Electrospun Poly(Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile) Nanofiber Mat as an Anion Exchange Membrane for Fuel Cell Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
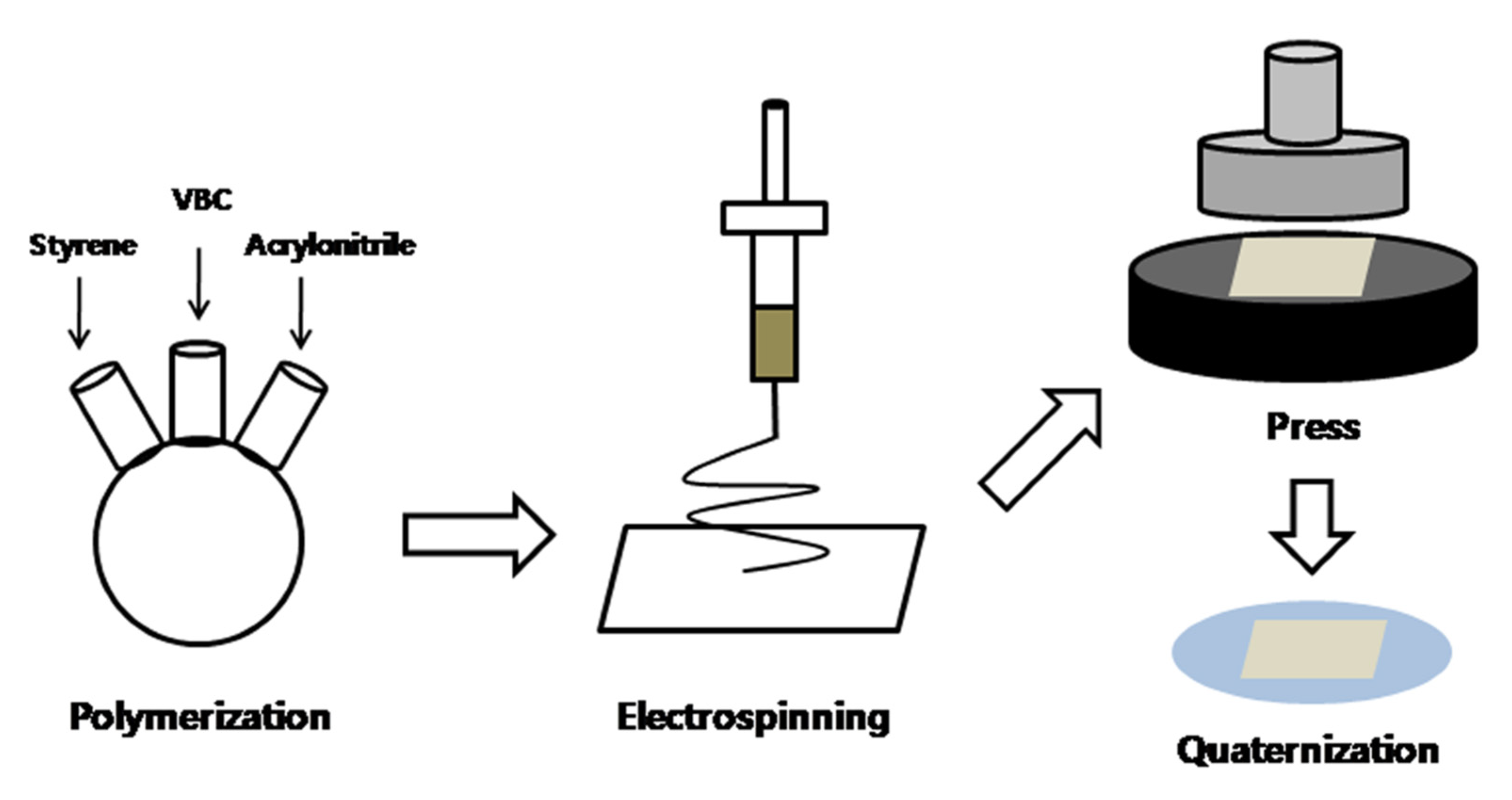
2.2. Synthesis of Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile
2.3. Fabrication of AEM by Electrospinning
2.4. Quaternization
2.5. Analysis
2.6. Characterization
2.7. Water-Uptake (WU),Swelling Ratio (SR), and Ion Exchange Capacity (IEC)
2.8. Ionic Conductivity (σ)
2.9. Alkaline Stability
3. Results and Discussion
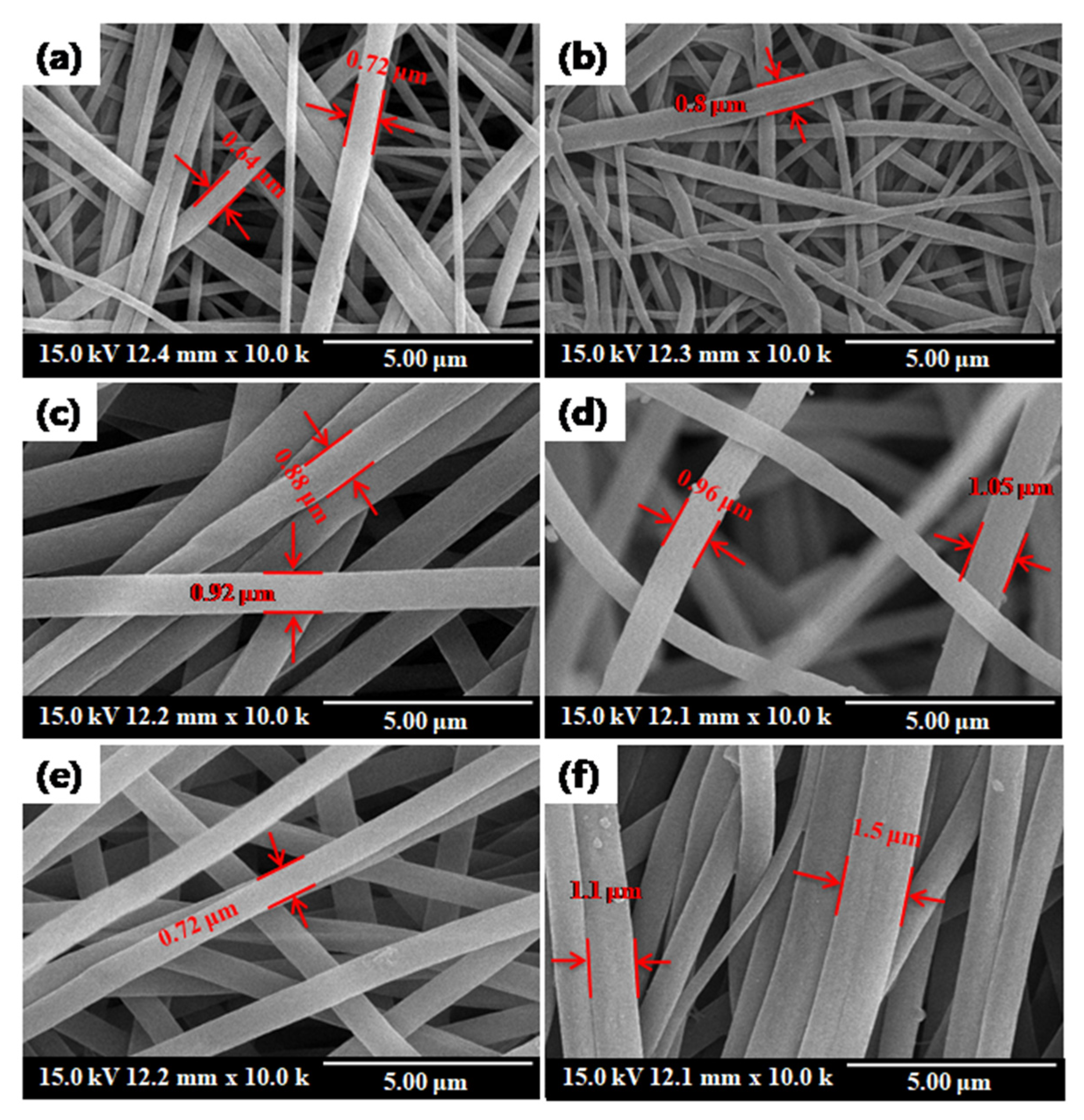
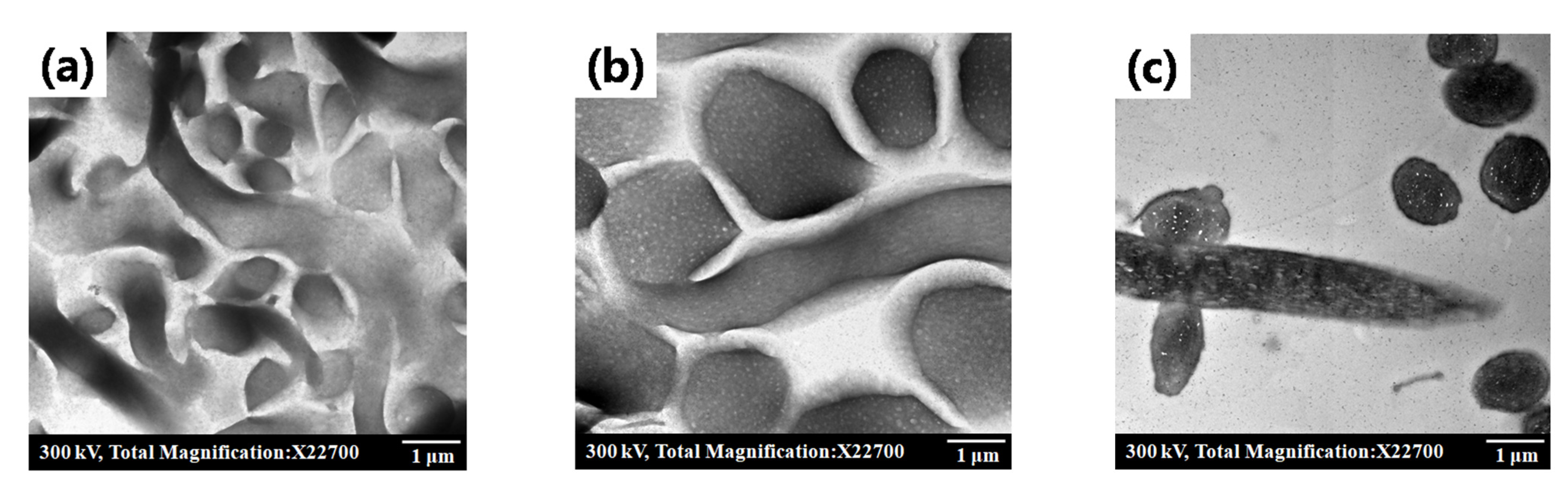
3.1. Characterization: Structure and Morphology
3.2. Membrane Properties: WU, SR, and IEC
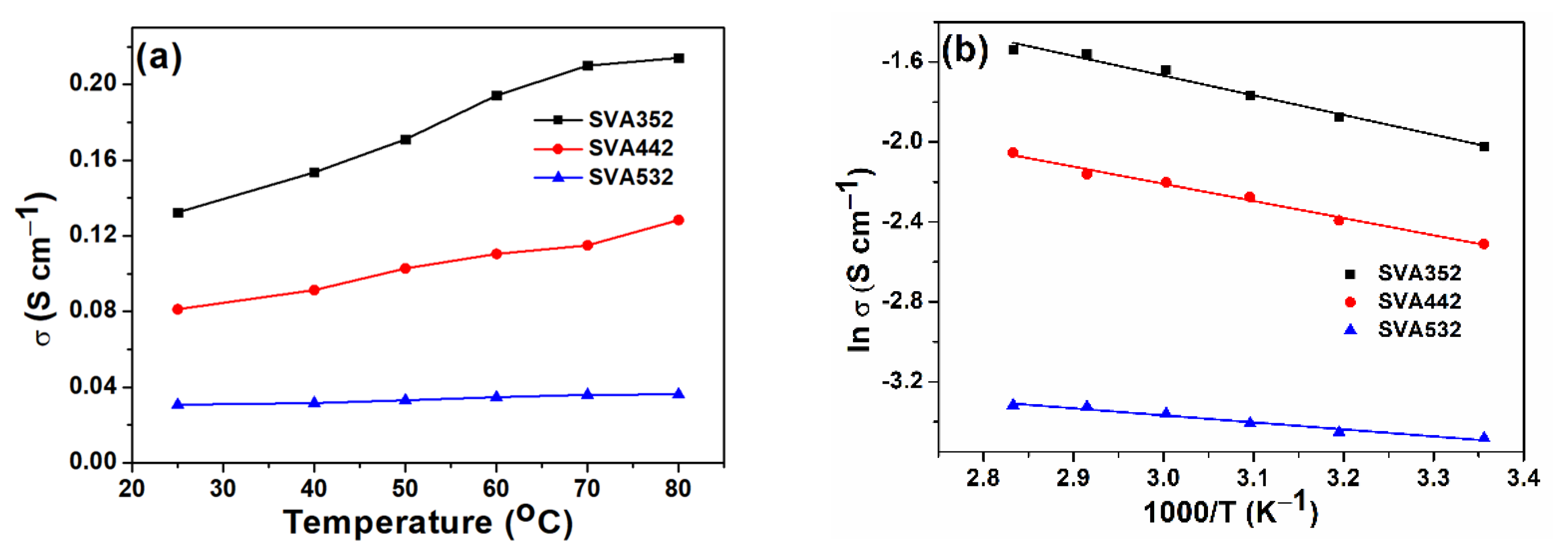
3.3. Ionic Conductivity
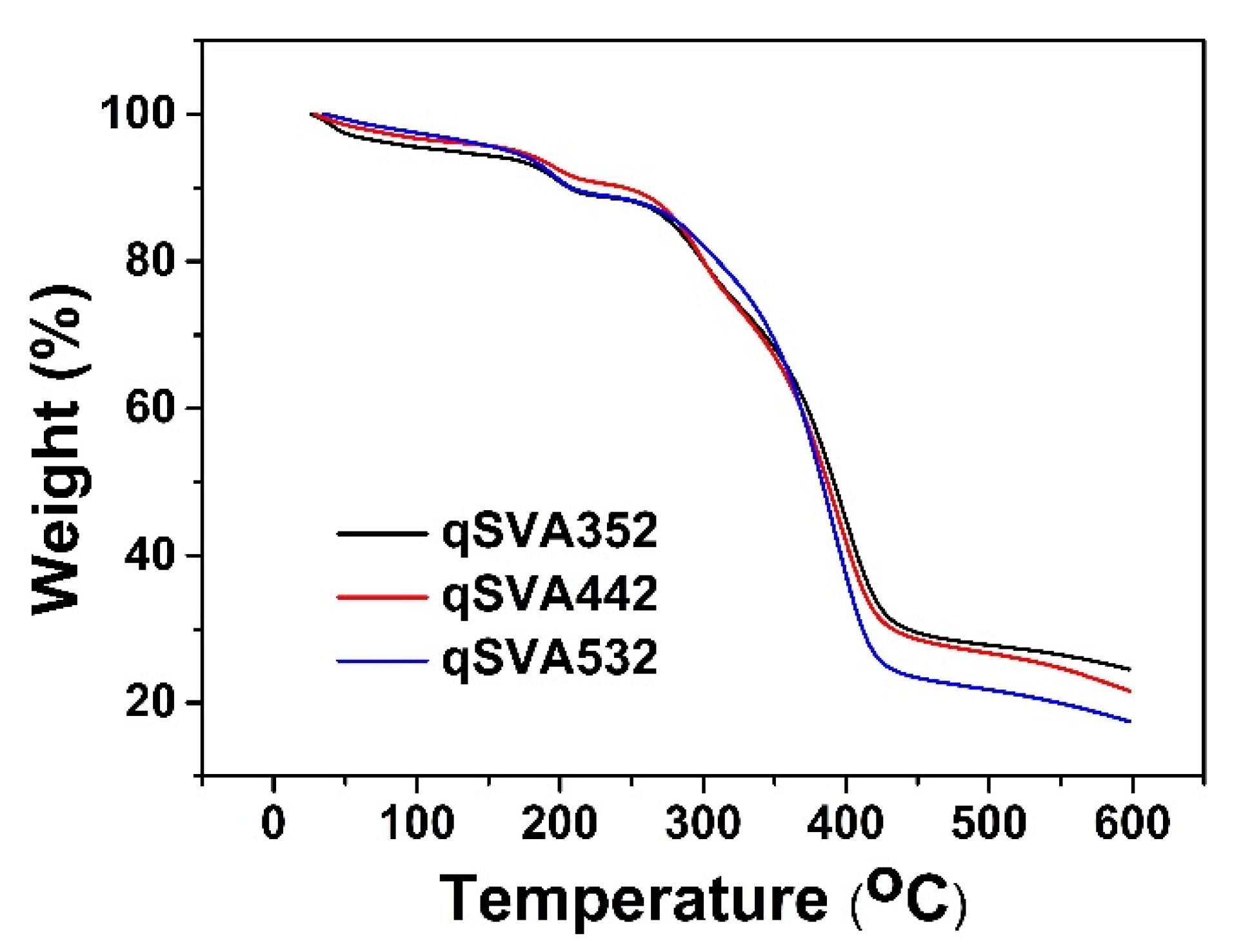
3.4. Thermal and Chemical Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olabi, A.; Wilberforce, T.; Abdelkareem, M.A. Fuel cell application in the automotive industry and future perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Li, B.; Mo, T.; Li, Y.; Feng, S.-P.; Qu, J.; Chu, P.K. Development and application of fuel cells in the automobile industry. J. Energy Storage 2021, 42, 103124. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Ning, X.; Tian, M.; Long, Y.; Ramakrishna, S. Recent advances in designing and tailoring nanofiber composite electrolyte membranes for high-performance proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 25225–25251. [Google Scholar] [CrossRef]
