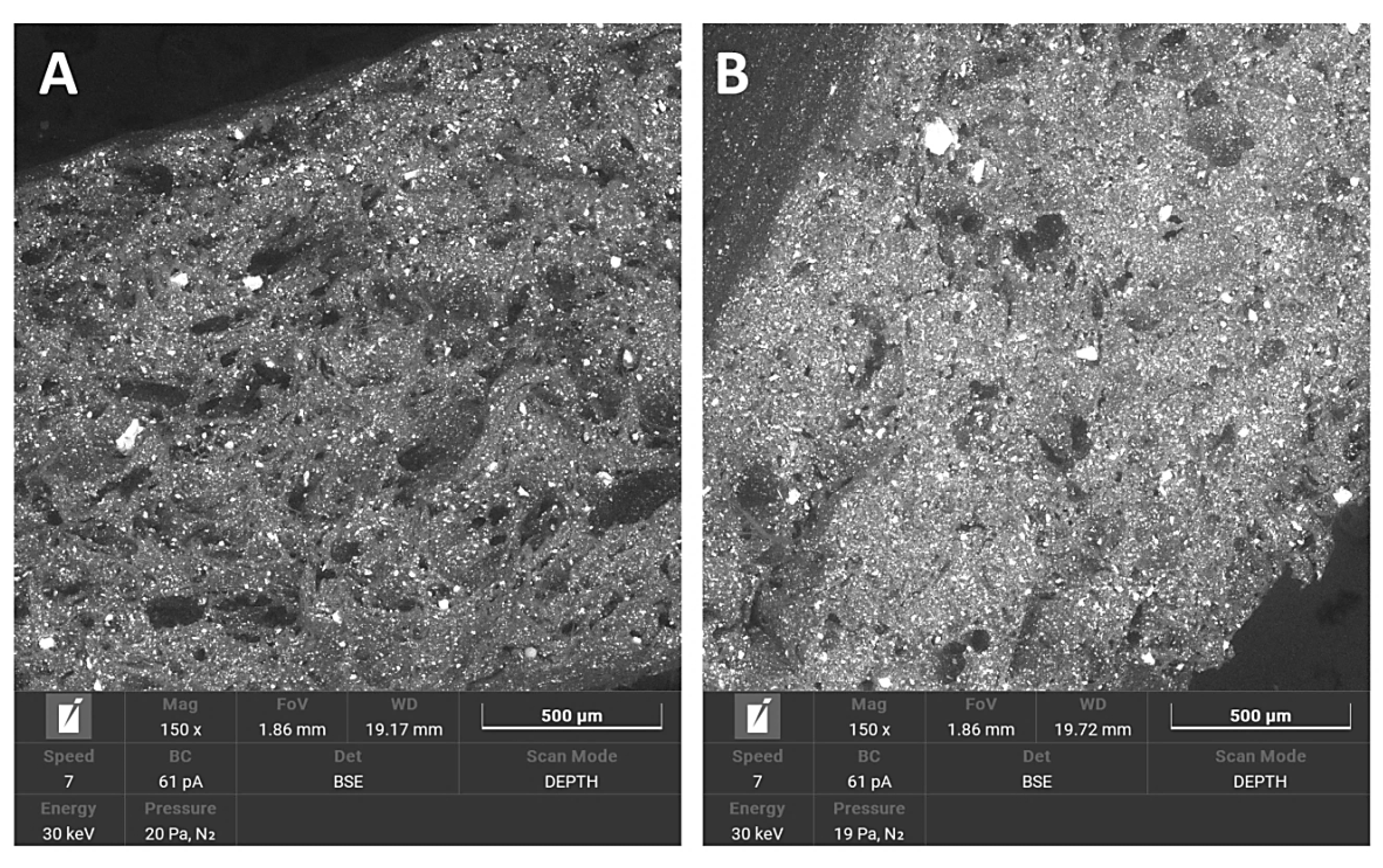
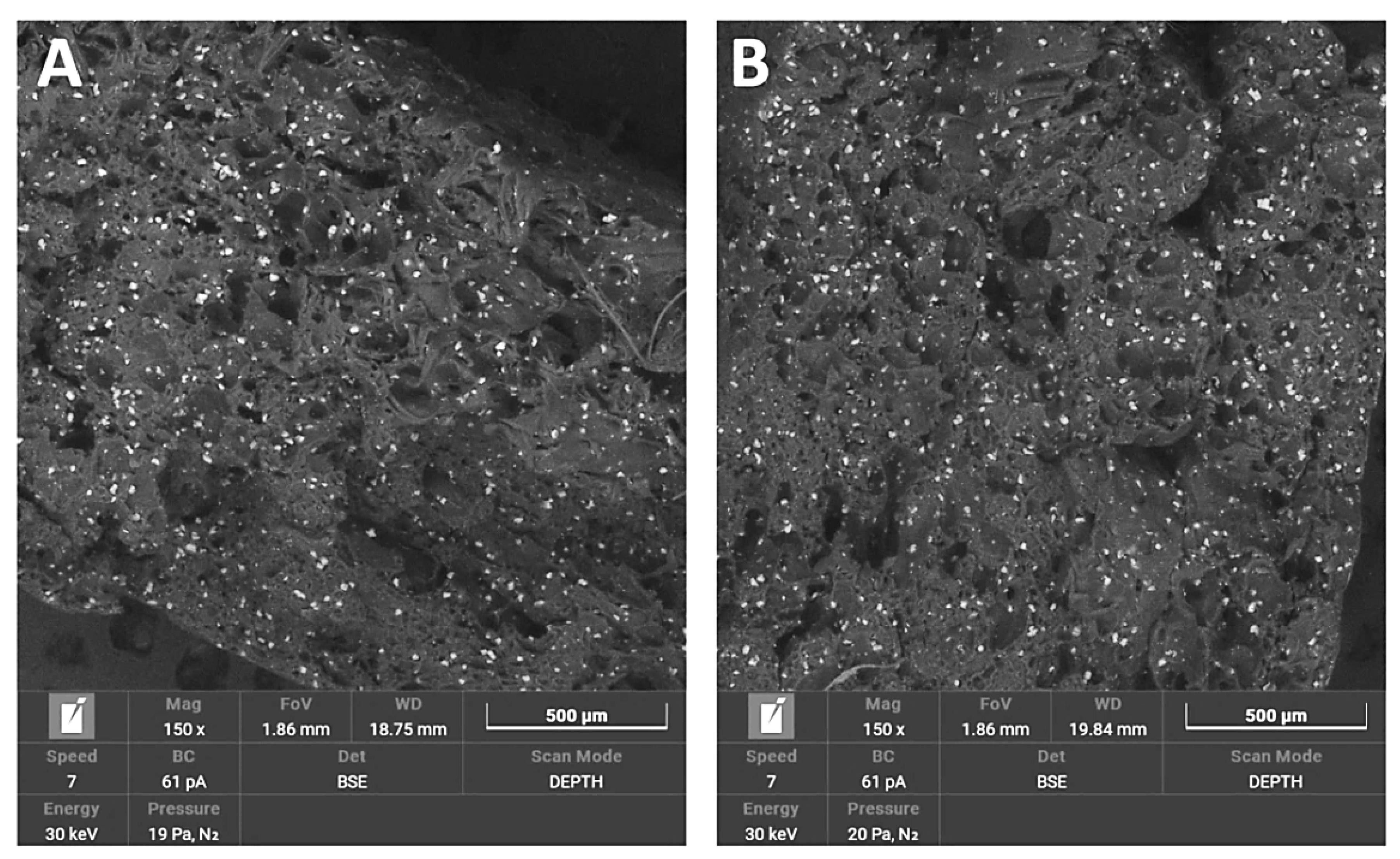
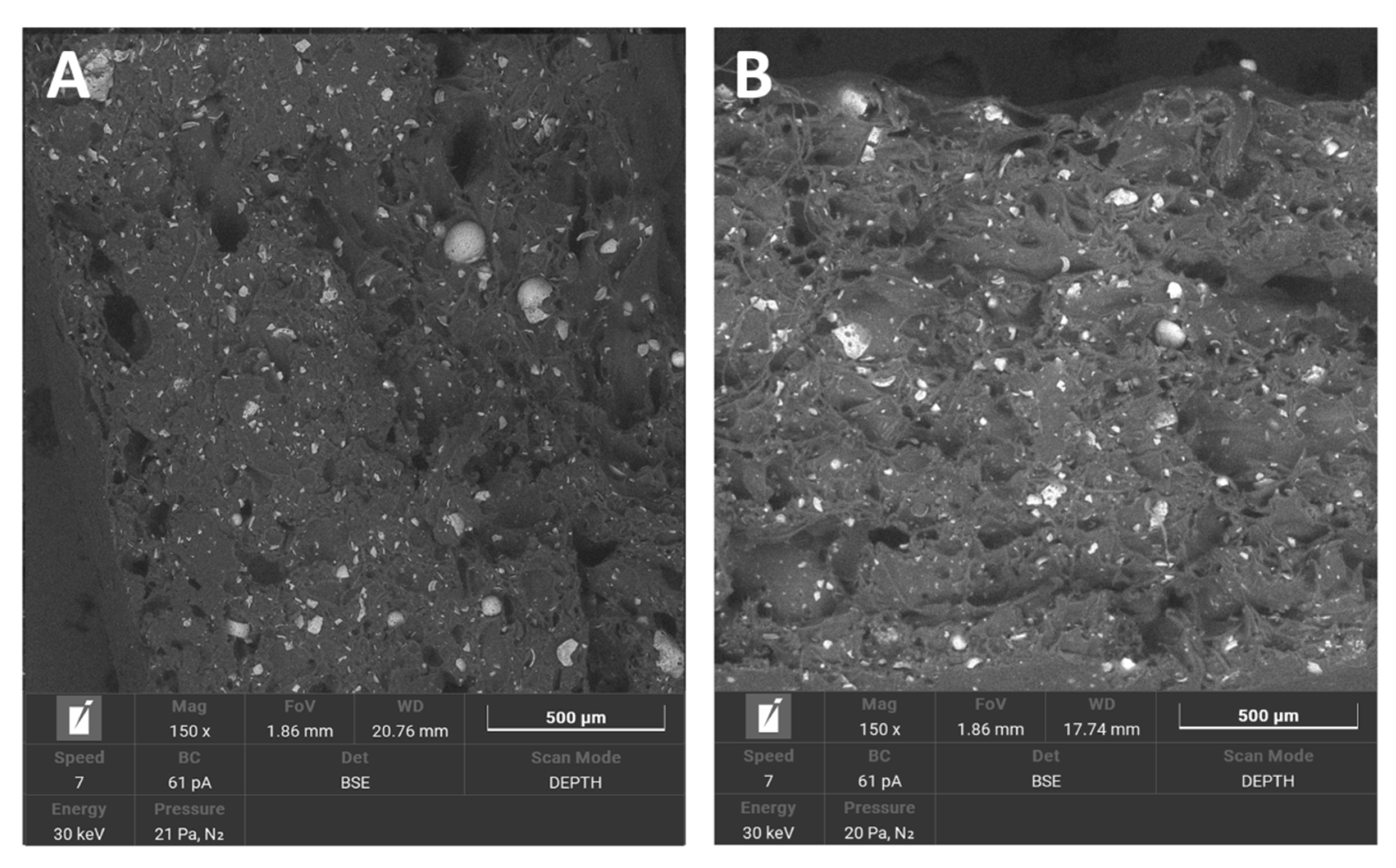
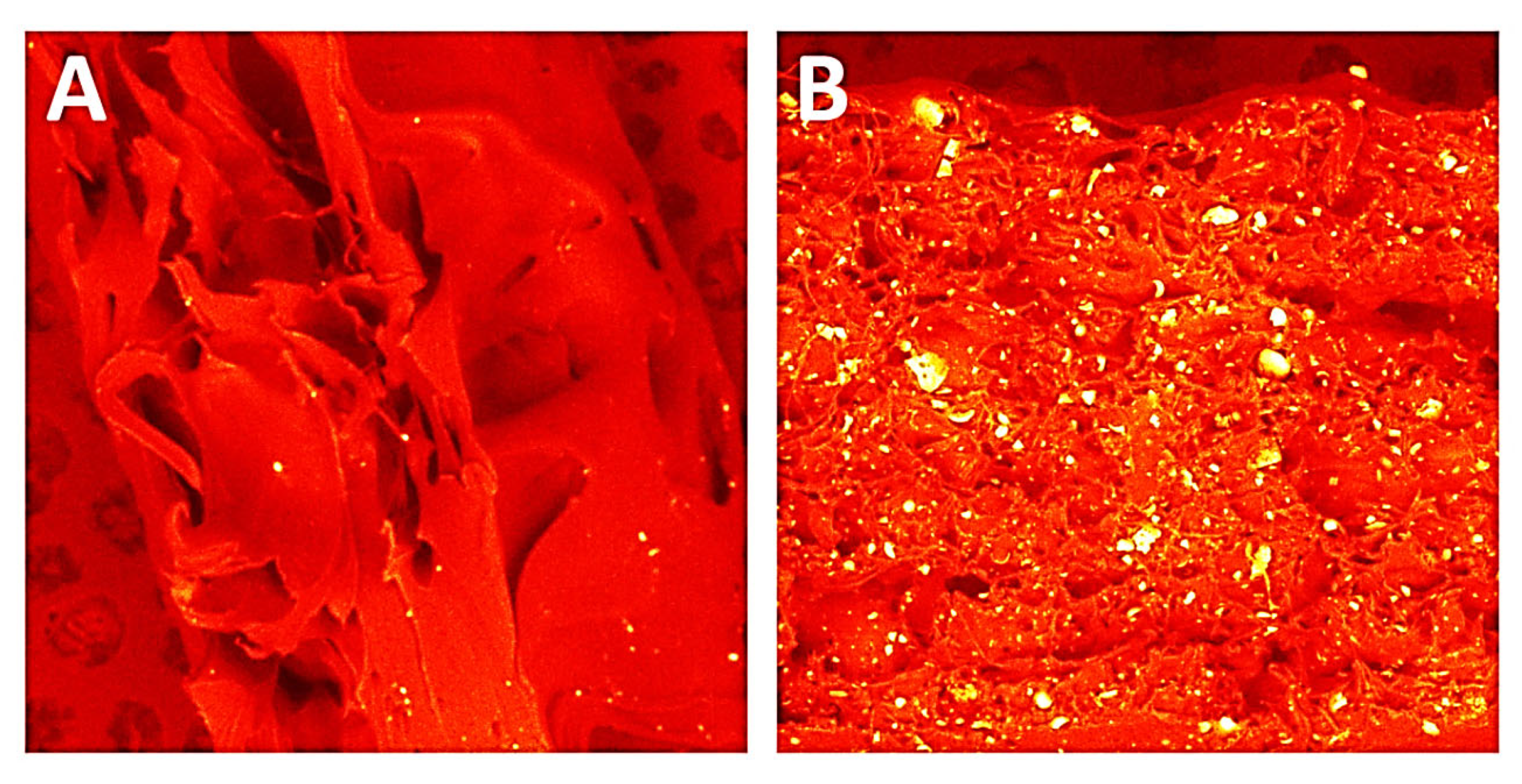
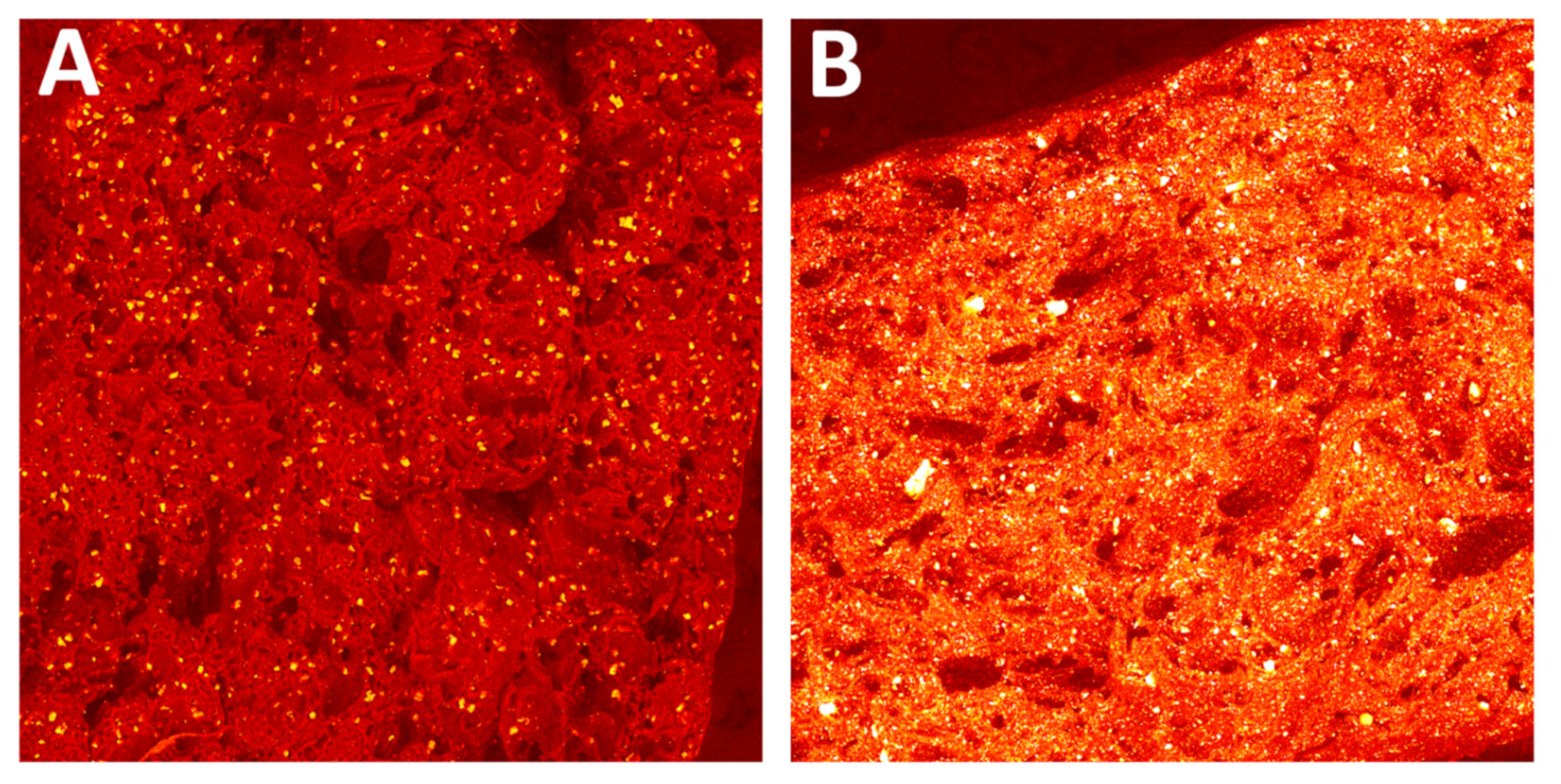
3.1.4. SEM/EDS
3.1.5. Saturation Test
3.2. Disscussion
- for composites with ZrO2, for composites without ageing the values were E = 0.63 GPa, UTS = 11 MPa, elongation 9 %, HVIT = 11, HIT, = 120 MPa; while for composites after ageing: E = 0.64 GPa, UTS = 18 MPa, elongation 24 %, HVIT = 9, HIT, = 100 MPa;
- for composites with Al2O3, for composites without ageing the values were E = 0.62 GPa, UTS = 13 MPa, elongation 14 %, HVIT = 17, HIT, = 175 MPa; while for composites after ageing: E = 0.48 GPa, UTS = 17 MPa, elongation 23 %, HVIT = 15, HIT, = 125 MPa;
- for composites with CS, for composites without ageing the values were E = 0.82 GPa, UTS = 21 MPa, elongation 10 %, HVIT = 29, HIT, = 302 MPa; while for composites after ageing: E = 0.68 GPa, UTS = 21 MPa, elongation 10 %, HVIT = 27, HIT, = 290 MPa;
4. Conclusions
- in all of the prepared composites, changes in the values of mechanical properties
- as a result of artificial ageing have been observed; in comparison to earlier studies [22,32], a decrease in the value of the Young’s Modulus as well as compression set and UTS have been observed; in contrast, the UTS of non-sterilised PA-12 samples increased after ageing; it is difficult to discern any correlation between the ageing process and changes in microhardness and hardness due to large variability; therefore, further studies are required;
