Design and Preparation of Polyimide/TiO2@MoS2 Nanofibers by Hydrothermal Synthesis and Their Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
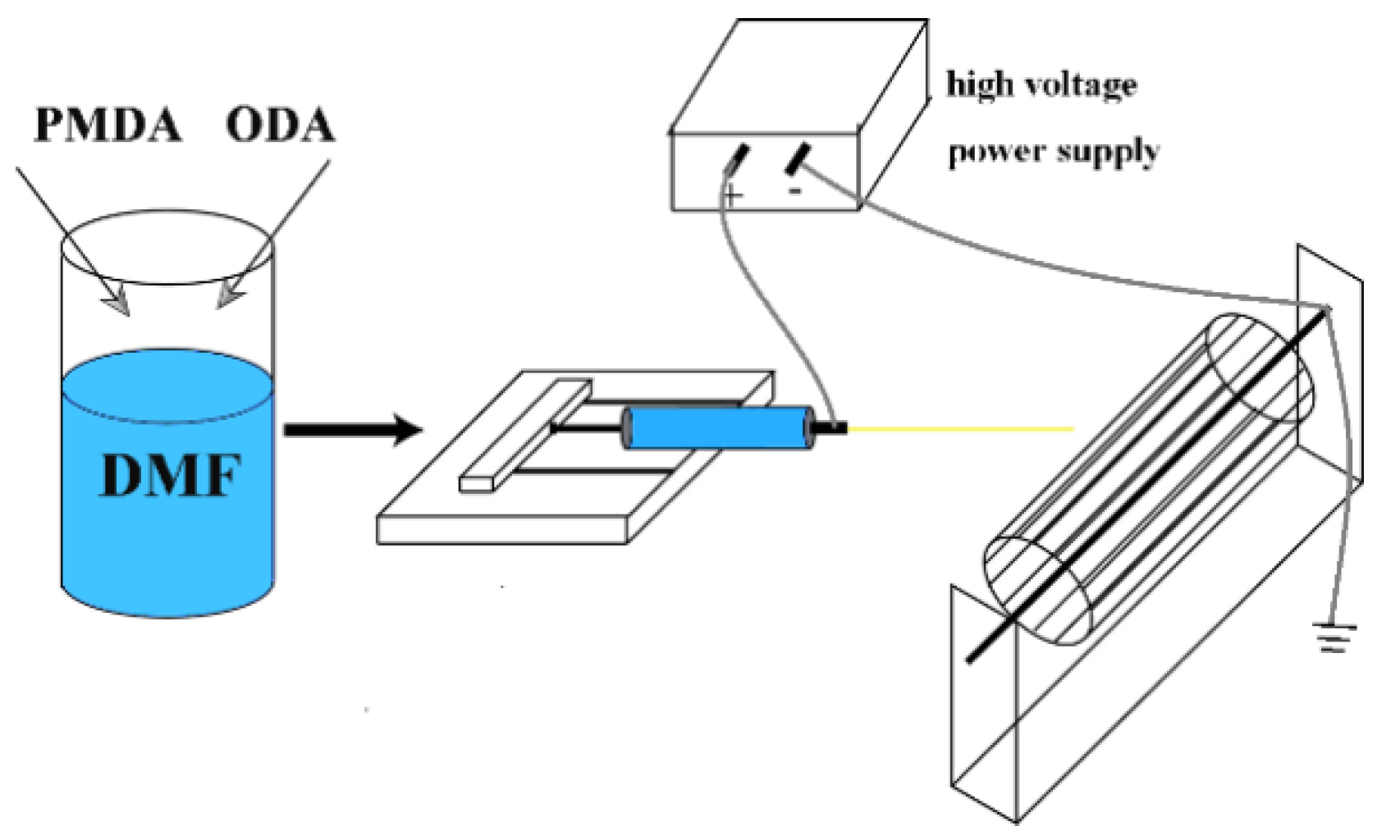
2.2. Preparation of Polyimide
2.3. Synthesis of the PI/TiO2 Nanofibers
2.4. Synthesis of the PI/TiO2@MoS2 Nanofibers
2.5. Characterization
3. Results and Discussion
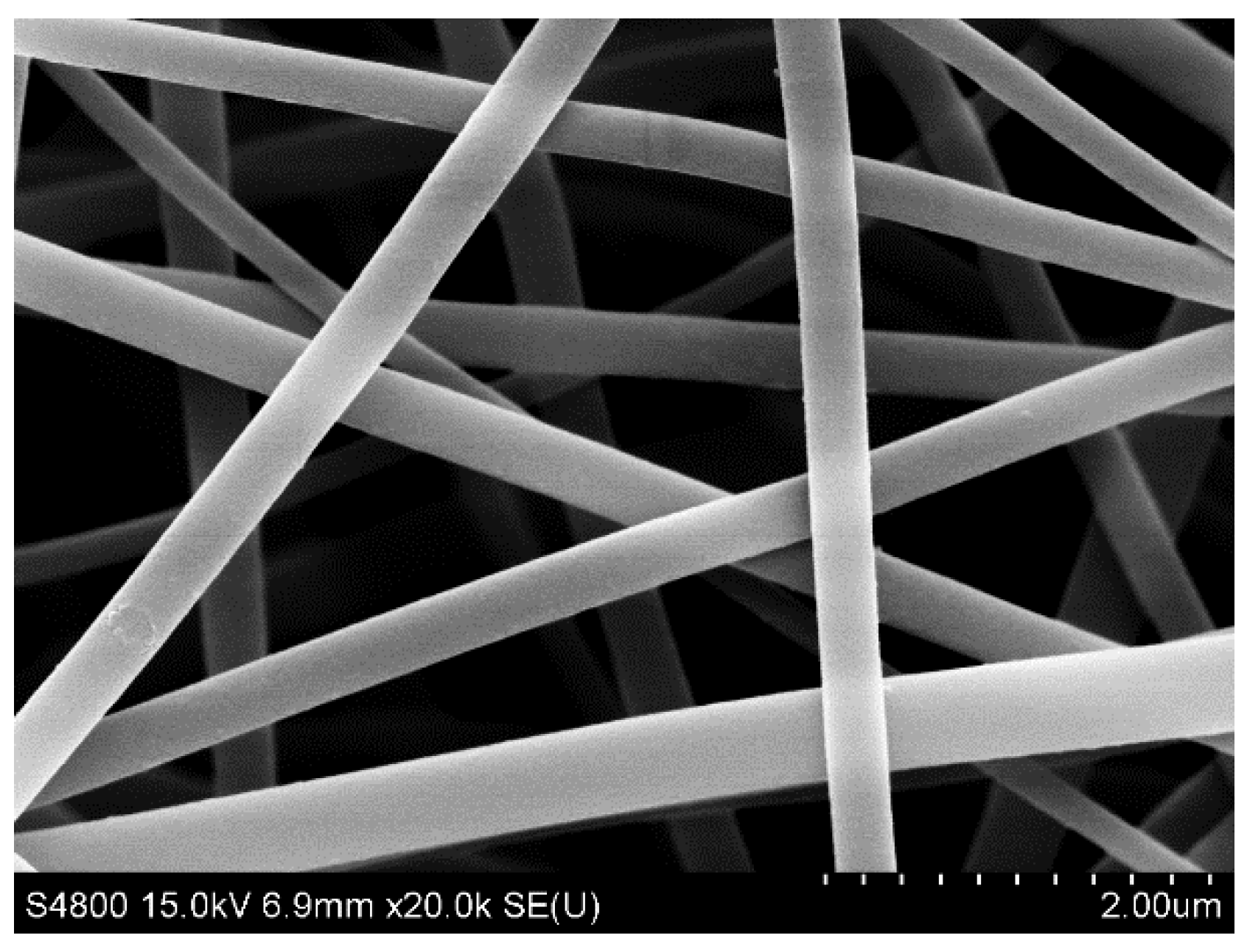
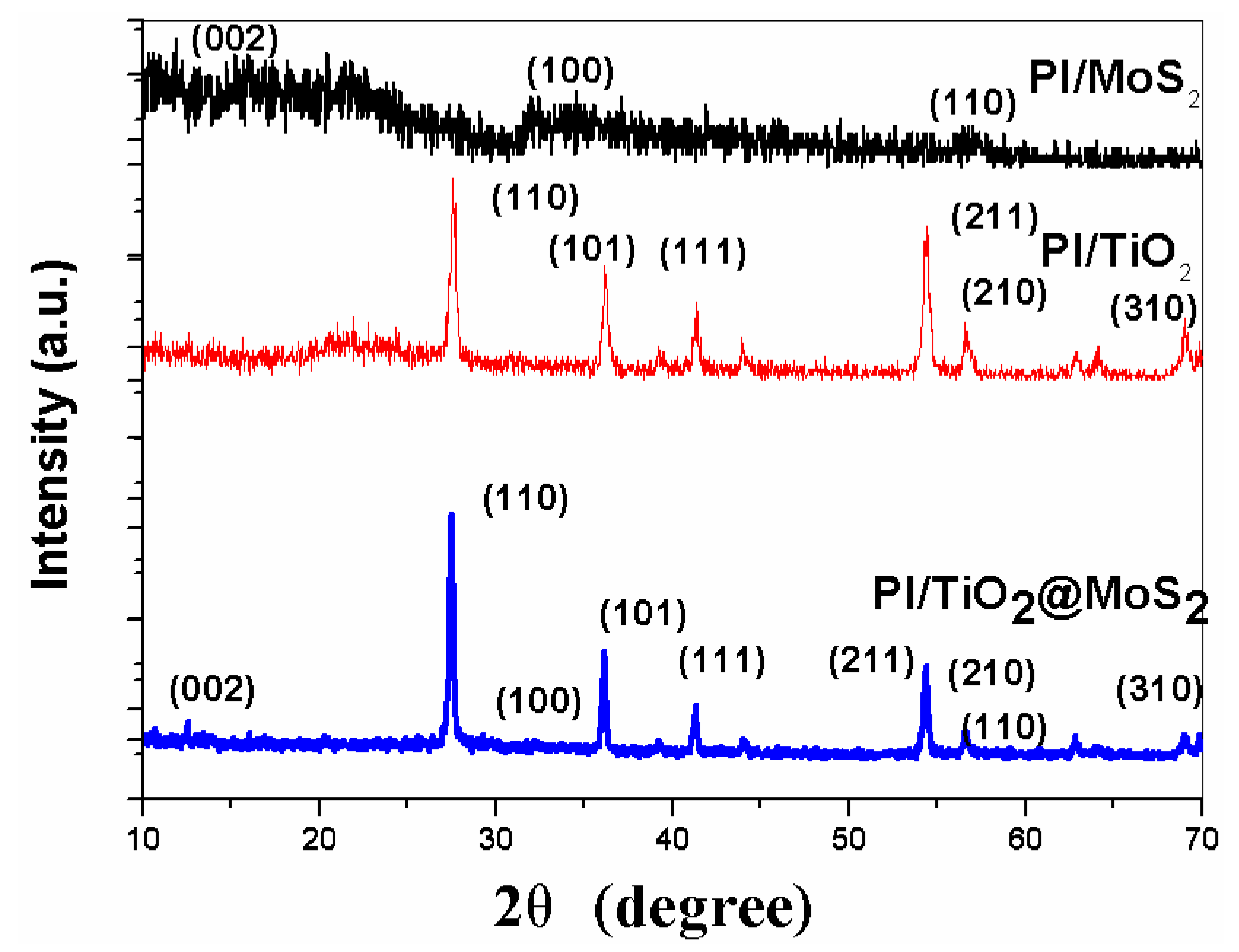
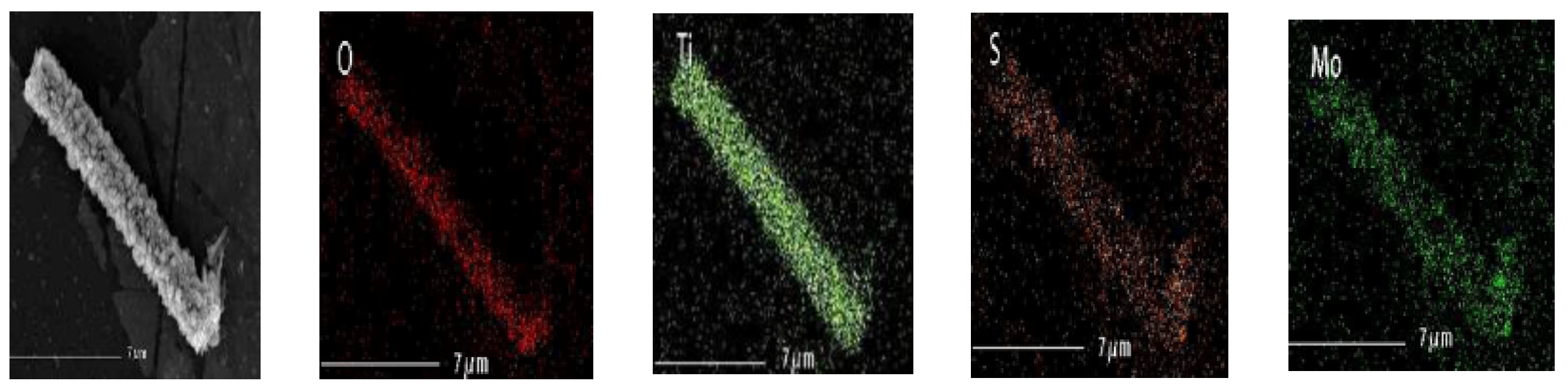
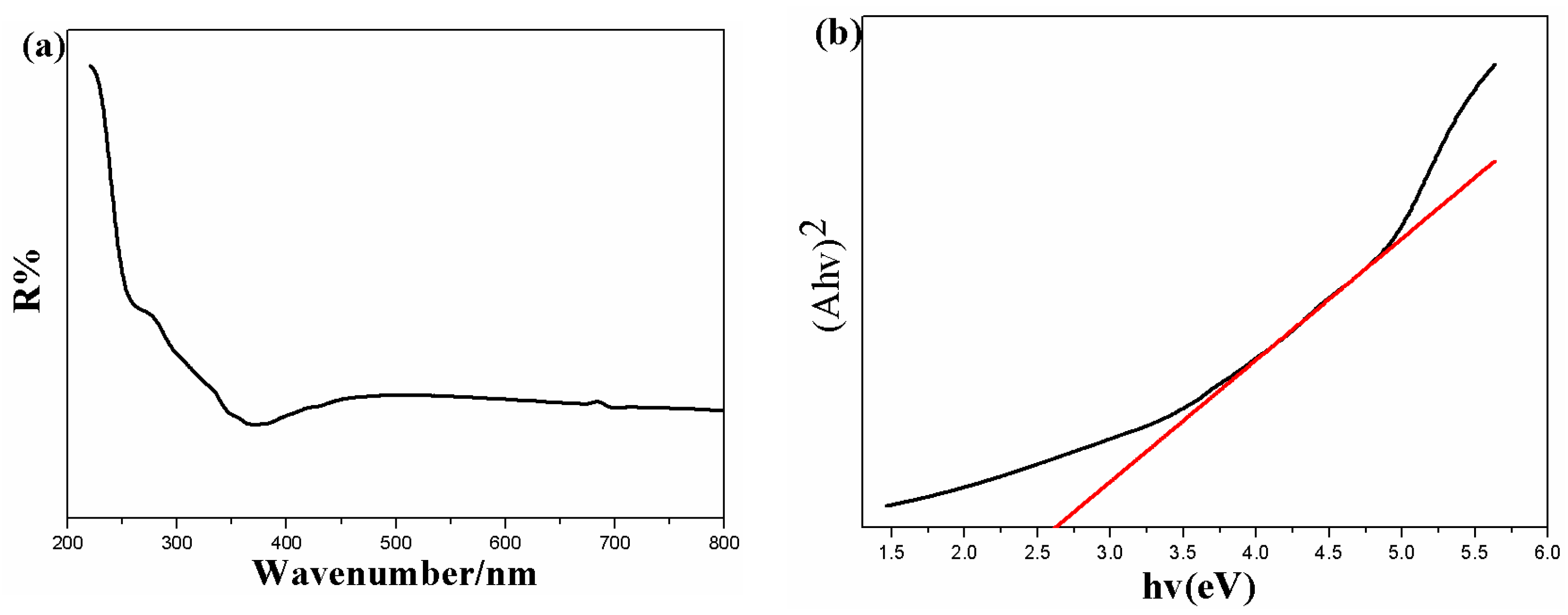
3.1. Characterization of the As-Prepared Photocatalysts
3.2. Photocatalytic Activity the As-Prepared Photocatalysts
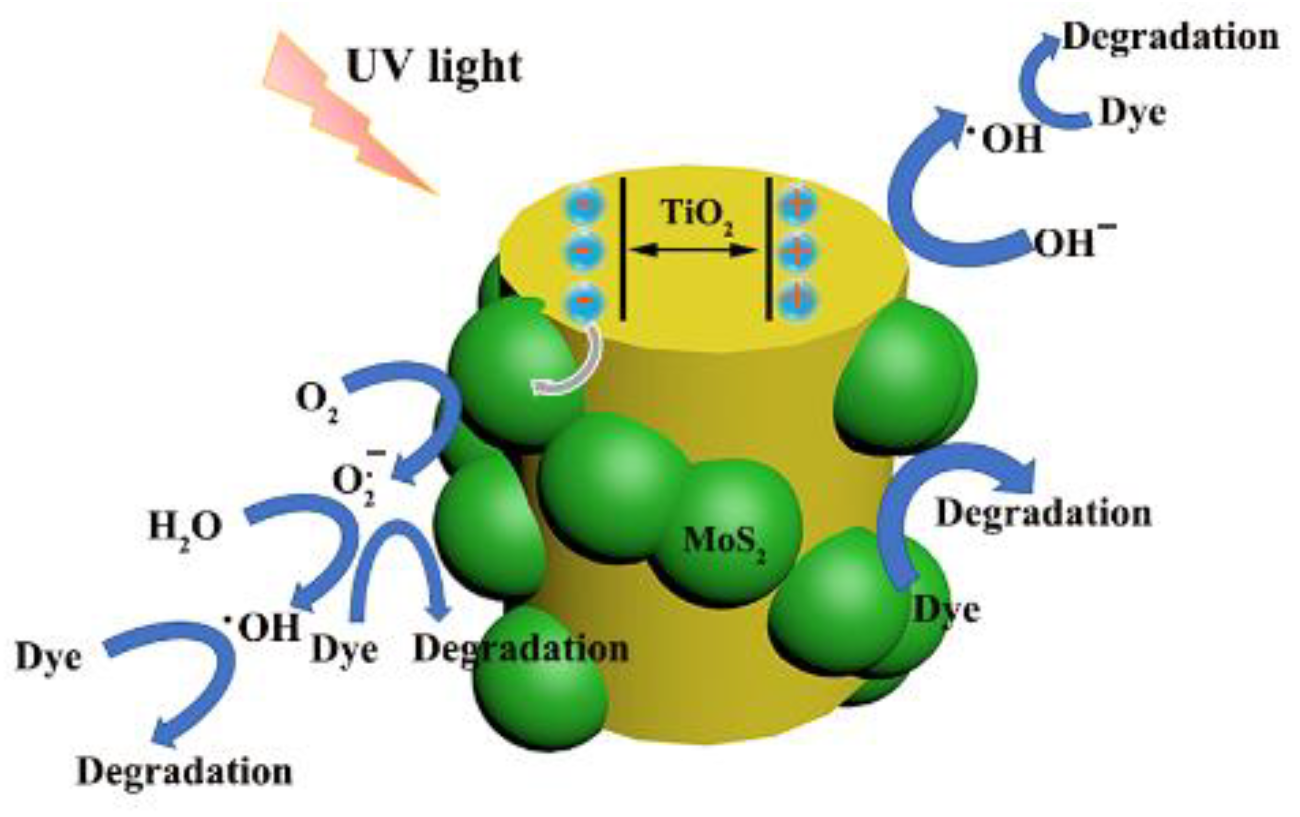
3.3. Possible Mechanism of the Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, M.; Hua, Y.Q.; Zhu, F.F.; Gu, W.; Jiang, J.H.; Shen, H.Q.; Shi, W.D. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B 2017, 202, 518–527. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.Q.; Liu, S.Q.; Sun, Y.G.; Xu, Y.J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.G.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Yousefia, A.T.; Do, T.O. Photocatalytic pathway toward degradation of environmental pharmaceutical pollutants: Structure, kinetics and mechanism approach. Catal. Sci. Technol. 