- Peng, S.; Xu, J.; Li, Z.; Jiang, S.; Munroe, P.; Xie, Z.-H.; Lu, H. A reactive-sputter-deposited TiSiN nanocomposite coating for the protection of metallic bipolar plates in proton exchange membrane fuel cells. Ceram. Int. 2020, 46, 2743–2757. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. Prospects for Alkaline Anion-Exchange Membranes in Low Temperature Fuel Cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef]
- Reshetenko, T.; Odgaard, M.; Schlueter, D.; Serov, A. Analysis of alkaline exchange membrane fuel cells performance at different operating conditions using DC and AC methods. J. Power Sources 2018, 375, 185–190. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Poldsepp, E.; Sarapuu, A.; Kikas, A.; Kisand, V.; Käärik, M.; Merisalu, M.; Treshchalov, A.; Leis, J.; Sammelselg, V.; et al. Nitrogen-doped carbide-derived carbon/carbon nanotube composites as cathode catalysts for anion exchange membrane fuel cell application. Appl. Catal. B Environ. 2020, 272, 119012. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Oshchepkov, A.; Braesch, G.; Bonnefont, A.; Savinova, E.R.; Chatenet, M. Recent Advances in the Understanding of Nickel-Based Catalysts for the Oxidation of Hydrogen-Containing Fuels in Alkaline Media. ACS Catal. 2020, 10, 7043–7068. [Google Scholar] [CrossRef]