- EO sterilisation tended to cause deterioration of mechanical properties. However, in the case of hardness changes there is a large variability, hence further research is needed on this topic;
- slight interaction with artificial saliva was found during ageing in PA-12 and composite samples as evidenced by the presence of K and Cl ions observed using EDS;
- artificial saliva testing with MS after ageing did not reveal the presence of PA-12 breakdown products or any composite components;
- in the case of PA-12 with ceramic fillers, the absorbability is a function of the filler density. The sample absorbability is greater with increased density and decreased volume.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; Rehman, S.; Matinlinna, J.K.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021, 33, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczny, D.S.; Antonowicz, M.; Paszenda, Z.K. Cenospheres and their applciations in biomedical engineering—A systematic review. Rev. Adv. Mater. Sci. 2020, 59, 115–130. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metlas for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Nakonieczny, D.S.; Ziębowicz, A.; Paszenda, Z.K.; Krawczyk, C. Trends and perspectives in modification of zirconium oxide for dental prosthetic applications—A review. Biocybern. Biomed. Eng. 2017, 37, 229–245. [Google Scholar] [CrossRef]
- Li, J.; Jansen, A.; Walboomers, X.F.; Beucken, J.J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, S.; Tanous, M.; Hajimahmoudi, M.; Mahgoli, H. Effect of ageing on fracture resistance and torque loss of restorations supported by zirconia and polyetheeetherketone abutments: An in vitro study. J. Prosthet. Dent. 2021, 125, 501.e1–501.e6. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Correia, M.S.T.; Noronha Oliviera, M.; Silva, F.S.; Henriques, B.; Oliveira, A.P.N.; Gomes, J.R. PEEK-matrix composites containing different content of natural silica fibers or particulate lithium-zirconium silicate glass fillers: Coefficient of friction and wear volume. Biotri 2020, 24, 100147. [Google Scholar] [CrossRef]
- Vasques, W.F.; Sa, T.A.; Martins, F.V.; Fonseca, E.M. Composite resin CAD-CAM restorations for a midline diastema closure: A clinical report. J. Prosthet. Dent. 2020, 127, 206–209. [Google Scholar] [CrossRef]
- Miura, S.; Fujisawa, M. Current status and perspective of CAD/CAM-produced resin composite crowns: A review of clinical effectiveness. Jpn. Dent. Sci. Rev. 2020, 56, 184–189. