Anion Exchange Membranes for Fuel Cell Application: A Review
Abstract
:1. Introduction
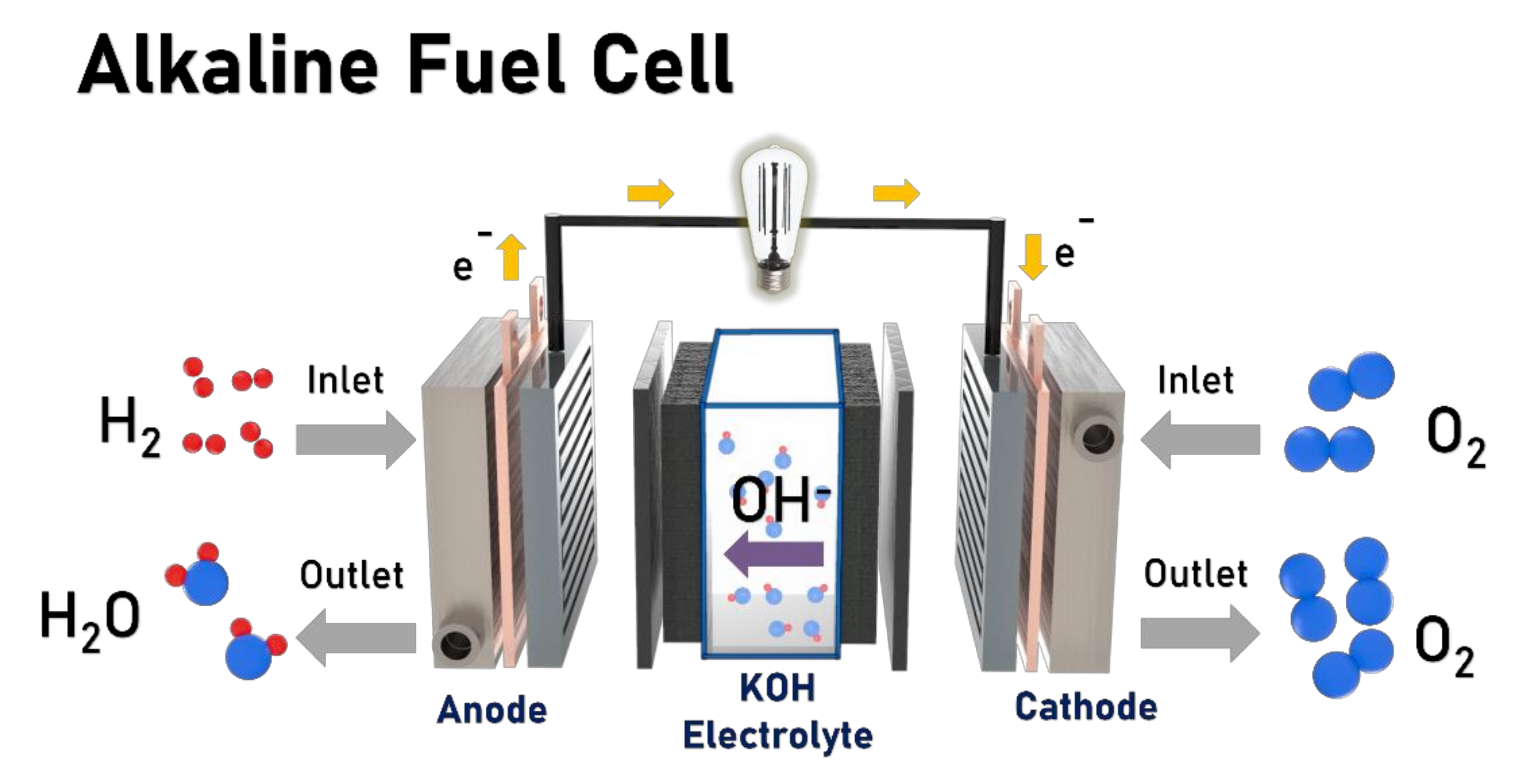
2. Overview of Fuel Cells
2.1. History
2.2. Principle of Fuel Cell Operation
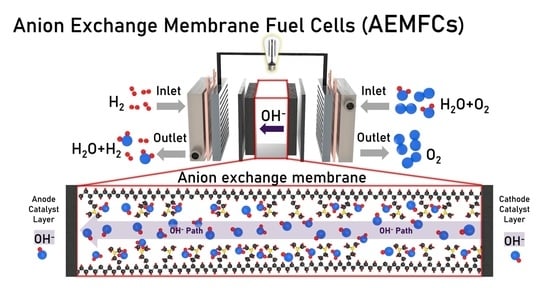
2.3. Anion Exchange Membrane Fuel Cells (AEMFCs)
Background
2.4. Challenges in AEMFC Operations
2.4.1. Ionomers
2.4.2. Water Management and Transport Properties
2.4.3. Contamination of AEM with CO2
2.5. Anion Exchange Membranes
2.5.1. Requirements of the Membrane
- (1)
- The hydrate ion conductivity of AEM, which can ensure high power density.
- (2)
- AEM with excellent chemical and mechanical stability in the AEMFC environment (temperature, humidity, alkali composition, etc.).
- (3)
- Easy to manufacture and inexpensive AEM.
2.5.2. Membrane Design
- (1)
- Physical grafting: In this process, the prepared membrane is bombarded with gamma radiation to generate reactive radicals which are then allowed to react with a quaternary group containing reactive sites. This process allows the introduction of quaternary ammonium or phosphonium groups’ membrane structure. The physical grafting techniques have been utilized to develop AEM based on perfluorocarbon membranes, as demonstrated by Varcoe, Slade, and co-workers [27]. Although this approach is convenient and clean, it is unable to produce the ionomer solution. An ionomer is crucial in the fabrication of membrane electrode assembly (MEA) [98,99].
- (2)
- Chemical grafting: In this approach, quaternary ammonia functional groups are grafted onto the polymer chain through chemical reaction on pre-functionalized polymer with chloromethyl or bromomethyl groups. Since in this method the functionalized polymer is solubilized in certain organic solvents, ionomer solutions can be readily obtained. Moreover, this process allows obtaining a high degree of quaternary amine functionalized AEM allowing the development of high ion-conducting AEMs. This efficient, complete approach has been widely adopted by several research groups. Polysulfone (PS) and its analogs are the most widely used polymer backbones, mainly because PS is a commercially mature product with outstanding stability and is capable of forming a flexible thin-film with high mechanical strength [100,101,102].
- (3)
- Polymerization: In this process, AEM starts from quaternary-ammonia-containing monomers and is synthesized through polymerization reactions from monomers functionalized with quaternary amine groups. [103,104]. The type of AEM is considered to demonstrate tailorability and versatility in terms of the fabrication of the membrane as reported by Coates and co-workers [105,106]. This type of process of AEM fabrication can result in a membrane with very high ion-exchange capacity (IEC) and controlled molecular weight; however, the overall AEM performance—in particular, the mechanical strength and thermal stability—need further exploration.