2017, 7, 4548–4569. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Chang, Z.; Zeng, J. Uniform deposition of Ag nanoparticles on ZnO nanorod arrays grown on polyimide/Ag nanofibers by electrospinning, hydrothermal, and photoreduction processes. Mater. Des. 2019, 181, 108069. [Google Scholar] [CrossRef]
- He, T.; Zhou, Z.; Xu, W.; Cao, Y.; Shi, Z.; Pan, W.P. Visible-light photocatalytic activity of semiconductor composites supported by electrospun fiber. Compos. Sci. Tech. 2010, 70, 1469–1475. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Wang, L.; Wang, X. Degradation of Rhodamine B and Safranin-T by MoO3: CeO2 Nanofibers and Air Using a Continuous Mode. CLEAN-Soil Air Water 2010, 38, 268–274. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Serra, M.; Corma, A.; Garcia, H. Contrasting photocatalytic activity of commercial TiO2 samples for hydrogen generation. Catal. Today 2014, 225, 52–54. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Rather, S.U.; Mehraj-ud-din, N.; Zacharia, R.; Hwang, S.W.; Kim, A.R.; Nahm, K.S. Hydrogen storage of nanostructured TiO2-impregnated carbon nanotubes. Int. J. Hydrog. Energy 2009, 34, 961–966. [Google Scholar] [CrossRef]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. AAPPS Bull. 2007, 17, 12–28. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Sheaf, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Roy, N.; Sohn, Y.; Pradhan, D. Synergy of Low-Energy {101} and High-Energy {001} TiO2 Crystal Facets for Enhanced Photocatalysis. ACS Nano 2013, 7, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Deokar, G.; Vancsó, P.; Arenal, R.; Ravaux, F.; Casanova-Cháfer, J.; Llobet, E.; Makarova, A.; Vyalikh, D.; Struzzi, C.; Lambin, P.; et al. MoS2–Carbon Nanotube Hybrid Material Growth and Gas Sensing. Adv. Mater. Interfaces 2017, 4, 1700801. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.M.; Wang, T.; Kang, Q.; Ouyang, S.X.; Ye, J.H. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niu, H.; Gu, W.; Cai, X.; Mao, B.; Li, D.; Shi, W. In-situ construction of hierarchical CdS/MoS2 microboxes for enhanced visible-light photocatalytic H2 production. Chem. Eng. J. 2018, 339, 117–124. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.X.; Zhang, W.D. MoS2/CdS Heterojunction with High Photoelectrochemical Activity for H2 Evolution under Visible Light: The Role of MoS2. J. Phys. Chem. C 2013, 117, 12949–12957. [Google Scholar] [CrossRef]
- Jia, T.; Kolpin, A.; Ma, C.; Chan, R.C.; Kwok, W.M.; Tsang, S.C. A graphene dispersed CdS–MoS2 nanocrystal ensemble for cooperative photocatalytic hydrogen production from water. Chem. Commun. 2014, 50, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, N.; Yang, Y.; Wang, G.Z. High Efficiency Photocatalysis for Pollutant Degradation with MoS2/C3N4 Heterostructures. Langmuir 2014, 30, 8965–8972. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hong, M.Z.; Zhang, F.W.; Zhuang, Z.Y.; Yu, Y. Recyclable Nanoscale Zero Valent Iron Doped g-C3N4/MoS2 for Efficient Photocatalysis of RhB and Cr(VI) Driven by Visible Light. ACS Sustain. Chem. Eng. 2016, 4, 4055–4063. [Google Scholar] [CrossRef]
- Tian, N.; Li, Z.; Xu, D.Y.; Li, Y.; Peng, W.C.; Zhang, G.L.; Zhang, F.B.; Fan, X.B. Utilization of MoS2 Nanosheets To Enhance the Photocatalytic Activity of ZnO for the Aerobic Oxidation of Benzyl Halides under Visible Light. Ind. Eng. Chem. Res. 2016, 55, 8726–8732. [Google Scholar] [CrossRef]