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Zimmermann, T.; Spintig, T.; Emara, M.A.; Lackamnn, J.; Muller, W.D.; Houshmand, A. Maximum insertion torque of novel implant-abutment-interface design for PEEK dental implants. J. Mech. Behav. Biomed. Mater. 2018, 77, 85–89. [Google Scholar] [CrossRef]
- Zimmermann, T.; Montero, A.F.; Lieblich, M.; Ferrari, B.; Gonzalez-Carrasco, J.L.; Muller, W.D.; Schwitalla, A.D. In vitro degradation of a biodegradable polyalactic acid/magnesium composite as potential bone augmentation materials in the presence of titanium and PEEK dental implants. Dent. Mater. 2018, 34, 1492–1500. [Google Scholar] [CrossRef]
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y.G. PEEK (Polyether-ether-ketone) and its composite materials in orthopedic implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Senthilvelan, T. Polymer composites in dentistry and orthopedic applications-a review. Mater. Today Proc. 2021, 46, 9707–9713. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Mozzoni, B.; Rivara, F.; Ghezzi, B.; Cutrera, M.; Lumetti, S.; Macaluso, G.M. Is selective protein adsorption on biomaterials a viable option to promote periodontal regeneration? Med. Hypo. 2019, 132, 109388. [Google Scholar] [CrossRef]
- Hukins, D.W.L.A.; Mahomed, A.; Kukureka, S.N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Hammerlich, K.J. General aging therory and simplified protocol for acclerated aging of medical devices. Med. Plast. Biomater. 1998, 5, 16–23. [Google Scholar]