2.6. Types of Anion Exchange Membrane
2.6.1. Polyvinyl Alcohol (PVA)-Based Membranes
2.6.2. Polysulfones (PS)
2.6.3. Polyphenylene-Based Membranes
2.6.4. Ionic Liquid-Based Membrane
2.6.5. Polyolefin-Based Membranes
Radiation Grafting
Direct Polymerization
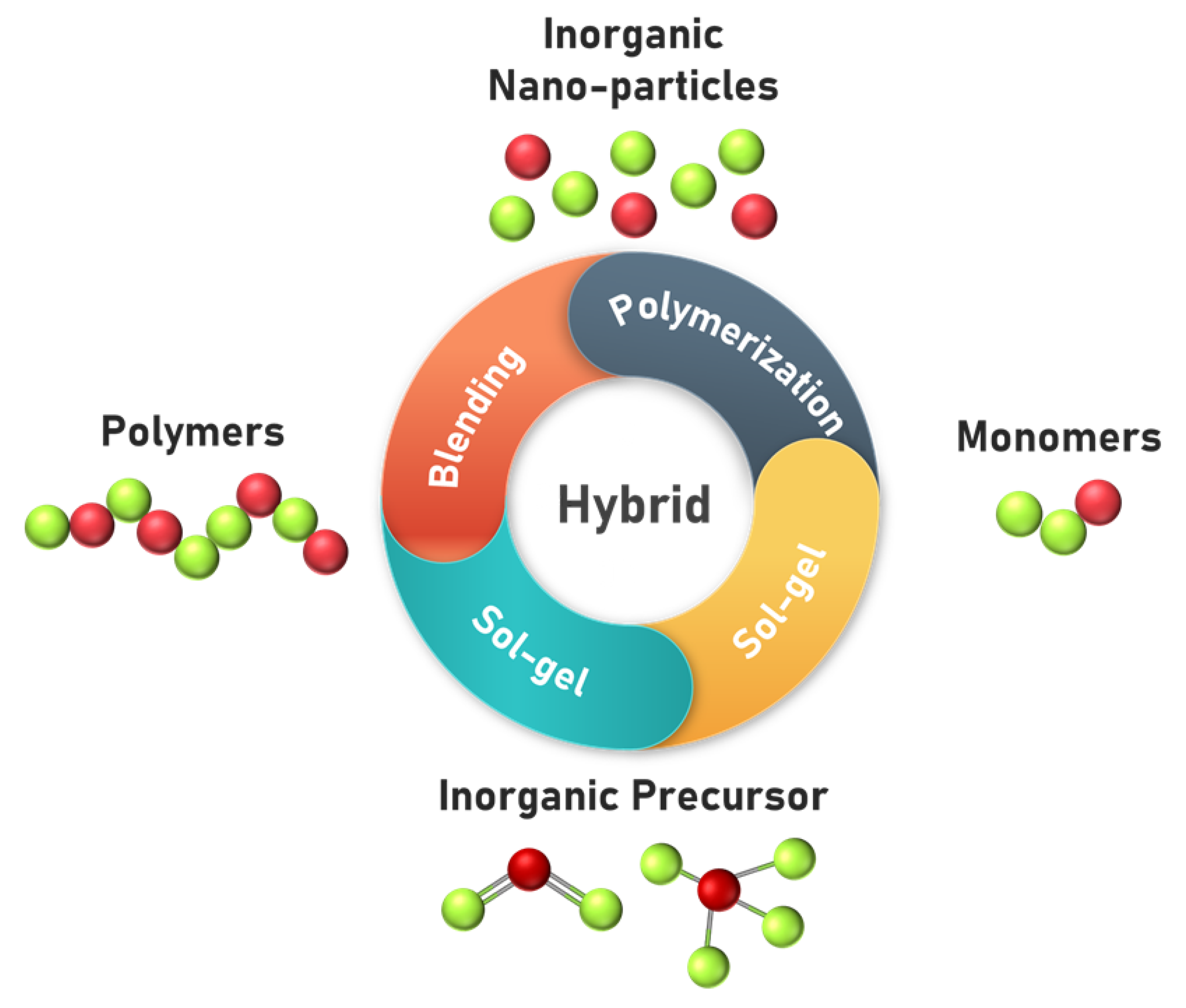
2.6.6. Organic–Inorganic-Based Membranes for AEMFC
3. Conventional Ion-Conducting Membranes: Pros and Cons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Kerres, J.A. Blended and Cross-Linked Ionomer Membranes for Application in Membrane Fuel Cells. Fuel Cells 2005, 5, 230–247. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and Fuel Cells: Towards a Sustainable Energy Future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Makky, A.; Baroutaji, A.; Carton, J.G.; Olabi, A.G. Developments of Electric Cars and Fuel Cell Hydrogen Electric Cars. Int. J. Hydrog. Energy 2017, 42, 25695–25734. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A Review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-Effective Iron-Based Aqueous Redox Flow Batteries for Large-Scale Energy Storage Application: A Review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Ralon, P.; Taylor, M.; Ilas, A.; Diaz-Bone, H.; Kairies, K. Electricity Storage and Renewables: Costs and Markets to 2030; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2017. [Google Scholar]
- Commission, E.T. Making the Hydrogen Economy Possible: Accelerating Clean Hydrogen in an Electrified Economy; Energy Transitions Commission: London, UK, 2021. [Google Scholar]
- Ewing, M.; Israel, B.; Jutt, T.; Talebian, H.; Stepanik, L. Hydrogen on the Path to Net-Zero Emissions; PEMBINA Institute: Calgary, AB, Canada, 2020. [Google Scholar]
- Kasimalla, V.K.; Velisala, V. A Review on Energy Allocation of Fuel Cell/Battery/Ultracapacitor for Hybrid Electric Vehicles. Int. J. Energy Res. 2018, 42, 4263–4283. [Google Scholar] [CrossRef]
- Balali, Y.; Stegen, S. Review of Energy Storage Systems for Vehicles Based on Technology, Environmental Impacts, and Costs. Renew. Sustain. Energy Rev. 2021, 135, 110185. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Xu, L.; Ouyang, M. Optimization for a Fuel Cell/Battery/Capacity Tram with Equivalent Consumption Minimization Strategy. Energy Convers. Manag. 2017, 134, 59–69. [Google Scholar] [CrossRef]
- Çabukoglu, E.; Georges, G.; Küng, L.; Pareschi, G.; Boulouchos, K. Fuel Cell Electric Vehicles: An Option to Decarbonize Heavy-Duty Transport? Results from a Swiss Case-Study. Transp. Res. D Transp. Environ. 2019, 70, 35–48. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B.D.; Chhina, H.; Taylor, A.K.; Gautam, S.; Schneider-Coppolino, M.; Duncan, K.L. Economic, Business, Technical, and Commercialization Hindrances for the Polymer Electrolyte Membrane Fuel Cell. In PEM Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2022; pp. 407–427. [Google Scholar]
- Pivovar, B. Catalysts for Fuel Cell Transportation and Hydrogen Related Uses. Nat. Catal. 2019, 2, 562–565. [Google Scholar] [CrossRef]
- Guerrero Moreno, N.; Cisneros Molina, M.; Gervasio, D.; Pérez Robles, J.F. Approaches to Polymer Electrolyte Membrane Fuel Cells (PEMFCs) and Their Cost. Renew. Sustain. Energy Rev. 2015, 52, 897–906. [Google Scholar] [CrossRef]
- Xu, F.; Su, Y.; Lin, B. Progress of Alkaline Anion Exchange Membranes for Fuel Cells: The Effects of Micro-Phase Separation. Front. Mater. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline Polymer Electrolyte Membranes for Fuel Cell Applications. Chem. Soc. Rev. 2013, 42, 5768. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, C.; Long, C.; Sang, J.; Tian, L.; Wang, F.; Wang, Z.; Zhu, H. Elastic and Durable Multi-cation-crosslinked Anion Exchange Membrane Based on Poly(Styrene-b-(Ethylene-Co-butylene)-b-styrene). J. Polym. Sci. 2020, 58, 2181–2196. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability Challenges of Anion Exchange Membrane Fuel Cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Acres, G.J. Recent Advances in Fuel Cell Technology and Its Applications. J. Power Sources 2001, 100, 60–66. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A. Solid Polymer Electrolyte Membranes for Fuel Cell Applications—A Review. J. Membr. Sci. 2005, 101, 10–26. [Google Scholar] [CrossRef]
- Agel, E.; Bouet, J.; Fauvarque, J.F. Characterization and Use of Anionic Membranes for Alkaline Fuel Cells. J. Power Sources 2001, 101, 267–274. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Gong, M. Fundamental Studies of a New Series of Anion Exchange Membranes: Membranes Prepared from Bromomethylated Poly(2,6-Dimethyl-1,4-Phenylene Oxide) (BPPO) and Pyridine. J. Membr. Sci. 2006, 279, 200–208. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. Prospects for Alkaline Anion-Exchange Membranes in Low Temperature Fuel Cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A Review on Membrane Fabrication: Structure, Properties and Performance Relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Tanimura, S.; Matsuoka, T. Proton Transfer in Nafion Membrane by Quantum Chemistry Calculation. J. Polym. Sci. B Polym. Phys. 2004, 42, 1905–1914. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kitano, N.; Morimoto, Y. Molecular Dynamics Simulations on O2 Permeation through Nafion Ionomer on Platinum Surface. Electrochim. Acta 2016, 188, 767–776. [Google Scholar] [CrossRef]
- Thompson, E.L.; Capehart, T.W.; Fuller, T.J.; Jorne, J. Investigation of Low-Temperature Proton Transport in Nafion Using Direct Current Conductivity and Differential Scanning Calorimetry. J. Electrochem. Soc. 2006, 153. [Google Scholar] [CrossRef]
- Paddison, S.J.; Paul, R. The Nature of Proton Transport in Fully Hydrated Nafion®. Phys. Chem. Chem. Phys. 2002, 4, 1158–1163. [Google Scholar] [CrossRef]
- Tandon, R.; Pintauro, P.N. Divalent/Monovalent Cation Uptake Selectivity in a Nafion Cation-Exchange Membrane: Experimental and Modeling Studies. J. Membr. Sci. 1997, 136, 207–219. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.N.K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Dicks, A.L.; Rand, D.A.J. Fuel Cell Systems Explained, 3rd ed.; Dicks, A.L., Rand, D.A.J., Eds.; John Wiley & Sons: Chichester, UK, 2018. [Google Scholar]
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs Directly Fed with Ethanol: A Current Status Comparative Review. J. Appl. Electrochem. 2013, 43, 119–136. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion Exchange Membrane Fuel Cells: Current Status and Remaining Challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly Quaternized Polystyrene Ionomers for High Performance Anion Exchange Membrane Water Electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Holewinski, A.; Idrobo, J.-C.; Linic, S. High-Performance Ag–Co Alloy Catalysts for Electrochemical Oxygen Reduction. Nat. Chem. 2014, 6, 828–834. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline Direct Alcohol Fuel Cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A Review of PEM Hydrogen Fuel Cell Contamination: Impacts, Mechanisms, and Mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic–Inorganic Nanocomposite Polymer Electrolyte Membranes for Fuel Cell Applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent Advancements in Applications of Alkaline Anion Exchange Membranes for Polymer Electrolyte Fuel Cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-Exchange Membranes in Electrochemical Energy Systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Lufrano, E.; Simari, C.; di Vona, M.L.; Nicotera, I.; Narducci, R. How the Morphology of Nafion-Based Membranes Affects Proton Transport. Polymers 2021, 13, 359. [Google Scholar] [CrossRef]
