The Anionic Polymerization of a tert-Butyl-Carboxylate-Activated Aziridine
Abstract
:1. Introduction
2. Materials and Methods
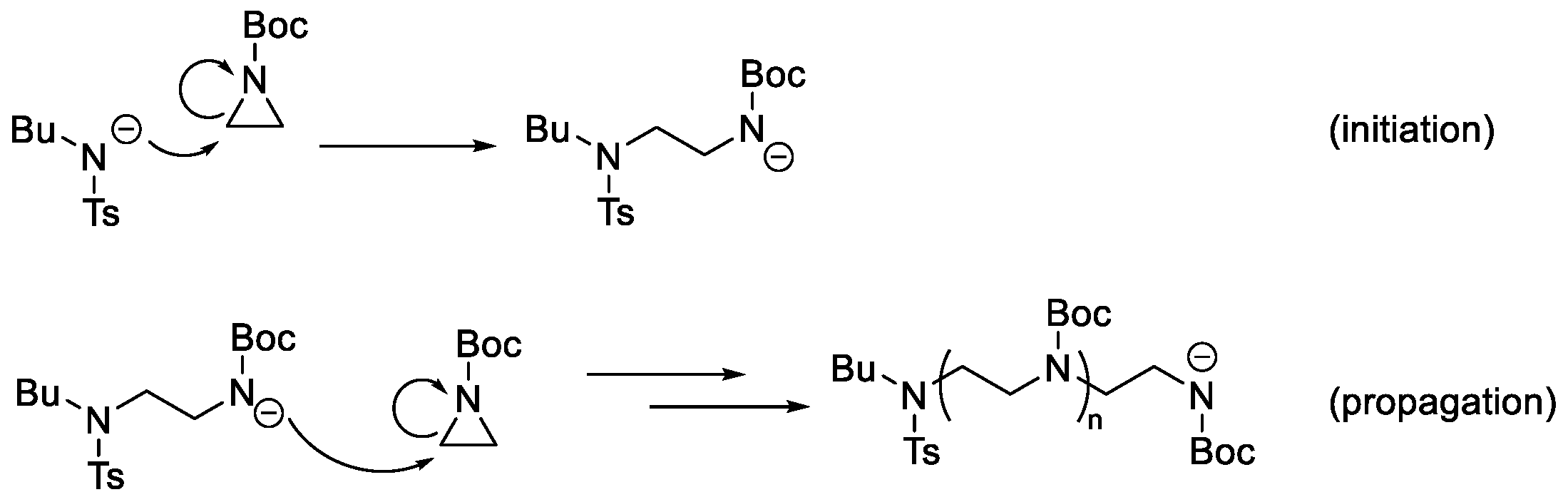
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grund, S.; Bauer, M.; Fischer, D. Polymers in Drug Delivery—State of the Art and Future Trends. Adv. Eng. Mater. 2011, 13, B61–B87. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blessing, T.; Kursa, M.; Holzhauser, R.; Kircheis, R.; Wagner, E. Different Strategies for Formation of PEGylated EGF-Conjugated PEI/DNA Complexes for Targeted Gene Delivery. Bioconjugate Chem. 2001, 12, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Höbel, S.; Loos, A.; Appelhans, D.; Schwarz, S.; Seidel, J.; Voit, B.; Aigner, A. Maltose- and maltotriose-modified, hyperbranched poly(ethylene imine)s (OM-PEIs): Physicochemical and biological properties of DNA and siRNA complexes. J. Control. Release 2011, 149, 146–158. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hiroishi, K.; Tokunoh, M.; Saegusa, T. Chelating properties of linear and branched poly(ethylenimines). Macromolecules 1987, 20, 1496–1500. [Google Scholar] [CrossRef]
- Von Zelewsky, A.; Barbosa, L.; Schläpfer, C.W. Poly(ethylenimines) as Brønsted bases and as ligands for metal ions. Coord. Chem. Rev. 1993, 123, 229–246. [Google Scholar] [CrossRef]
- Bayer, E.; Spivakov, B.Y.; Geckeler, K. Poly(ethyleneimine) as complexing agent for separation of metal ions using membrane filtration. Polym. Bull. 1985, 13, 307–311. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Khunsupat, R.; Chen, T.T.; Jones, C.W. Poly(allylamine)–Mesoporous Silica Composite Materials for CO2 Capture from Simulated Flue Gas or Ambient Air. Ind. Eng. Chem. Res. 2011, 50, 14203–14210. [Google Scholar] [CrossRef]
- Hicks, J.C.; Drese, J.H.; Fauth, D.J.; Gray, M.L.; Qi, G.; Jones, C.W. Designing Adsorbents for CO2 Capture from Flue Gas-Hyperbranched Aminosilicas Capable of Capturing CO2 Reversibly. J. Am. Chem. Soc. 2008, 130, 2902–2903. [Google Scholar] [CrossRef]
- Li, P.; Ge, B.; Zhang, S.; Chen, S.; Zhang, Q.; Zhao, Y. CO2 Capture by Polyethylenimine-Modified Fibrous Adsorbent. Langmuir 2008, 24, 6567–6574. [Google Scholar] [CrossRef]
- Tong, Z.; Ho, W.S.W. New sterically hindered polyvinylamine membranes for CO2 separation and capture. J. Membr. Sci. 2017, 543, 202–211. [Google Scholar] [CrossRef]
- Xu, X.; Myers, M.B.; Versteeg, F.G.; Pejcic, B.; Heath, C.; Wood, C.D. Direct air capture (DAC) of CO2 using polyethylenimine (PEI) “snow”: A scalable strategy. Chem. Commun. 2020, 56, 7151–7154. [Google Scholar] [CrossRef]