- Maxwell, A.; Sims, G.; Broughton, W.R. Review of Accelarated Ageing Methods and Liftime Prediction Techniques for Polymeric Materials. 2005. Npl Report, Depc Mpr 016. Available online: https://www.semanticscholar.org/paper/Review-of-accelerated-ageing-methods-and-lifetime-Maxwell-Broughton/21f8eab8dae9fc64ada70d5de4d0b43ea2a19fc3 (accessed on 11 May 2022).
- Standard ISO 13356; Implants for Surgery—Ceramic Materials Based on Yttria-Stabilized Tetragonal Zirconia (Y-TZP). 2015. Available online: https://www.iso.org/standard/62373.html (accessed on 11 May 2022).
- Simha Martynková, G.; Slíva, A.; Kratošová, G.; Cech Barabaszova, K.; Studentova, S.; Klusak, J.; Brozova, S.; Dokoupil, T.; Holesova, S. Polyamide 12 Materials Study of Morpho-Structural Changes during Laser Sintering of 3D Printing. Polymers 2021, 13, 810. [Google Scholar] [CrossRef]
- Alterary, S.S.; Alyabes, R.M.; Alshahrani, A.A.; Monirah, A.A.A. Unfunctionalized and Functionalized Multiwalled Carbon Nanotubes/Polyamide Nanocomposites as Selective-Layer Polysulfone Membranes. Polymers 2022, 14, 1544. [Google Scholar] [CrossRef]
- Standard ISO 527; Plastics—Determination of Mechanical Properties in Static Tension—Part 1: General Principles. ISO: Geneva, Switzerland, 2012.