- Cooper, K. Characterizing Through-Plane and In-Plane Ionic Conductivity of Polymer Electrolyte Membranes. ECS Trans. 2011, 41, 1371–1380. [Google Scholar] [CrossRef]
- López-Fernández, E.; Sacedón, C.G.; Gil-Rostra, J.; Yubero, F.; González-Elipe, A.R.; de Lucas-Consuegra, A. Recent Advances in Alkaline Exchange Membrane Water Electrolysis and Electrode Manufacturing. Molecules 2021, 26, 6326. [Google Scholar] [CrossRef] [PubMed]
- Kordesh, K.; Weissenbacher, M. Rechargeable Alkaline Manganese Dioxide/Zinc Batteries. J. Power Sources 1994, 51, 61–78. [Google Scholar] [CrossRef]
- Gülzow, E. Alkaline Fuel Cells: A Critical View. J. Power Sources 1996, 61, 99–104. [Google Scholar] [CrossRef]
- Easton, E.B.; Astill, T.D.; Holdcroft, S. Properties of Gas Diffusion Electrodes Containing Sulfonated Poly(Ether Ether Ketone). J. Electrochem. Soc. 2005, 152, 752–758. [Google Scholar] [CrossRef]
- Kim, D.S.; Fujimoto, C.H.; Hibbs, M.R.; Labouriau, A.; Choe, Y.-K.; Kim, Y.S. Resonance Stabilized Perfluorinated Ionomers for Alkaline Membrane Fuel Cells. Macromolecules 2013, 46, 7826–7833. [Google Scholar] [CrossRef]
- Ramani, V.; Swier, S.; Shaw, M.T.; Weiss, R.A.; Kunz, H.R.; Fenton, J.M. Membranes and MEAs Based on Sulfonated Poly(Ether Ketone Ketone) and Heteropolyacids for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2008, 155, 532–537. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Alkaline Direct Alcohol Fuel Cells Using an Anion Exchange Membrane. J. Power Sources 2005, 150, 27–31. [Google Scholar] [CrossRef]
- Zhou, J.; Ünlü, M.; Anestis-Richard, I.; Kim, H.; Kohl, P.A. Solvent Processible, High-Performance Partially Fluorinated Copoly(Arylene Ether) Alkaline Ionomers for Alkaline Electrodes. J. Power Sources 2011, 196, 7924–7930. [Google Scholar] [CrossRef]
- UÜnluü, M.; Zhou, J.; Kohl, P.A. Study of Alkaline Electrodes for Hybrid Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2010, 157, B1391. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Fujigaya, T.; Yanagi, H.; Nakashima, N. Very High Performance Alkali Anion-Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2011, 21, 1089–1094. [Google Scholar] [CrossRef]
- Yang, D.; Yu, H.; Li, G.; Zhao, Y.; Liu, Y.; Zhang, C.; Song, W.; Shao, Z. Fine Microstructure of High Performance Electrode in Alkaline Anion Exchange Membrane Fuel Cells. J. Power Sources 2014, 267, 39–47. [Google Scholar] [CrossRef]
- Ahlfield, J.; Huang, G.; Liu, L.; Kaburagi, Y.; Kim, Y.; Kohl, P.A. Anion Conducting Ionomers for Fuel Cells and Electrolyzers. J. Electrochem. Soc. 2017, 164, F1648–F1653. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Shehzad, M.A.; Zhu, Y.; Wang, L.; Ge, X.; Zhang, J.; Yang, Z.; Wu, L.; Varcoe, J.R.; Xu, T. Ionomer Cross-Linking Immobilization of Catalyst Nanoparticles for High Performance Alkaline Membrane Fuel Cells. Chem. Mater. 2019, 31, 7812–7820. [Google Scholar] [CrossRef]
- Huang, G.; Mandal, M.; Peng, X.; Yang-Neyerlin, A.C.; Pivovar, B.S.; Mustain, W.E.; Kohl, P.A. Composite Poly(Norbornene) Anion Conducting Membranes for Achieving Durability, Water Management and High Power (3.4 W/Cm2) in Hydrogen/Oxygen Alkaline Fuel Cells. J. Electrochem. Soc. 2019, 166, F637–F644. [Google Scholar] [CrossRef] [Green Version]
- Ul Hassan, N.; Mandal, M.; Huang, G.; Firouzjaie, H.A.; Kohl, P.A.; Mustain, W.E. Achieving High-Performance and 2000 h Stability in Anion Exchange Membrane Fuel Cells by Manipulating Ionomer Properties and Electrode Optimization. Adv. Energy Mater. 2020, 10, 2001986. [Google Scholar] [CrossRef]
- Dekel, D.R.; Rasin, I.G.; Page, M.; Brandon, S. Steady State and Transient Simulation of Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 191–204. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takeguchi, T.; Takahashi, H.; Ueda, W. Water Transport during Ion Conduction in Anion-Exchange and Cation-Exchange Membranes. J. Electrochem. Soc. 2009, 156, B831–B835. [Google Scholar] [CrossRef] [Green Version]
- Gutru, R.; Turtayeva, Z.; Xu, F.; Maranzana, G.; Vigolo, B.; Desforges, A. A Comprehensive Review on Water Management Strategies and Developments in Anion Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 19642–19663. [Google Scholar] [CrossRef]
- Huo, S.; Deng, H.; Chang, Y.; Jiao, K. Water Management in Alkaline Anion Exchange Membrane Fuel Cell Anode. Int. J. Hydrog. Energy 2012, 37, 18389–18402. [Google Scholar] [CrossRef]
- Pivovar, B.S.; Smyrl, W.H.; Cussler, E.L. Electro-Osmosis in Nafion 117, Polystyrene Sulfonic Acid, and Polybenzimidazole. J. Electrochem. Soc. 2005, 152, A53. [Google Scholar] [CrossRef]
- Tschinder, T.; Schaffer, T.; Fraser, S.D.; Hacker, V. Electro-Osmotic Drag of Methanol in Proton Exchange Membranes. J. Appl. Electrochem. 2007, 37, 711–716. [Google Scholar] [CrossRef]
- Zhang, H.; Ohashi, H.; Tamaki, T.; Yamaguchi, T. Water Movement in a Solid-State Alkaline Fuel Cell Affected by the Anion-Exchange Pore-Filling Membrane Properties. J. Phys. Chem. C 2013, 117, 16791–16801. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Zhang, Y.; Liang, Y.; Wang, X.; Yi, B. An Ultrathin Self-Humidifying Membrane for PEM Fuel Cell Application: Fabrication, Characterization, and Experimental Analysis. J. Phys. Chem. B 2006, 110, 14240–14248. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Knobbe, M.W. A Liquid Water Management Strategy for PEM Fuel Cell Stacks. J. Power Sources 2003, 114, 70–79. [Google Scholar] [CrossRef]
- Varcoe, J.R. Investigations of the Ex Situ Ionic Conductivities at 30 °C of Metal-Cation-Free Quaternary Ammonium Alkaline Anion-Exchange Membranes in Static Atmospheres of Different Relative Humidities. Phys. Chem. Chem. Phys. 2007, 9, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Hickner, M.A. Water-Mediated Transport in Ion-Containing Polymers. J. Polym. Sci. B Polym. Phys. 2011, 50, 9–20. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Springer, T.E.; Davey, J.; Jestel, R.; Lopez, C.; Valerio, J.; Gottesfeld, S. A Comparative Study of Water Uptake by and Transport Through Ionomeric Fuel Cell Membranes. J. Electrochem. Soc. 1993, 140, 1981–1985. [Google Scholar] [CrossRef]
- Manke, I.; Hartnig, C.; Grünerbel, M.; Lehnert, W.; Kardjilov, N.; Haibel, A.; Hilger, A.; Banhart, J.; Riesemeier, H. Investigation of Water Evolution and Transport in Fuel Cells with High Resolution Synchrotron X-ray Radiography. Appl. Phys. Lett. 2007, 90, 174105. [Google Scholar] [CrossRef]
- Sinha, P.K.; Halleck, P.; Wang, C.-Y. Quantification of Liquid Water Saturation in a PEM Fuel Cell Diffusion Medium Using X-Ray Microtomography. Electrochem. Solid State Lett. 2006, 9, A344–A348. [Google Scholar] [CrossRef]