- Gleede, T.; Reisman, L.; Rieger, E.; Mbarushimana, P.C.; Rupar, P.A.; Wurm, F.R. Aziridines and azetidines: Building blocks for polyamines by anionic and cationic ring-opening polymerization. Polym. Chem. 2019, 10, 3257–3283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Chen, D.; Wang, X.; Xie, X. Insight into the synthesis of branched polyethylenimines from 2-haloethylamine via a one-pot two-stage process. Polymer 2022, 255, 125113. [Google Scholar] [CrossRef]
- Jäger, M.; Schubert, S.; Ochrimenko, S.; Fischer, D.; Schubert, U.S. Branched and linear poly(ethylene imine)-based conjugates: Synthetic modification, characterization, and application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef]
- Kagiya, T.; Narisawa, S.; Maeda, T.; Fukui, K. Ring-opening polymerization of 2-substituted 2-oxazolines. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 441–445. [Google Scholar] [CrossRef]
- Lambermont-Thijs, H.M.L.; van der Woerdt, F.S.; Baumgaertel, A.; Bonami, L.; Du Prez, F.E.; Schubert, U.S.; Hoogenboom, R. Linear Poly(ethylene imine)s by Acidic Hydrolysis of Poly(2-oxazoline)s: Kinetic Screening, Thermal Properties, and Temperature-Induced Solubility Transitions. Macromolecules 2010, 43, 927–933. [Google Scholar] [CrossRef]
- Fischer, W.; Brissault, B.; Prévost, S.; Kopaczynska, M.; Andreou, I.; Janosch, A.; Gradzielski, M.; Haag, R. Synthesis of Linear Polyamines with Different Amine Spacings and their Ability to Form dsDNA/siRNA Complexes Suitable for Transfection. Macromol. Biosci. 2010, 10, 1073–1083. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Brissault, B.; Leborgne, C.; Guis, C.; Danos, O.; Cheradame, H.; Kichler, A. Linear Topology Confers in Vivo Gene Transfer Activity to Polyethylenimines. Bioconjugate Chem. 2006, 17, 759–765. [Google Scholar] [CrossRef]
- Kichler, A. Gene transfer with modified polyethylenimines. J. Gene Med. 2004, 6, S3–S10. [Google Scholar] [CrossRef]
- Stewart, I.C.; Lee, C.C.; Bergman, R.G.; Toste, F.D. Living Ring-Opening Polymerization of N-Sulfonylaziridines: Synthesis of High Molecular Weight Linear Polyamines. J. Am. Chem. Soc. 2005, 127, 17616–17617. [Google Scholar] [CrossRef]
- Gleede, T.; Rieger, E.; Homann-Müller, T.; Wurm, F.R. 4-Styrenesulfonyl-(2-methyl) aziridine: The First Bivalent Aziridine-Monomer for Anionic and Radical Polymerization. Macromol. Chem. Phys. 2018, 219, 1700145. [Google Scholar] [CrossRef]
- Rieger, E.; Gleede, T.; Weber, K.; Manhart, A.; Wagner, M.; Wurm, F.R. The living anionic polymerization of activated aziridines: A systematic study of reaction conditions and kinetics. Polym. Chem. 2017, 8, 2824–2832. [Google Scholar] [CrossRef] [Green Version]
- Rieger, E.; Alkan, A.; Manhart, A.; Wagner, M.; Wurm, F.R. Sequence-controlled polymers via simultaneous living anionic copolymerization of competing monomers. Macromol. Rapid Commun. 2016, 37, 833–839. [Google Scholar] [CrossRef]
- Rieger, E.; Blankenburg, J.; Grune, E.; Wagner, M.; Landfester, K.; Wurm, F.R.J.A.C.I.E. Controlling the polymer microstructure in anionic polymerization by compartmentalization. Angew. Chem. Int. Ed. 2018, 57, 2483–2487. [Google Scholar] [CrossRef]