- Nakonieczny, D.S.; Kern, F.; Dufner, L.; Antonowicz, M.; Matus, K. Alumina and Zirconia reinforced polyamide PA-12 composites for biomedical additive manufacturing. Materials 2021, 14, 6201. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Kern, F.; Dufner, L.; Dubiel, A.; Antonowicz, M.; Matus, K. Effect of calcination temperatures on the phase composition, morphology and thermal properties of ZrO2 and Al2O3 for biomedical applications modified with APTES (3-aminopropyltriethoxysilane). Materials 2021, 14, 6651. [Google Scholar] [CrossRef]
- ASTM F1980-16; Standard Guide for Accelrated Aging of Sterile Barrier Systems for Medical Devices. Available online: https://webstore.ansi.org/Standards/ASTM/ASTMF198016?gclid=Cj0KCQjw852XBhC6ARIsAJsFPN3-dL0trM4eUIMm5Iw4JMec5P1U25WjXnlWPSv-rGGCSW5jiig30pEaAhrmEALw_wcB (accessed on 11 May 2022).
- Madej-Kiełbik, L.; Kośla, K.; Zielińska, D.; Chmal-Fudali, E.; Maciejewska, M. Effect of Accelerated Ageing on the Mechanical and Structural Properties of the Material System Used in Protectors. Polymers 2019, 11, 1263. [Google Scholar] [CrossRef] [Green Version]
- Olivier, W.C.; Pharr, G.M.L. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Mousa, A.; Heinrich, G.; Wagenknecht, U.; Kretzschmar, B.; Landwehr, A.U. The Application of Di-isocyanate Modified Agro-polymer as Filler For XNBR/PA-12 Thermoplastic Elastomer Composites. J. Macromol. Sci. Part A 2012, 49, 385–396. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Paggi, R.A.; Lago, A.; Beal, V.E. Microstructural and mechanical characterization of PA12/MWCNTs nanocomposite manufactured by selective laser sintering. Poly. Test. 2011, 30, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Kuracina, R.; Szabová, Z.; Buranská, E.; Pastierova, A.; Gogola, E.; Buransky, I. Determination of Fire Parameters of Polyamide 12 Powder for Additive Technologies. Polymers 2021, 13, 3014. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 381, 388–391. [Google Scholar] [CrossRef]
- Culler, S.R.; Ishida, I.; Koenig, J.L. Structure of silane coupling agents adsorber on silicon powder. J. Colloid Interface Sci. 1985, 106, 334–345. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Antonowicz, M.; Heim, T.; Swinarew, A.S.; Nuckowski, P.; Matus, K.; Lemanowicz, M. Cenospheres-reinforced PA-12 composite: Preparation, physicochemical properties and soaking tests. Polymers 2022, 14, 2332. [Google Scholar] [CrossRef]
- Horakova, J.; Mikes, P.; Saman, A.; Jencova, V.; Klapstova, A.; Svarcova, T.; Ackermann, M.; Novotny, V.; Suchy, T.; Lukas, D. The effect of ethylene oxide sterilization on electrospun vascular grafts made from biodegradable polyesters. Mater. Sci. Eng. C 2018, 92, 132–142. [Google Scholar] [CrossRef]
- Sethy, S.; Samantaray, S.K.; Satapathy, B.K. Dynamic crystallization behavior of PA-12/PP-MWCNT nanocomposites: Non-isothermal kinetics approach. J. Polym. Eng. 2021, 42, 87–99. [Google Scholar] [CrossRef]
- ZRO-T6 IMERYS, MSDS, IMERYS. Available online: https://www.imerys.com/ (accessed on 11 May 2022).
- Sumitomo, Sumicorundum AA-18, MSDS. Sumitomo. Available online: https://www.sumitomocorp.com/en/jp (accessed on 11 May 2022).