- Nam, J.H.; Lee, K.-J.; Hwang, G.-S.; Kim, C.-J.; Kaviany, M. Microporous Layer for Water Morphology Control in PEMFC. Int. J. Heat Mass Transf. 2009, 52, 2779–2791. [Google Scholar] [CrossRef]
- Satija, R.; Jacobson, D.L.; Arif, M.; Werner, S.A. In Situ Neutron Imaging Technique for Evaluation of Water Management Systems in Operating PEM Fuel Cells. J. Power Sources 2004, 129, 238–245. [Google Scholar] [CrossRef]
- Gong, X.; Bandis, A.; Tao, A.; Meresi, G.; Wang, Y.; Inglefield, P.T.; Jones, A.A.; Wen, W.-Y. Self-Diffusion of Water, Ethanol and Decafluropentane in Perfluorosulfonate Ionomer by Pulse Field Gradient NMR. Polymer 2001, 42, 6485–6492. [Google Scholar] [CrossRef]
- Weinzierl, C.; Krewer, U. Model-Based Analysis of Water Management at Anode of Alkaline Direct Methanol Fuel Cells. Chem. Eng. Sci. 2016, 143, 181–193. [Google Scholar] [CrossRef]
- Grew, K.N.; Ren, X.; Chu, D. Effects of Temperature and Carbon Dioxide on Anion Exchange Membrane Conductivity. Electrochem. Solid State Lett. 2011, 14, B127. [Google Scholar] [CrossRef]
- Zheng, Y.; Omasta, T.J.; Peng, X.; Wang, L.; Varcoe, J.R.; Pivovar, B.S.; Mustain, W.E. Quantifying and Elucidating the Effect of CO2 on the Thermodynamics, Kinetics and Charge Transport of AEMFCs. Energy Environ. Sci. 2019, 12, 2806–2819. [Google Scholar] [CrossRef] [Green Version]
- Yanagi, H.; Fukuta, K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). ECS Trans. 2008, 16, 257–262. [Google Scholar] [CrossRef]
- Matsui, Y.; Saito, M.; Tasaka, A.; Inaba, M. Influence of Carbon Dioxide on the Performance of Anion-Exchange Membrane Fuel Cells. ECS Trans. 2010, 25, 105–110. [Google Scholar] [CrossRef]
- Mamlouk, M.; Horsfall, J.A.; Williams, C.; Scott, K. Radiation Grafted Membranes for Superior Anion Exchange Polymer Membrane Fuel Cells Performance. Int. J. Hydrog. Energy 2012, 37, 11912–11920. [Google Scholar] [CrossRef]
- Suzuki, S.; Muroyama, H.; Matsui, T.; Eguchi, K. Influence of CO2 Dissolution into Anion Exchange Membrane on Fuel Cell Performance. Electrochim. Acta 2013, 88, 552–558. [Google Scholar] [CrossRef]
- Pandey, T.P.; Peters, B.D.; Liberatore, M.W.; Herring, A.M. Insight on Pure vs Air Exposed Hydroxide Ion Conductivity in an Anion Exchange Membrane for Fuel Cell Applications. ECS Trans. 2014, 64, 1195–1200. [Google Scholar] [CrossRef]
- Divekar, A.G.; Park, A.M.; Owczarczyk, Z.R.; Seifert, S.; Pivovar, B.S.; Herring, A.M. A Study of Carbonate Formation Kinetics and Morphological Effects Observed on OH—Form of Pfaem When Exposed to Air Containing CO2. ECS Trans. 2017, 80, 1005–1011. [Google Scholar] [CrossRef]
- Krewer, U.; Weinzierl, C.; Ziv, N.; Dekel, D.R. Impact of Carbonation Processes in Anion Exchange Membrane Fuel Cells. Electrochim. Acta 2018, 263, 433–446. [Google Scholar] [CrossRef]
- Peng, J.; Roy, A.L.; Greenbaum, S.G.; Zawodzinski, T.A. Effect of CO2 Absorption on Ion and Water Mobility in an Anion Exchange Membrane. J. Power Sources 2018, 380, 64–75. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, G.; Wang, L.; Varcoe, J.R.; Kohl, P.A.; Mustain, W.E. Effect of Reacting Gas Flowrates and Hydration on the Carbonation of Anion Exchange Membrane Fuel Cells in the Presence of CO2. J. Power Sources 2020, 467, 228350. [Google Scholar] [CrossRef]
- Zheng, Y.; Irizarry Colón, L.N.; Ul Hassan, N.; Williams, E.R.; Stefik, M.; LaManna, J.M.; Hussey, D.S.; Mustain, W.E. Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells. Membranes 2021, 11, 102. [Google Scholar] [CrossRef]
- Ravindra, A. Potrekar Novel Polybenzimidazoles as Polymer Electrolytes for Fuel Cell. Ph.D Thesis, University of Pune, Pune, India, 2009. [Google Scholar]
- Ye, Y.; Elabd, Y.A. Chemical Stability of Anion Exchange Membranes for Alkaline Fuel Cells. In Polymers for Energy Storage and Delivery: Polyelectrolytes for Batteries and Fuel Cells; ACS Publishing: London, UK, 2012; pp. 233–251. [Google Scholar]
- Arges, C.G.; Zhang, L. Anion Exchange Membranes’ Evolution toward High Hydroxide Ion Conductivity and Alkaline Resiliency. ACS Appl. Energy Mater. 2018, 1, 2991–3012. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, L.; Han, J.; Hickner, M.A. Mechanically Tough and Chemically Stable Anion Exchange Membranes from Rigid-Flexible Semi-Interpenetrating Networks. Chem. Mater. 2015, 27, 6689–6698. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T.; Lam How Yee, E. An Alkaline Polymer Electrochemical Interface: A Breakthrough in Application of Alkaline Anion-Exchange Membranes in Fuel Cells. Chem. Commun. 2006, 13, 1428–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamain, C.; Poynton, S.D.; Slade, R.C.T.; Carroll, B.; Varcoe, J.R. Development of Cathode Architectures Customized for H2/O2 Metal-Cation-Free Alkaline Membrane Fuel Cells. J. Phys. Chem. C 2007, 111, 18423–18430. [Google Scholar] [CrossRef]
- Pan, J.; Lu, S.; Li, Y.; Huang, A.; Zhuang, L.; Lu, J. High-Performance Alkaline Polymer Electrolyte for Fuel Cell Applications. Adv. Funct. Mater. 2010, 20, 312–319. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, S.-H.; Yim, S.-D.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Performance of Solid Alkaline Fuel Cells Employing Anion-Exchange Membranes. J. Power Sources 2008, 178, 620–626. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Zhang, S. Novel Hydroxide-Conducting Polyelectrolyte Composed of an Poly(Arylene Ether Sulfone) Containing Pendant Quaternary Guanidinium Groups for Alkaline Fuel Cell Applications. Macromolecules 2010, 43, 3890–3896. [Google Scholar] [CrossRef]
- Singh, M.; Singh, R.K.; Chandra, S. Ionic Liquids Confined in Porous Matrices: Physicochemical Properties and Applications. Prog. Mater. Sci. 2014, 64, 73–120. [Google Scholar] [CrossRef]
- Guo, M.; Fang, J.; Xu, H.; Li, W.; Lu, X.; Lan, C.; Li, K. Synthesis and Characterization of Novel Anion Exchange Membranes Based on Imidazolium-Type Ionic Liquid for Alkaline Fuel Cells. J. Membr. Sci. 2010, 362, 97–104. [Google Scholar] [CrossRef]
- Lin, B.; Qiu, L.; Lu, J.; Yan, F.C. Cross-Linked Alkaline Ionic Liquid-Based Polymer Electrolytes for Alkaline Fuel Cell Applications. Chem. Mater. 2010, 22, 6718–6725. [Google Scholar] [CrossRef]
- Ye, Y.; Sharick, S.; Davis, E.; Winey, K.I.; Elabd, Y.A. High Hydroxide Conductivity in Polymerized Ionic Liquid Block Copolymers. ACS Macro Lett. 2013, 2, 575–580. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion Exchange Hybrid Membranes from PVA and Multi-Alkoxy Silicon Copolymer Tailored for Diffusion Dialysis Process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Wang, E.; Shin, G.; Han, J.-E.; Mather, P.T. Poly(Vinyl Alcohol) (PVA)/Sulfonated Polyhedral Oligosilsesquioxane (SPOSS) Hybrid Membranes for Direct Methanol Fuel Cell Applications. Polym. Adv. Technol. 2007, 18, 535–543. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Cheng, Q.; Zhang, S.; Zhang, B. Preparation and Properties of Poly(Vinyl Alcohol)/Silica Nanocomposites Derived from Copolymerization of Vinyl Silica Nanoparticles and Vinyl Acetate. Eur. Polym. J. 2007, 43, 1123–1131. [Google Scholar] [CrossRef]