- Sangkheaw, P.; Therdthianwong, S.; Therdthianwong, A.; Wongyao, N.; Yongprapat, S. Enhancement of anode performance for alkaline-acid direct glycerol fuel cells. Renew. Energy 2020, 161, 395–407. [Google Scholar] [CrossRef]
- Nhung, L.T.T.; Kim, I.Y.; Yoon, Y.S. Quaternized chitosan-based anion exchange membrane composited with quaternized poly (vinylbenzyl chloride)/polysulfone blend. Polymers 2020, 12, 2714. [Google Scholar] [CrossRef]
- Das, G.; Dongho, K.; Kim, C.Y.; Yoon, H.H. Graphene oxide crosslinked poly(phenylene oxide) nanocomposite as high-performance anion-conducting membrane. J. Ind. Eng. Chem. 2019, 72, 380–389. [Google Scholar] [CrossRef]
- Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef]
- Chu, X.; Miao, S.; Zhou, A.; Liu, S.; Liu, L.; Li, N. A strategy to design quaternized poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes by atom transfer radical coupling. J. Membr. Sci. 2022, 649, 120397. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.; Wei, L.; Jiang, H.; Wu, M. Anion exchange membranes for aqueous acid-based redox flow batteries: Current status and challenges. Appl. Energy 2019, 233, 622–643. [Google Scholar] [CrossRef]