- Touris, A.; Turcios, A.; Mintz, E.; Pulugurtha, S.R.; Thor, P.; Jolly, M.; Jalgaonkar, U. Effect of molecular weight and hydration on the tensile properties of polyamide 12. Res. Mater. 2020, 8, 100149. [Google Scholar] [CrossRef]
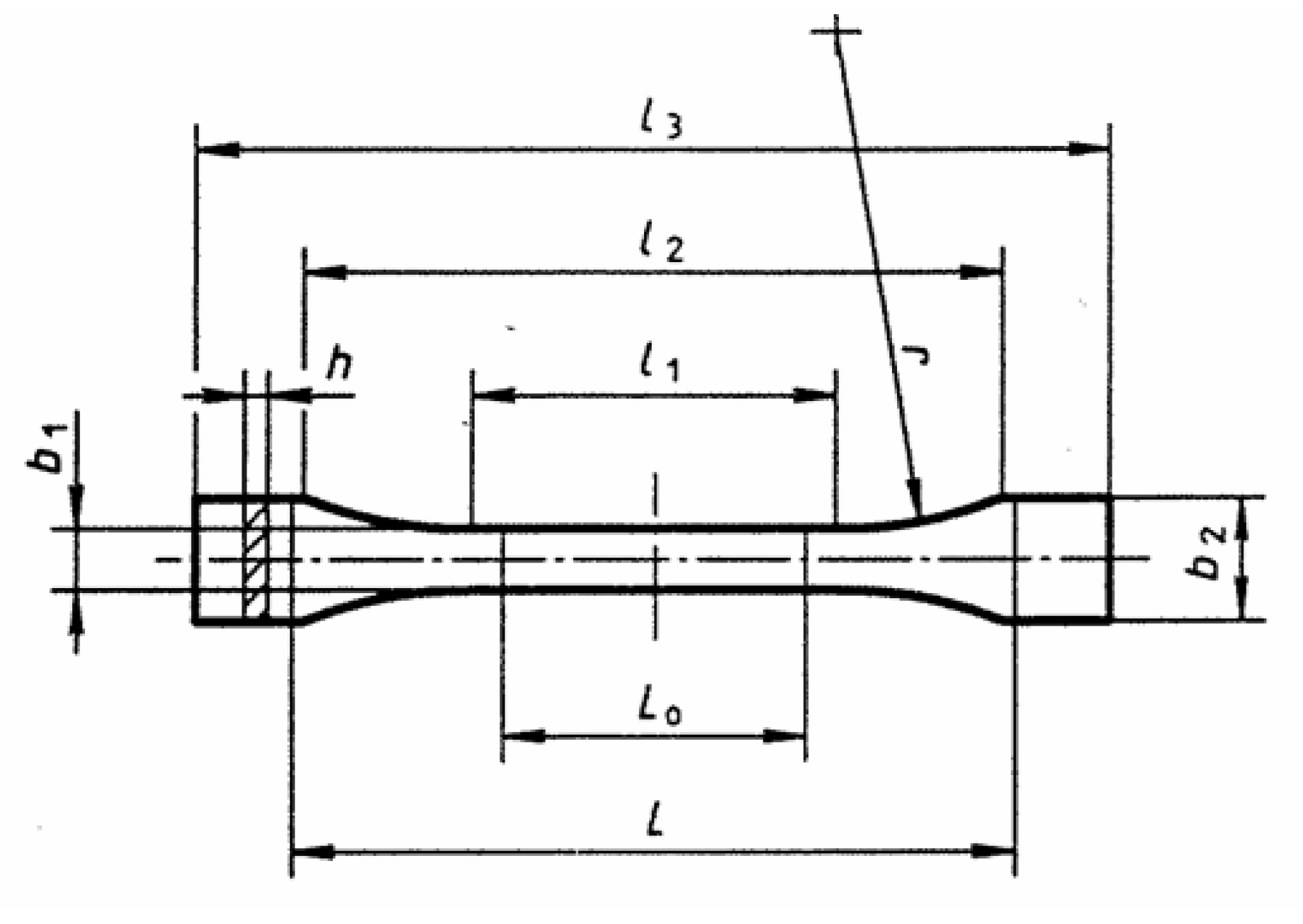
| Dimensions of the Sample | Dimensions, mm |
|---|---|
| l3—overall length | 75 |
| l1—length of narrow parallel-sided portion | 30.5 |
| r—radius | 37 |
| l2—distance between broad parallel-sided portions | 57.5 |
| b2—width at ends | 10 |
| b1—width of narrow portion | 5 |
| h—thickness | 2.35 |
| L0—gauge length | 25 |
| L—initial distance between grips | 54 |
| Printing Parameters | ||
|---|---|---|
| Material | PA-ZrO2 | PA-Al2O3 |
| Nozzle diameter (mm) | 0.5 mm | 0.5 mm |
| Layer thickness (mm) | 0.35 mm | 0.35 mm |
| Build orientation | × (horizontally) | × (horizontally) |
| Infill density | 100% | 100% |
| Infill pattern | Linear aligned (0°) | Linear aligned (0°) |
| Outer layers | 2 | 2 |
| Extruder temp. (°C) | 210 °C | 210 °C |
| Samples | Description | Non-Sterilised Samples | Sterilised Samples |
|---|---|---|---|
| PA12 | Pure polyamide | PA12_NS (5 samples) | PA12_S (5 samples) |
| PA12_ZrO2 | Polyamide and zirconia | PA12_ZrO2_NS (5 samples) | PA12_ZrO2_S (5 samples) |
| PA12_Al2O3 | Polyamide and alumina | PA12_Al2O3_NS (5 samples) | PA12_Al2O3_S (5 samples) |
| PA12_CS | Polyamide and cenosphere | PA12_CS_NS (5 samples) | PA12_CS_S (5 samples) |
| Compound | Na2HPO4 | NaCl | KSCN | KH2PO4 | NaHCO3 | KCl |
|---|---|---|---|---|---|---|
| Concentration, g/L | 0.260 | 0.700 | 0.330 | 0.200 | 1.500 | 1.200 |
| Settings | Characteristics |
|---|---|
| Polarity: | ES+ |
| Analyzer: | Resolution Mode |
| Capillary (kV): | 40,000 |
| Sampling Cone: | 200,000 |
| Extraction Cone: | 40,000 |
| Source Temperature (°C): | 120 |
| Desolvation Temperature (°C): | 200 |
| Cone Gas Flow (L/Hr): | 50.0 |
| Desolvation Gas Flow (L/Hr): | 500.0 |
| PA12_NS | PA12_S | PA12 ZrO2_NS | PA12 ZrO2_S | PA12 Al2O3_NS | PA12 Al2O3_S | PA12 CS_NS | PA12 CS_S | |
|---|---|---|---|---|---|---|---|---|
| Vickers hardness, HVIT | 12 ± 2 | 11 ± 2 | 9 ± 2 | 16 ± 9 | 15 ± 1 | 13 ± 4 | 27 ± 2 | 22 ± 4 |
| Microhardness HIT, MPa | 122 ± 19 | 113 ± 9 | 100 ± 20 | 174 ± 18 | 125 ± 9 | 142 ± 31 | 290 ± 7 | 240 ±10 |
| Wave Number, cm−1 | Characteristics |
|---|---|
| 1500 | C=O |
| 1539–1645 | N–H, C–CO–NH2, C=O |
| 1540 | –NH |
| 1650 | –CO |
| 2800 | alkyl groups |
| 2900 | alkyl groups |
| 3500 | –OH |
| 3750 | free–NH |
| Samples | Wave Number, cm−1 | Characteristics |
|---|---|---|
| PA12_ZrO2_NS/S PA12_Al2O3_NS/S PA12_CS_NS/S | 460 | Si–O–Si |
| 1000 ÷ 1100 | Si–O | |