- Binsu, V.V.; Nagarale, R.K.; Shahi, V.K. Phosphonic Acid Functionalized Aminopropyl Triethoxysilane–PVA Composite Material: Organic–Inorganic Hybrid Proton-Exchange Membranes in Aqueous Media. J. Mater. Chem. 2005, 15, 4823–4831. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Proton Conductivity and Methanol Transport Behavior of Cross-Linked PVA/PAA/Silica Hybrid Membranes. Solid State Ion. 2005, 176, 117–126. [Google Scholar] [CrossRef]
- Lewandowski, A.; Skorupska, K.; Malinska, J. Novel Poly(Vinyl AlcoholIKOhH2O Alkaline Polymer Electrolyte. Solid State Ion. 2000, 133, 265–271. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, S.-J. Preparation of Composite Alkaline Polymer Electrolyte. Mater. Lett. 2002, 57, 873–881. [Google Scholar] [CrossRef]
- Pandit, S.; Khilari, S.; Bera, K.; Pradhan, D.; Das, D. Application of PVA–PDDA Polymer Electrolyte Composite Anion Exchange Membrane Separator for Improved Bioelectricity Production in a Single Chambered Microbial Fuel Cell. Chem. Eng. J. 2014, 257, 138–147. [Google Scholar] [CrossRef]
- Merle, G.; Hosseiny, S.S.; Wessling, M.; Nijmeijer, K. New Cross-Linked PVA Based Polymer Electrolyte Membranes for Alkaline Fuel Cells. J. Membr. Sci. 2012, 409–410, 191–199. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. Preparation and Characterization of PVA/PAA Membranes for Solid Polymer Electrolytes. J. Membr. Sci. 2006, 275, 127–133. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, H.Z.; Yang, C.C. Modification and Characterization of Semi-Crystalline Poly(Vinyl Alcohol) with Interpenetrating Poly(Acrylic Acid) by UV Radiation Method for Alkaline Solid Polymer Electrolytes Membrane. J. Membr. Sci. 2008, 322, 74–80. [Google Scholar] [CrossRef]
- Subramania, A.; Kalyana Sundaram, N.T.; Sukumar, N. Development of PVA Based Micro-Porous Polymer Electrolyte by a Novel Preferential Polymer Dissolution Process. J. Power Sources 2005, 141, 188–192. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, S.-J.; Wu, G.-M. Study of Ionic Transport Properties of Alkaline Poly(Vinyl) Alcohol-Based Polymer Electrolytes. Mater. Chem. Phys. 2005, 92, 251–255. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.; Zeng, Q.H.; Liu, Q.L. Preparation and Characterization of Cross-Linked Quaternized Poly(Vinyl Alcohol) Membranes for Anion Exchange Membrane Fuel Cells. J. Membr. Sci. 2008, 311, 319–325. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Liu, Y.; Sheng, J. Highly Stable Hydroxyl Anion Conducting Membranes Poly(Vinyl Alcohol)/Poly(Acrylamide-Co-Diallyldimethylammonium Chloride) (PVA/PAADDA) for Alkaline Fuel Cells: Effect of Cross-Linking. Int. J. Hydrog. Energy 2012, 37, 4580–4589. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Lin, R.; Ma, J.; Liu, J. Alkaline Solid Polymer Electrolyte Membranes Based on Structurally Modified PVA/PVP with Improved Alkali Stability. Polymer 2010, 51, 4850–4859. [Google Scholar] [CrossRef]
- Ye, L.; Zhai, L.; Fang, J.; Liu, J.; Li, C.; Guan, R. Synthesis and Characterization of Novel Cross-Linked Quaternized Poly(Vinyl Alcohol) Membranes Based on Morpholine for Anion Exchange Membranes. Solid State Ion. 2013, 240, 1–9. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Bhat, S.D. Anion Exchange Membrane from Polyvinyl Alcohol Functionalized with Quaternary Ammonium Groups via Alkyl Spacers. Ionics 2018, 24, 1097–1109. [Google Scholar] [CrossRef]
- Chikh, L.; Delhorbe, V.; Fichet, O. (Semi-)Interpenetrating Polymer Networks as Fuel Cell Membranes. J. Membr. Sci. 2011, 368, 1–17. [Google Scholar] [CrossRef]
- Zuo, D.; Gong, Y.; Yan, Q.; Zhang, H. Preparation and Characterization of Hydroxyl Ion-Conducting Interpenetrating Polymer Network Based on PVA and PEI. J. Polym. Res. 2016, 23, 126. [Google Scholar] [CrossRef]
- Ei-Hibri, M.J.; Shari, W. Polyartlerhersulfones. In Axelrad Handbook of Thermoplastics, 2nd ed.; Olabisi, O., Adewale, K., Eds.; CRC Press: London, UK, 2016; Chapter 14. [Google Scholar]
- Nebipasagil, A. Synthesis and Characterization of Hydrophilic-Hydrophobic Poly(Arylene Ether Sulfone) Random and Segmented Copolymers for Membrane Applications. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2014. Volume 10668827. [Google Scholar]
- Zhang, F.; Zhang, H.; Qu, C. Imidazolium Functionalized Polysulfone Anion Exchange Membrane for Fuel Cell Application. J. Mater. Chem. 2011, 21, 12744. [Google Scholar] [CrossRef]
- Duan, X.; Wang, C.; Wang, T.; Xie, X.; Zhou, X.; Ye, Y. A Polysulfone-Based Anion Exchange Membrane for Phosphoric Acid Concentration and Purification by Electro-Electrodialysis. J. Mater. Chem. 2018, 552, 86–94. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Preparation of Anion Exchange Membrane by Efficient Functionalization of Polysulfone for Electrodialysis. J. Mater. Chem. 2020, 596, 117591. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Akbari, A.; Mehrnia, M.R. Preparation of Polysulfone Nanofiltration Membranes by UV-Assisted Grafting Polymerization for Water Softening. Desalination 2010, 263, 217–225. [Google Scholar] [CrossRef]
- Sun, C.-C.; Zhou, M.-Y.; Yuan, J.-J.; Yan, Y.; Song, Y.-Z.; Fang, L.-F.; Zhu, B.-K. Membranes with Negatively-Charged Nanochannels Fabricated from Aqueous Sulfonated Polysulfone Nanoparticles for Enhancing the Rejection of Divalent Anions. J. Mater. Chem. 2020, 602, 117692. [Google Scholar] [CrossRef]
- Weiber, E.A.; Jannasch, P. Polysulfones with Highly Localized Imidazolium Groups for Anion Exchange Membranes. J. Mater. Chem. 2015, 481, 164–171. [Google Scholar] [CrossRef]
- Bauer, B.; Strathmann, H.; Effenberger, F. Anion-Exchange Membranes with Improved Alkaline Stability. Desalination 1990, 79, 125–144. [Google Scholar] [CrossRef]
- Zschocke, P.; Quellmalz, D. Novel Ion Exchange Membranes Based on an Aromatic Polyethersulfone. J. Mater. Chem. 1985, 22, 325–332. [Google Scholar] [CrossRef]
- Einsla, B.R.; Chempath, S.; Pratt, L.; Boncella, J.; Rau, J.; Macomber, C.; Pivovar, B. Stability of Cations for Anion Exchange Membrane Fuel Cells. Transactions 2019, 11, 1173–1180. [Google Scholar] [CrossRef]
- Chempath, S.; Einsla, B.R.; Pratt, L.R.; Macomber, C.S.; Boncella, J.M.; Rau, J.A.; Pivovar, B.S. Mechanism of Tetraalkylammonium Headgroup Degradation in Alkaline Fuel Cell Membranes. J. Phys. Chem. C 2008, 112, 3179–3182. [Google Scholar] [CrossRef]
- Cope, A.C.; Mehta, A.S. Mechanism of the Hofmann Elimination Reaction: An Ylide Intermediate in the Pyrolysis of a Highly Branched Quaternary Hydroxide. J. Am. Chem. Soc. 1963, 85, 1949–1952. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Golding, B.T.; Sadeghi, M.; Cao, Y.; Yu, E.H.; Scott, K. A Polytetrafluoroethylene-Quaternary 1,4-Diazabicyclo-[2.2.2]-Octane Polysulfone Composite Membrane for Alkaline Anion Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2011, 36, 10022–10026. [Google Scholar] [CrossRef]
- Rao, A.H.N.; Thankamony, R.L.; Kim, H.-J.; Nam, S.; Kim, T.-H. Imidazolium-Functionalized Poly(Arylene Ether Sulfone) Block Copolymer as an Anion Exchange Membrane for Alkaline Fuel Cell. Polymer 2013, 54, 111–119. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Diat, O.; Gebel, G. Fuel Cells: Proton Channels. Nature Materials 2008, 7, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liao, X.; Xu, J.; Chen, D.; Zhang, H. Novel Anion-Conducting Interpenetrating Polymer Network of Quaternized Polysulfone and Poly(Vinyl Alcohol) for Alkaline Fuel Cells. Int. J. Hydrog. Energy 2016, 41, 5816–5823. [Google Scholar] [CrossRef]