- Hong, J.G.; Park, T.-W. Electrochemical characterizations and reverse electrodialysis performance of hybrid anion exchange membranes for salinity gradient energy. J. Electroanal. Chem. 2018, 817, 134–140. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, S.; Shahi, V.K. Cross-linked poly (vinyl alcohol)—Poly (acrylonitrile-co-2-dimethylamino ethylmethacrylate) based anion-exchange membranes in aqueous media. J. Phys. Chem. B 2010, 114, 198–206. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, J.; Wu, Z.; Zhou, J.; Li, Z.; Gao, S.; Shen, C. Crosslinked Anion Exchange Membranes Based on Styrene/Acrylonitrile/Vinylimidazole Copolymer and PPO. J. Electrochem. Soc. 2021, 168, 094506. [Google Scholar] [CrossRef]
- Ponce-González, J.; Varcoe, J.R.; Whelligan, D.K. Commercial monomer availability leading to missed opportunities? Anion-exchange membranes made from meta-vinylbenzyl chloride exhibit an alkali stability enhancement. ACS Appl. Energy Mater. 2018, 1, 1883–1887. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Si, Z.; Qiu, L.; Chu, F.; Zhao, J.; Yan, F. Bis-imidazolium-based anion-exchange membranes for alkaline fuel cells. J. Power Sources 2012, 217, 329–335. [Google Scholar] [CrossRef]
- Zulfi, A.; Munir, M.M.; Hapidin, D.A.; Rajak, A.; Edikresnha, D.; Iskandar, F.; Khairurrijal, K. Air filtration media from electrospun waste high-impact polystyrene fiber membrane. Mater. Res. Express 2018, 5, 035049. [Google Scholar] [CrossRef]