| 1484 | –NH2 | |
| 1500 | C=O | |
| 1539 ÷ 1645 | N–H, C–CO–NH2, C=O | |
| 1540 | –NH | |
| 1562 | –NH2 | |
| 1650 | –CO | |
| 2800 | alkyl groups | |
| 2900 | alkyl groups | |
| 3500 | –OH | |
| 3750 | free–NH |
| Sample | Chemical Composition, wt% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Al | Zr | N | Fe | Na | K | Mg | P | Si | S | Cl | |
| PA12 * | 84.7 | 15.3 | - | - | - | - | - | - | - | - | - | - | - |
| PA12_NS/S | 81.8 | - | - | - | - | - | 1.2 | 2 | - | - | - | - | - |
| PA12_ZrO2 * | 78.7 | 14.4 | - | 3.6 | 3.3 | - | - | - | - | - | - | - | - |
| PA12_ZrO2_NS/S | 76.0 | 15.1 | - | 3.5 | 3.2 | - | 0.5 | 0.7 | - | 0.3 | - | - | 0.7 |
| PA12_Al2O3 * | 75.1 | 20.1 | 3.0 | - | 1.8 | - | - | - | - | - | - | - | - |
| PA12_Al2O3_NS/S | 75.2 | 17.2 | 3.1 | - | 1.6 | - | 1.0 | 0.9 | - | 0.2 | - | - | 0.8 |
| PA12_CS * | 78.7 | 14.4 | 0.8 | - | 3.3 | 0.3 | - | - | 1.2 | - | 1.3 | - | - |
| PA12_CS_NS/S | 75.0 | 15.1 | 0.7 | - | 3.8 | 0.3 | 0.8 | 0.6 | 1.1 | 0.5 | 1.1 | - | 1.0 |
| Sample | Mass before the Absorption Measurement, g | Mass after Measurement of Absorption, g | Absorbability, % * |
|---|---|---|---|
| PA12 | 0.710 | 0.753 | 5.71 |
| PA12_ZrO2 | 0.854 | 0.867 | 1.50 |
| PA12_Al2O3 | 0.979 | 1.001 | 2.20 |
| PA12_CS | 0.793 | 0.801 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakonieczny, D.S.; Antonowicz, M.; SimhaMartynkova, G.; Kern, F.; Pazourková, L.; Erfurt, K.; Hüpsch, M. PA-12-Zirconia-Alumina-Cenospheres 3D Printed Composites: Accelerated Ageing and Role of the Sterilisation Process for Physicochemical Properties. Polymers 2022, 14, 3152. https://doi.org/10.3390/polym14153152
Nakonieczny DS, Antonowicz M, SimhaMartynkova G, Kern F, Pazourková L, Erfurt K, Hüpsch M. PA-12-Zirconia-Alumina-Cenospheres 3D Printed Composites: Accelerated Ageing and Role of the Sterilisation Process for Physicochemical Properties. Polymers. 2022; 14(15):3152. https://doi.org/10.3390/polym14153152
Chicago/Turabian StyleNakonieczny, Damian S., Magdalena Antonowicz, Gražyna SimhaMartynkova, Frank Kern, Lenka Pazourková, Karol Erfurt, and Michał Hüpsch. 2022. "PA-12-Zirconia-Alumina-Cenospheres 3D Printed Composites: Accelerated Ageing and Role of the Sterilisation Process for Physicochemical Properties" Polymers 14, no. 15: 3152. https://doi.org/10.3390/polym14153152
APA StyleNakonieczny, D. S., Antonowicz, M., SimhaMartynkova, G., Kern, F., Pazourková, L., Erfurt, K., & Hüpsch, M. (2022). PA-12-Zirconia-Alumina-Cenospheres 3D Printed Composites: Accelerated Ageing and Role of the Sterilisation Process for Physicochemical Properties. Polymers, 14(15), 3152. https://doi.org/10.3390/polym14153152