- Pérez-Prior, M.T.; Ureña, N.; Tannenberg, M.; del Río, C.; Levenfeld, B. DABCO-Functionalized Polysulfones as Anion-Exchange Membranes for Fuel Cell Applications: Effect of Crosslinking. J. Polym. Sci. B Polym. Phys. 2017, 55, 1326–1336. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Wang, C.; Lee, Y.M.; Guiver, M.D. Phenyltrimethylammonium Functionalized Polysulfone Anion Exchange Membranes. Macromolecules 2012, 45, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Ureña, N.; Pérez-Prior, M.T.; del Rio, C.; Várez, A.; Levenfeld, B. New Amphiphilic Semi-Interpenetrating Networks Based on Polysulfone for Anion-Exchange Membrane Fuel Cells with Improved Alkaline and Mechanical Stabilities. Polymer 2021, 226, 123824. [Google Scholar] [CrossRef]
- Lin, B.; Xu, F.; Chu, F.; Ren, Y.; Ding, J.; Yan, F. Bis-Imidazolium Based Poly(Phenylene Oxide) Anion Exchange Membranes for Fuel Cells: The Effect of Cross-Linking. J. Mater. Chem. A 2019, 7, 13275–13283. [Google Scholar] [CrossRef]
- Pan, J.; Han, J.; Zhu, L.; Hickner, M.A. Cationic Side-Chain Attachment to Poly(Phenylene Oxide) Backbones for Chemically Stable and Conductive Anion Exchange Membranes. Chem. Mater. 2017, 29, 5321–5330. [Google Scholar] [CrossRef]
- Xue, J.; Liu, X.; Zhang, J.; Yin, Y.; Guiver, M.D. Poly(Phenylene Oxide)s Incorporating FDdwsSpirocyclic Quaternary Ammonium Cation/Cation Strings for Anion Exchange Membranes. J. Membr. Sci. 2020, 595, 117507. [Google Scholar] [CrossRef]
- Carlson, A.; Eriksson, B.; Olsson, J.S.; Lindbergh, G.; Lagergren, C.; Jannasch, P.; Wreland Lindström, R. Fuel Cell Evaluation of Anion Exchange Membranes Based on Poly(Phenylene Oxide) with Different Cationic Group Placement. Sustain. Energy Fuels 2020, 4, 2274–2283. [Google Scholar] [CrossRef]
- Lee, S.-B.; Min, C.-M.; Jang, J.; Lee, J.-S. Enhanced Conductivity and Stability of Anion Exchange Membranes Depending on Chain Lengths with Crosslinking Based on Poly(Phenylene Oxide). Polymer 2020, 192, 122331. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Ma, L.; Bai, L.; Zhang, A.; Qaisrani, N.A.; Yan, X.; Zhang, F.; He, G. Dual-Side-Chain-Grafted Poly(Phenylene Oxide) Anion Exchange Membranes for Fuel-Cell and Electrodialysis Applications. ACS Sustain. Chem. Eng. 2021, 9, 8611–8622. [Google Scholar] [CrossRef]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the Use of Ionic Liquids as Electrolyte Membranes in Fuel Cells. J. Membr. Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Elabd, Y.A. Relative Chemical Stability of Imidazolium-Based Alkaline Anion Exchange Polymerized Ionic Liquids. Macromolecules 2011, 44, 8494–8503. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Si, Z.; Qiu, L.; Chu, F.; Zhao, J.; Yan, F. Bis-Imidazolium-Based Anion-Exchange Membranes for Alkaline Fuel Cells. J. Power Sources 2012, 217, 329–335. [Google Scholar] [CrossRef]
- Fang, J.; Lyu, M.; Wang, X.; Wu, Y.; Zhao, J. Synthesis and Performance of Novel Anion Exchange Membranes Based on Imidazolium Ionic Liquids for Alkaline Fuel Cell Applications. J. Power Sources 2015, 284, 517–523. [Google Scholar] [CrossRef]
- Ouadah, A.; Xu, H.; Luo, T.; Gao, S.; Wang, X.; Fang, Z.; Jing, C.; Zhu, C. A Series of Poly(Butylimidazolium) Ionic Liquid Functionalized Copolymers for Anion Exchange Membranes. J. Power Sources 2017, 371, 77–85. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized Aryl Ether-Free Polyaromatics for Alkaline Membrane Fuel Cells: Synthesis, Properties, and Performance-a Topical Review. J. Mater. Chem. A 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Caire, B.R.; Vandiver, M.A.; Pandey, T.P.; Herring, A.M.; Liberatore, M.W. Accelerated Mechanical Degradation of Anion Exchange Membranes via Hydration Cycling. J. Electrochem. Soc. 2016, 163, H964–H969. [Google Scholar] [CrossRef] [Green Version]
- Narducci, R.; Chailan, J.-F.; Fahs, A.; Pasquini, L.; Di Vona, M.L.; Knauth, P. Mechanical Properties of Anion Exchange Membranes by Combination of Tensile Stress–Strain Tests and Dynamic Mechanical Analysis. J. Polym. Sci. B Polym. Phys. 2016, 54, 1180–1187. [Google Scholar] [CrossRef]
- Peng, X.; Omasta, T.J.; Magliocca, E.; Wang, L.; Varcoe, J.R.; Mustain, W.E. Nitrogen-Doped Carbon–CoOx Nanohybrids: A Precious Metal Free Cathode That Exceeds 1.0 W Cm−2 Peak Power and 100 h Life in Anion-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2019, 58, 1046–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Kashyap, V.; Ng, B.; Kurungot, S.; Wang, L.; Varcoe, J.R.; Mustain, W.E. High-Performing PGM-Free AEMFC Cathodes from Carbon-Supported Cobalt Ferrite Nanoparticles. Catalysts 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Bellini, M.; Miller, H.A.; Varcoe, J.R. A High Conductivity Ultrathin Anion-Exchange Membrane with 500+ h Alkali Stability for Use in Alkaline Membrane Fuel Cells That Can Achieve 2 W Cm-2 at 80 °C. J. Mater. Chem. A 2018, 6, 15404–15412. [Google Scholar] [CrossRef] [Green Version]
- Sherazi, T.A.; Yong Sohn, J.; Moo Lee, Y.; Guiver, M.D. Polyethylene-Based Radiation Grafted Anion-Exchange Membranes for Alkaline Fuel Cells. J. Membr. Sci. 2013, 441, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Danks, T.N.; Slade, R.C.T.; Varcoe, J.R. Alkaline Anion-Exchange Radiation-Grafted Membranes for Possible Electrochemical Application in Fuel Cells. J. Mater. Chem. 2003, 13, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Magliocca, E.; Cunningham, E.L.; Mustain, W.E.; Poynton, S.D.; Escudero-Cid, R.; Nasef, M.M.; Ponce-González, J.; Bance-Souahli, R.; Slade, R.C.T.; et al. An Optimised Synthesis of High Performance Radiation-Grafted Anion-Exchange Membranes. Green Chem. 2017, 19, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.J.; Robertson, N.J.; Kostalik, H.A., IV; Lobkovsky, E.B.; Mutolo, P.F.; Abruña, H.D.; Coates, G.W. A Ring-Opening Metathesis Polymerization Route to Alkaline Anion Exchange Membranes: Development of Hydroxide-Conducting Thin Films from an Ammonium-Functionalized Monomer. J. Am. Chem. Soc. 2009, 131, 12888–12889. [Google Scholar] [CrossRef]
- Chen, W.; Mandal, M.; Huang, G.; Wu, X.; He, G.; Kohl, P.A. Highly Conducting Anion-Exchange Membranes Based on Cross-Linked Poly(Norbornene): Ring Opening Metathesis Polymerization. ACS Appl. Energy Mater. 2019, 2, 2458–2468. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Wang, Y.; An, L.; Guiver, M.D.; Li, N. Highly Stable Anion Exchange Membranes Based on Quaternized Polypropylene. J. Mater. Chem. A 2015, 3, 12284–12296. [Google Scholar] [CrossRef]
- Zhu, M.; Su, Y.; Wu, Y.; Zhang, M.; Wang, Y.; Chen, Q.; Li, N. Synthesis and Properties of Quaternized Polyolefins with Bulky Poly(4-Phenyl-1-Butene) Moieties as Anion Exchange Membranes. J. Membr. Sci. 2017, 541, 244–252. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Highly Efficient Ring-Opening Metathesis Polymerization (ROMP) Using New Ruthenium Catalysts Containing N-Heterocyclic Carbene Ligands. Angew. Chem. 2000, 39, 2903–2906. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP Toolbox Upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- You, W.; Hugar, K.M.; Coates, G.W. Synthesis of Alkaline Anion Exchange Membranes with Chemically Stable Imidazolium Cations: Unexpected Cross-Linked Macrocycles from Ring-Fused ROMP Monomers. Macromolecules 2018, 51, 3212–3218. [Google Scholar] [CrossRef]