- Gao, X.; Han, S.; Zhang, R.; Liu, G.; Wu, J. Progress in electrospun composite nanofibers: Composition, performance and applications for tissue engineering. J. Mater. Chem. B 2019, 7, 7075–7089. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Şahiner, N.; Pekel, N.; Güven, O. Radiation synthesis, characterization and amidoximation of N-vinyl-2-pyrrolidone/acrylonitrile interpenetrating polymer networks. React. Funct. Polym. 1999, 39, 139–146. [Google Scholar] [CrossRef]
- Vengatesan, S.; Santhi, S.; Jeevanantham, S.; Sozhan, G. Quaternized poly (styrene-co-vinylbenzyl chloride) anion exchange membranes for alkaline water electrolysers. J. Power Sources 2015, 284, 361–368. [Google Scholar] [CrossRef]
- Koo, J.S.; Kwak, N.-S.; Hwang, T.S. Synthesis and properties of an anion-exchange membrane based on vinylbenzyl chloride–styrene–ethyl methacrylate copolymers. J. Membr. Sci. 2012, 423, 293–301. [Google Scholar]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Kallem, P.; Yanar, N.; Choi, H. Nanofiber-based proton exchange membranes: Development of aligned electrospun nanofibers for polymer electrolyte fuel cell applications. ACS Sustain. Chem. Eng. 2018, 7, 1808–1825. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.; Wu, H.; Deng, X.; Yang, Q.; Liu, P.; Hong, Y.; Zhu, A.; Liu, Q. Dual hydrophobic modifications toward anion exchange membranes with both high ion conductivity and excellent dimensional stability. J. Membr. Sci. 2020, 595, 117521. [Google Scholar] [CrossRef]