- Zhang, C.M.; Chen, G.; Li, C.M.; Sun, J.X.; Lv, C.D.; Fan, S.; Xing, W.N. In Situ Fabrication of Bi2WO6/MoS2/RGO Heterojunction with Nanosized Interfacial Contact via Confined Space Effect toward Enhanced Photocatalytic Properties. ACS Sustain. Chem. Eng. 2016, 4, 5936–5942. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Lin, Y.; Wu, H.; Yuan, R.; Li, Z. MoS2 as non-noble-metal co-catalyst for photocatalytic hydrogen evolution over hexagonal ZnIn2S4 under visible light irradiations. Appl. Catal. B 2014, 144, 521–527. [Google Scholar] [CrossRef]
- Jiang, J.; Carlson, M.A.; Teusink, M.J.; Wang, H.J.; MacEwan, M.R.; Xie, J.W. Expanding two-dimensional electrospun nanofiber membranes in the third dimension by a modified gas-foaming technique. ACS Biomater. Sci. Eng. 2015, 1, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Vanangamudi, A.; Dumee, L.F.; Duke, M.C.; Yang, X. Nanofiber composite membrane with intrinsic Janus surface for reversed-protein-fouling ultrafiltration. ACS Appl. Mater. Interfaces 2017, 9, 18328–18337. [Google Scholar] [CrossRef] [PubMed]
- Kristopher, W.; Dobosz Kolewe, K.M.K.; Rieger, A.; Chang, C.; Emrick, T.; Schiffman, J.D. Antifouling electrospun nanofiber mats functionalized with polymer zwitterions. ACS Appl. Mater. Interfaces 2016, 8, 27585–27593. [Google Scholar]