- You, W.; Padgett, E.; MacMillan, S.N.; Muller, D.A.; Coates, G.W. Highly Conductive and Chemically Stable Alkaline Anion Exchange Membranes via ROMP of Trans-Cyclooctene Derivatives. Prog. Polym. Sci. 2019, 116, 9729–9734. [Google Scholar] [CrossRef] [Green Version]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-Stable Anion Exchange Membranes: A Review of Synthetic Approaches. J. Am. Chem. Soc. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Zha, Y.; Disabb-Miller, M.L.; Johnson, Z.D.; Hickner, M.A.; Tew, G.N. Metal-Cation-Based Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 4493–4496. [Google Scholar] [CrossRef]
- Zhu, T.; Tang, C. Crosslinked Metallo-Polyelectrolytes with Enhanced Flexibility and Dimensional Stability for Anion-Exchange Membranes. Polym. Chem. 2020, 11, 4542–4546. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Mountz, D.A.; Reuschle, D.A.; Blackwell, R.I. Self-Assembled Organic/Inorganic Hybrids as Membrane Materials. Electrochim. Acta 2004, 50, 565–569. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Shin, W.; Singh, P.K. Progress in Ionic Organic-Inorganic Composite Membranes for Fuelcell Applications. Polym. Chem. 2010, 1, 388–408. [Google Scholar] [CrossRef]
- Caseri, W.R. Nanocomposites of Polymers and Inorganic Particles: Preparation, Structure and Properties. Mater. Sci. Technol. 2006, 22, 807–817. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V.K.; Pintauro, P.N. The Chalkboard: Anion Exchange Membrane Fuel Cells. Electrochem. Soc. Interface 2010, 19, 31–35. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Winey, K.I.; Vaia, R.A. Polymer Nanocomposites. MRS Bull. 2007, 32, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Deka, B.K.; Lee, S.H.; Park, Y.-B.; Yoon, Y.S. Poly(Vinyl Alcohol)/Silica Nanoparticles Based Anion-Conducting Nanocomposite Membrane for Fuel-Cell Applications. Macromol. Res. 2015, 23, 256–264. [Google Scholar] [CrossRef]
- Das, G.; Park, B.J.; Yoon, H.H. A Bionanocomposite Based on 1,4-Diazabicyclo-[2.2.2]-Octane Cellulose Nanofiber Cross-Linked-Quaternary Polysulfone as an Anion Conducting Membrane. J. Mater. Chem. A 2016, 4, 15554–15564. [Google Scholar] [CrossRef]
- Das, G.; Kim, C.Y.; Kang, D.H.; Kim, B.H.; Yoon, H.H. Quaternized Polysulfone Cross-Linked N,N-Dimethyl Chitosan-Based Anion-Conducting Membranes. Polymers 2019, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Dongho, K.; Kim, C.Y.; Yoon, H.H. Graphene Oxide Crosslinked Poly(Phenylene Oxide) Nanocomposite as High-Performance Anion-Conducting Membrane. J. Ind. Eng. Chem. 2019, 72, 380–389. [Google Scholar] [CrossRef]
- Das, G.; Park, B.J.; Kim, J.; Kang, D.; Yoon, H.H. Quaternized Cellulose and Graphene Oxide Crosslinked Polyphenylene Oxide Based Anion Exchange Membrane. Sci. Rep. 2019, 9, 9572. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Zhang, H.; Hou, L. Preparation and Characterization of Quaternized Poly(Vinyl Alcohol)/Chitosan/MoS(2) Composite Anion Exchange Membranes with High Selectivity. Carbohydr. Polym. 2018, 180, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, X.; Fu, Y.; Hu, B.; Bai, Y.; Lü, C. Quaternized Polyhedral Oligomeric Silsesquioxanes (QPOSS) Modified Polysulfone-Based Composite Anion Exchange Membranes. Solid State Ion. 2017, 309, 170–179. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Meng, Y. Novel Quaternized Poly(Arylene Ether Sulfone)/Nano-ZrO2 Composite Anion Exchange Membranes for Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2013, 5, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Das, G.; Yoon, H.H.; Kim, I.T. A Composite Anion Conducting Membrane Based on Quaternized Cellulose and Poly(Phenylene Oxide) for Alkaline Fuel Cell Applications. Polymers 2020, 12, 2676. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Lin, B.; Qiu, L.; Yan, F. Alkaline Imidazolium- and Quaternary Ammonium-Functionalized Anion Exchange Membranes for Alkaline Fuel Cell Applications. J. Mater. Chem. 2012, 22, 1040–1045. [Google Scholar] [CrossRef]
- Khoiruddin, D.A.; Subajgo, I.G.W. Surface Modification of Ion-Exchange Membranes: Methods, Characteristics, and Performance. J. Appl. Polym. Sci. 2017, 134, 45540. [Google Scholar] [CrossRef] [Green Version]
- Boschet, F.; Ameduri, B. Development of Anion Exchange Membranes: A Step towards Non-Noble Metal Fuel Cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar]
- Mishra, A.K.; Bose, S.; Kuila, T.; Kim, N.H.; Lee, J.H. Silicate-Based Polymer-Nanocomposite Membranes for Polymer Electrolyte Membrane Fuel Cells. Prog. Polym. Sci. 2012, 37, 842–869. [Google Scholar] [CrossRef]
- Pivovar, B.; Kim, Y.S. 2019 Anion Exchange Membrane Workshop Summary Report; NREL/TP-5900-77240; National Renewable Energy Lab. (NREL), Golden, CO: Dallas, TX, USA, 2019. [Google Scholar]












| Type | Energy Density (Wh/L) | Power Density (W/L) | Nominal Voltage (V) | Life Cycle | Depth of Discharge (%) | Round Trip Efficiency | Estimated Cost (USD/kWh) |
|---|---|---|---|---|---|---|---|
| Li-ion | 200–400 | 1500–10,000 | 4.3 | 10,000 | 95 | 96 | 200–1260 |
| Lead-acid | 50–80 | 10–400 | 2.0 | 1500 | 50 | 82 | 15–475 |
| VRFB | 25–33 | 1–2 | 1.4 | 13,000 | 100 | 70 | 315–1050 |
| Type | Membrane Brand Name | Manufacturer, Country | Thickness (µm) | Conductivity (mS/cm) | Ref. |
|---|---|---|---|---|---|
| Cation | Nafion 117 | Dupont, USA | 183 | 76.5 ± 1.7 | [45] |
| Nafion 212 | Dupont, USA | 50.8 | 157.0 ± 2.1 | ||
| Gore-select® | Gore, USA | 18 | 100 | [46] | |
| Gore-select® | Gore, USA | 35 | 96 | ||
| Anion | Fumasep® FAA-3 | Fumatech, Germany | 45–50 | 40–45 | [47] |
| Tokuyama A201 | Tokuyama, Japan | 28 | 42 | ||
| Sustainion® 37–50 | Dioxide Materials, USA | 50 | 70 |
| Manufacturer | Brand/Commercial Name |
|---|---|
| Surrey | SION1 |
| Tokayama, Japan | AS-4 anion exchange ionomer |
| Fuma, Germany | Fumion/FAA-3-SOLUT-10 |
| Xergy, USA | Pention™/Pention-D72 |
| Dioxide Materials, USA | Sustainion®/XB-7 |
| Membrane/Manufacturer | Structure | IEC (meq g−1) | Thickness (mm) | Resistance (cm2) |
|---|---|---|---|---|
| Tokuyama Co., Ltd., Tokyo, Japan | PS/DVB | 1.4–1.7 | 0.12–0.18 | 2.0–3.5 |
| RAI Research Corp., Hauppauge, NY, USA | LDPF | 0.9 | 0.24 | 4.0–7.0 |
| CSMCRI, Bhavnagar, India | LDPE/HDPE (IPN) | 0.8–0.9 | 0.16–0.18 | 2.0–4.0 |
| Solvay S.A., Bruxelles, Belgium | Morgane ADP | 1.3–1.7 | 0.13–0.17 | 1.8–2.9 |
| PCA Polymerchemie Altmeier GmbH, Heusweiler, Germany | PC 100 D | 1.2 quat. | 0.08–0.1 | 5 |
| Membrane | Thickness (µm) | Conductivity (mS/cm) | Condition | Ref. |
|---|---|---|---|---|
| PVA (43.3 wt.%)–KOH (35.7 wt.%)–H2O (21 wt.%) | 800 | 0.13 | 25 °C | [112] |
| PVA (30 wt.%)–KOH (40 wt%)–H2O (30 wt.%) | 480 | 47.1 | 30 °C | [113] |
| PVA (60 wt.%)–polydiallyldimethylammonium chloride (PDDA) | 110 | 22 | 25 °C | [114] |
| PVA (5 wt.%)–KOH (10 wt.%)–PEGDGE (5 wt.%) | 250 ± 50 | 220 ± 3 | 25 °C | [115] |