- Wang, H.; Du, X.; Zhang, H.; Shen, H.; Liu, Q.; Wang, Z. Synthesis and characterization of long-side-chain type quaternary ammonium-functionalized poly (ether ether ketone) anion exchange membranes. Int. J. Hydrogen Energy 2021, 46, 8156–8166. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Anionic multiblock copolymer membrane based on vinyl addition polymerization of norbornenes: Applications in anion-exchange membrane fuel cells. J. Membr. Sci. 2019, 570, 394–402. [Google Scholar] [CrossRef]
- Ren, X.; Price, S.C.; Jackson, A.C.; Pomerantz, N.; Beyer, F.L. Highly Conductive Anion Exchange Membrane for High Power Density Fuel-Cell Performance. ACS Appl. Mater. Interfaces 2014, 6, 13330–13333. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Li, T.; Yan, X.; Zhang, F.; Wang, X.; Wu, X.; Pang, B.; He, G. A rod-coil grafts strategy for N-spirocyclic functionalized anion exchange membranes with high fuel cell power density. J. Power Sources 2021, 490, 229544. [Google Scholar] [CrossRef]
- Yang, Z.; Ran, J.; Wu, B.; Wu, L.; Xu, T. Stability challenge in anion exchange membrane for fuel cells. Curr. Opin. Chem. Eng. 2016, 12, 22–30. [Google Scholar] [CrossRef]


| Sample | WU (%) | SRip (%) | SRtp (%) | IEC (mmol g−1) |