- Li, H.; Wang, Y.; Chen, G.; Sang, Y.; Jiang, H.; He, J.; Liu, H. Few-layered MoS2 nanosheets wrapped ultrafine TiO2 nanobelts with enhanced photocatalytic property. Nanoscale 2016, 8, 6101–6109. [Google Scholar] [CrossRef]
- Chang, Z.; Zeng, J. Immobilization seeding layers using precursor for fabricating core–shell polyimide/Cu–BTC hierarchical nanofibers with high gas separation and adsorption of methylene blue from aqueous solution. Macromol. Chem. Phys. 2016, 217, 1007–1013. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Lee, W.; Kim, C.U.; Kim, E.J.; Hong, S.W.; Choi, K.J. Preparation, characterization, and application of TiO2-patterned polyimide film as a photocatalyst for oxidation of organic contaminants. J. Hazard. Mater. 2017, 340, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhou, Z.; Wei, H.; Yang, Z.; Wang, Z.; Zhang, Y. Rapid large-scale preparation of ZnO nanowires for photocatalytic application. Nanoscale Res. Lett. 2011, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Fu, K.; Su, Z. Fabrication of 3D MoS2-TiO2@ PAN electro-spun membrane for efficient and recyclable photocatalytic degradation of organic dyes. Mater. Sci. Eng. B 2021, 269, 115179. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Kakiuchi, K.; Imai, H. Growth of Submicrometer-Scale Rectangular Parallelepiped Rutile TiO2 Films in Aqueous TiCl3 Solutions under Hydrothermal Conditions. J. Am. Chem. Soc. 2004, 126, 7790–7791. [Google Scholar] [CrossRef]
- Cheng, H.M.; Ma, J.M.; Zhao, Z.G.; Qi, L.M. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Kumar, A.; Madaria, A.R.; Zhou, C.W. Growth of Aligned Single-Crystalline Rutile TiO2 Nanowires on Arbitrary Substrates and Their Application in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 7787–7792. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, C.L.; Li, X.H.; Miao, F.J.; Wang, K.X.; Lu, N.; Liu, Y.C. 3D MoS2 nanosheet/TiO2 nanofiber heterostructures with enhanced photocatalytic activity under UV irradiation. J. Alloy.Compd. 2016, 686, 137–144. [Google Scholar] [CrossRef]
- Sonkusare, V.N.; Chaudhary, R.G.; Bhusari, G.S.; Mondal, A.; Potbhare, A.K.; Mishra, R.K.; Juneja, H.D.; Abdala, A.A. Mesoporous Octahedron-Shaped Tricobalt Tetroxide Nanoparticles for Photocatalytic Degradation of Toxic Dyes. ACS Omega 2020, 5, 7823–7835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Jing, T.; Dong, M.Y.; Pan, D.; Guo, J.; Tian, J.Z.; Wu, M.; Naik, N.; Huang, M.N.; Guo, Z.H. A Visible Light Driven Photoelectrochemical Chloramphenicol Aptasensor Based on a Gold Nanoparticle-Functionalized 3D Flower-like MoS2/TiO2 Heterostructure. Langmuir 2022, 38, 2276–2286. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.; Sun, X.; Liao, Z.; Liu, Q.; Han, J. Design and Preparation of Polyimide/TiO2@MoS2 Nanofibers by Hydrothermal Synthesis and Their Photocatalytic Performance. Polymers 2022, 14, 3230. https://doi.org/10.3390/polym14163230
Chang Z, Sun X, Liao Z, Liu Q, Han J. Design and Preparation of Polyimide/TiO2@MoS2 Nanofibers by Hydrothermal Synthesis and Their Photocatalytic Performance. Polymers. 2022; 14(16):3230. https://doi.org/10.3390/polym14163230
Chicago/Turabian StyleChang, Zhenjun, Xiaoling Sun, Zhengzheng Liao, Qiang Liu, and Jie Han. 2022. "Design and Preparation of Polyimide/TiO2@MoS2 Nanofibers by Hydrothermal Synthesis and Their Photocatalytic Performance" Polymers 14, no. 16: 3230. https://doi.org/10.3390/polym14163230
APA StyleChang, Z., Sun, X., Liao, Z., Liu, Q., & Han, J. (2022). Design and Preparation of Polyimide/TiO2@MoS2 Nanofibers by Hydrothermal Synthesis and Their Photocatalytic Performance. Polymers, 14(16), 3230. https://doi.org/10.3390/polym14163230