| PVA (10 wt.%)–PAA (7.5 wt.%)–KOH (32 wt.%) | 450 | 301 | 25 °C | [116] |
| PVAAA (PVA60 wt.%, AA40 wt.%)–KOH (40 wt.%) | - | 312 | 25 °C | [117] |
| PVA(50 wt.%)-PVC(50 wt.%) | 150–200 | 540.1 | 25 °C | [118] |
| PVA–KOH | 480 | 47.2 | 25 °C | [119] |
| PVA/PECH (1:1) blend | 530 | 1 | 25 °C | |
| PVA/TEAC (1:1) blend | 460 | 23.1 | 25 °C | |
| Crosslinked QAPVA | - | 7.34 | 30, in DI water | [120] |
| PVA/PAADDA | 90–110 (by composition mass) | 0.74–12 | 30–90 °C, in DI water | [121] |
| PVA/PVP/KOH-d (PVA/PVP, 1:0.5 by mass) | 60–80 | 530 | 25 °C, in 8M KOH solution. | [122] |
| Crosslinked and quaternized poly(vinyl alcohol) (CLQPVA) | - | 52.1 | °C, in DI water (RH 100%) | [123] |
| QAPVA-hexadecyl trimethylammonium bromide (HDT) (15%) | - | 4.84 | 30 °C, in DI water | [124] |
| PVA/PEI | - | 4.87 | 80 °C | [126] |
| Membrane | Thickness (µm) | Conductivity (mS/cm) | Condition | Ref. |
|---|---|---|---|---|
| PTFE-quaternary 1,4-diazabicyclo-[2.2.2]-octane (DABCO) polysulfone (PTFE-QDPSU) composite membrane | 30 | 51 | 55 °C, in DI water, RH 100% | [140] |
| Imidazolium-functionalized PES membrane (EI-PES) | 25–30 | 100 | 80 °C, in DI water | [141] |
| Ammonium-functionalized membrane (QA-PES) | 73 | |||
| QPSF-PVA (7:3 wt.%) | - | 25.2 | 60 °C, | [144] |
| QPSF-PVA (6:4 wt.%) | 18.2 | |||
| DABCO-functionalized polysulfones membrane (PSU-DABCO–OH 58%) | 115 | 0.157 | 25 °C, in 0.1 M KOH solution | [145] |
| Crosslinked DABCO-functionalized polysulfones membrane (C–PSU–DABCO–OH 133%) | 0.167 | |||
| Phenyltrimethylammonium functionalized polysulfone membrane (PSf-PTMA) | - | 58 | 80 °C | [146] |
| functionalized PSU (degree of crosslinking 20%, crosslinked polymer/PSU: 6/4) | - | 0.01 | 25 °C, in 0.1 M KOH solution | [147] |
| sIPN-MIm–OH functionalized PSU (degree of crosslinking 15%, crosslinked polymer/PSU: 9/1) | - | 0.09 | ||
| sIPN-DMIm–OH functionalized PSU (degree of crosslinking 5%, crosslinked polymer/PSU: 6/4) | - | 0.04 |
| Membrane | Thickness (µm) | Conductivity (mS/cm) | Condition | Ref. |
|---|---|---|---|---|
| Bis-imidazolium based poly(phenylene oxide) membrane | - | 54.04 | 90 °C, in DI water | [148] |
| Crosslinked bis-imidazolium based poly(phenylene oxide) membrane | - | Approximately 32 | ||
| NC4Q-PPO-40 | 50 ± 3 | 73.9 | 80 °C | [149] |
| NC5Q-PPO-60 | 96.1 | |||
| PPO-SDSU-36 | 50 ± 5 | 51.6 | 60 °C, in DI water | [150] |
| PPO-DDSU-27 | 47.2 | |||
| PPO5-TMA-1.9 | 30 ± 5 | 110 | 60 °C in DI water (RH 90%) | [151] |
| PPO5-Pip-1.8 | 45 ± 5 | 93 | ||
| Hexyl acyl chain and a crosslinked cQPH | - | 105 | 80 °C, RH 100% | [152] |
| Dual-grafted mono-quaternized PPO 16C25-3O25 | 90 | 21.3 | 30 °C | [153] |
| Dual-grafted tri-quaternized PPO 3QA16C16-3O16 | 50 |
| Membrane | Thickness (µm) | Conductivity (mS/cm) | Condition | Ref. |
|---|---|---|---|---|
| AmimCl:MMA = 6:1 (mole ratio) | 33.1 | 33.3 | 30 °C, in DI water | [104] |
| [PVMIm][OH] 40 -DVB 2 | 50 | 55.8 ± 5.5 | 60 °C, in DI water, RH 100% | [105] |
| Poly(MEBIm-OH) | 80–200 | 9.6 | 30 °C, RH 90% | [155] |
| [PABMHM]40[OH]2 | 45 | 25 | 90 °C | [156] |
| [VBI]Br:Styrene = 1:1.5 Feed ratio of (IILs/Styrene, mole) | 45 | 22.6 | 30 °C, in 0.1M/L NaOH solution | [157] |
| Crosslinked b-VIB/p-MS | - | 35.7 | 25 °C | [158] |
| Type | Membrane | Thickness (µm) | Conductivity (mS/cm) | Condition | Ref. |
|---|---|---|---|---|---|
| Radiation grafting | Polyethylene-based membrane (PE-g–PVBC–TOH) | 85–95 | 47.5 | 90 °C, in DI water | [165] |
| F1NOH (FEP-g-PVBTMAOH membranes, g = 25.6%) | 90–100 | 10–20 | 25 °C | [166] | |
| F2NOH (FEP-g-PVBTMAOH membranes, g = 22.7%) | 75–82 | ||||
| Aminated poly(LDPE-g-VBC, g = 68%) | 40 | 180–320 | 20–80 °C, RH 100% | [86] | |
| Aminated poly(HDPE-g-VBC, g = 47–56%) | 40 | 140–280 | |||
| Aminated poly(ETFE-g-VBC, g = 28%) | 25 | 5–20 | |||
| ETFE-based RG-AEM(30 kGy) | 47 ± 2 | 68 ± 3 (Cl−) | 80 °C, RH 100% | [167] | |
| Direct polymerization | Imidazolium-fused cyclooctene monomer was prepared and subjected to ROMP applied to AAEM-1 | – | 29 ± 2 | 22 °C, in DI water | [174] |
| Imidazolium-fused cyclooctene monomer was prepared and subjected to ROMP applied to AAEM-2 | – | 37 ± 2 | |||
| Random copolymer from a trans-cyclooctene–fused imidazolium monomer (HC–[1]498[2]200) | – | 134 | 80 °C | [175] | |
| ROMP of a bis(terpyridine)ruthenium(II) complex-functionalized norbornene 4 based membrane: DCPD (1:5 ratio) | 103 ± 5 | 28.6 | 30 °C | [177] |
| Membrane | Thickness (µm) | Conductivity (mS/cm) | Condition | Ref. |
|---|---|---|---|---|
| PVA/DGBE-15/SiO2-5 | 140 | 7.14 | 25 °C, in DI water | [185] |
| QPSfQC15 | - | 128 | 80 °C, in DI water | [186] |
| Dimethyl chitosan crosslinked polysulfone QPSfDMC2 | - | 54.15 ± 2.10 | 25 °C, in DI water | [187] |
| Quaternized chitosan was amalgamated with 1,4-diazoniabicycle-[2.2.2]-octane-functionalized PS to obtain crosslinked membrane | - | 151 | 80 °C, in DI water | [188] |
| Quaternized chitosan and graphene oxide with DABCO as the filler in a PPO matrix GO/cellulose/PPO (1/1/100 wt.%) | - | 215 | 80 °C, in DI water | [189] |
| Quaternized poly(vinyl alcohol)/chitosan/MoS2 composite—QPVA/CS/MoS2-0.2 | 150 | 31.53 | 25 °C | [190] |
| Quaternized polyhedral oligomeric silsesquioxanes (QPOSS) into the quaternized polysulfone (QPSU) membrane QPSU-3%-QPOSS | 30–45 | 53.6 | 80 °C | [191] |
| Quaternized poly(arylene ether sulfone)/nano-ZrO2 composite (ZrO2 content more than 7.5%) | - | >41.4 | 80 °C | [192] |
| Loading of the quaternized cellulose in the quaternized PPO (qPPO) matrix: qPPO/DG-Cel7 (7 wt.% of cellulose functionalized with DG) | - | 164 | 80 °C, in DI water | [193] |
| Type | Proposed Milestones |
|---|---|
| 2022 | AEM fuel cell initial performance 0.65V at 1000 mA cm−2 on H2/O2 (maximum pressure of 1.5 atm) in MEA with total < 0.2 mgPGM cm−2 and < 10% voltage degradation over 1000 h, T > 80 °C |
| 2023 | CO2 tolerance: < 65 mV loss for steady-state operation at 1.5 A cm−2 in H2/air scrubbed to 2 ppm CO2 |
| 2024 | Catalyst durability: H2/CO2-scrubbed air after accelerated stress test < 40% loss after 10,000 cycles from 0.6 V to 0.95 V Membrane durability: 1000 h open circuit voltage hold at 70% RH and ≥80 °C |
| 2025 | 1 W cm−2 at 0.65 V; H2/CO2-free air with total PGM loading < 0.125 mg cm−2. T > 80 °C, P ≤ 250 kPa |
| 2030 | AEM fuel cell peak power performance > 600 mW cm−2 under H2/air (maximum pressure of 1.5 atm) in PGM-free MEA |
| Ultimate | 1 W cm−2 at rated power (~0.65 V at 95 °C), PGM-free MEA, T ≥ 80 °C, P ≤ 250 kPa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. https://doi.org/10.3390/polym14061197
Das G, Choi J-H, Nguyen PKT, Kim D-J, Yoon YS. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers. 2022; 14(6):1197. https://doi.org/10.3390/polym14061197
Chicago/Turabian StyleDas, Gautam, Ji-Hyeok Choi, Phan Khanh Thinh Nguyen, Dong-Joo Kim, and Young Soo Yoon. 2022. "Anion Exchange Membranes for Fuel Cell Application: A Review" Polymers 14, no. 6: 1197. https://doi.org/10.3390/polym14061197
APA StyleDas, G., Choi, J.-H., Nguyen, P. K. T., Kim, D.-J., & Yoon, Y. S. (2022). Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers, 14(6), 1197. https://doi.org/10.3390/polym14061197