|---|---|---|---|---|
| qSVA352 | 425 | 7.8 | 50 | 1.862 |
| qSVA442 | 281 | 3.7 | 33 | 1.319 |
| qSVA532 | 222 | 2.7 | 14 | 0.786 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Lee, J.S.; Yoon, H.H.; Sharma, C.M.; Das, G.; Yoon, Y.S. Electrospun Poly(Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile) Nanofiber Mat as an Anion Exchange Membrane for Fuel Cell Applications. Polymers 2022, 14, 3236. https://doi.org/10.3390/polym14163236
Kang D, Lee JS, Yoon HH, Sharma CM, Das G, Yoon YS. Electrospun Poly(Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile) Nanofiber Mat as an Anion Exchange Membrane for Fuel Cell Applications. Polymers. 2022; 14(16):3236. https://doi.org/10.3390/polym14163236
Chicago/Turabian StyleKang, Dongho, Ji Su Lee, Hyon Hee Yoon, Chinta Mani Sharma, Gautam Das, and Young Soo Yoon. 2022. "Electrospun Poly(Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile) Nanofiber Mat as an Anion Exchange Membrane for Fuel Cell Applications" Polymers 14, no. 16: 3236. https://doi.org/10.3390/polym14163236
APA StyleKang, D., Lee, J. S., Yoon, H. H., Sharma, C. M., Das, G., & Yoon, Y. S. (2022). Electrospun Poly(Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile) Nanofiber Mat as an Anion Exchange Membrane for Fuel Cell Applications. Polymers, 14(16), 3236. https://doi.org/10.3390/polym14163236