- Thomi, L.; Wurm, F.R. Living Anionic Polymerization of Functional Aziridines. Macromol. Symp. 2015, 349, 51–56. [Google Scholar] [CrossRef]
- Homann-Müller, T.; Rieger, E.; Alkan, A.; Wurm, F.R. N-Ferrocenylsulfonyl-2-methylaziridine: The first ferrocene monomer for the anionic (co) polymerization of aziridines. Polym. Chem. 2016, 7, 5501–5506. [Google Scholar] [CrossRef] [Green Version]
- Bakkali-Hassani, C.; Rieger, E.; Vignolle, J.; Wurm, F.R.; Carlotti, S.; Taton, D. The organocatalytic ring-opening polymerization of N-tosyl aziridines by an N-heterocyclic carbene. Chem. Commun. 2016, 52, 9719–9722. [Google Scholar] [CrossRef] [Green Version]
- Bakkali-Hassani, C.; Coutouly, C.; Gleede, T.; Vignolle, J.; Wurm, F.R.; Carlotti, S.; Taton, D. Selective initiation from unprotected aminoalcohols for the N-heterocyclic carbene-organocatalyzed ring-opening polymerization of 2-methyl-N-tosyl aziridine: Telechelic and block copolymer synthesis. Macromolecules 2018, 51, 2533–2541. [Google Scholar] [CrossRef]
- Gleede, T.; Rieger, E.; Liu, L.; Bakkali-Hassani, C.; Wagner, M.; Carlotti, S.; Taton, D.; Andrienko, D.; Wurm, F.R. Alcohol- and Water-Tolerant Living Anionic Polymerization of Aziridines. Macromolecules 2018, 51, 5713–5719. [Google Scholar] [CrossRef]
- Bakkali-Hassani, C.; Rieger, E.; Vignolle, J.; Wurm, F.R.; Carlotti, S.; Taton, D. Expanding the scope of N-heterocyclic carbene-organocatalyzed ring-opening polymerization of N-tosyl aziridines using functional and non-activated amine initiators. Eur. Polym. J. 2017, 95, 746–755. [Google Scholar] [CrossRef]
- Mbarushimana, P.C.; Liang, Q.; Allred, J.M.; Rupar, P.A. Polymerizations of Nitrophenylsulfonyl-Activated Aziridines. Macromolecules 2018, 51, 977–983. [Google Scholar] [CrossRef]
- Reisman, L.; Mbarushimana, C.P.; Cassidy, S.J.; Rupar, P.A. Living Anionic Copolymerization of 1-(Alkylsulfonyl)aziridines to Form Poly(sulfonylaziridine) and Linear Poly(ethylenimine). ACS Macro Lett. 2016, 5, 1137–1140. [Google Scholar] [CrossRef]
- Reisman, L.; Rowe, E.A.; Jackson, E.M.; Thomas, C.; Simone, T.; Rupar, P.A. Anionic Ring-Opening Polymerization of N-(tolylsulfonyl)azetidines To Produce Linear Poly(trimethylenimine) and Closed-System Block Copolymers. J. Am. Chem. Soc. 2018, 140, 15626–15630. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, Z.; Wang, H.; Gebru, H.; Chen, S.; Zhu, H.; Wei, F.; Guo, K. Organocatalyzed Anionic Ring-Opening Polymerizations of N-Sulfonyl Aziridines with Organic Superbases. ACS Macro Lett. 2017, 6, 1331–1336. [Google Scholar] [CrossRef]
- Kang, S.; Moon, H.K.; Yoon, H.J. Diaziridyl Ether of Bisphenol A. Macromolecules 2018, 51, 4068–4076. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, H.; Wang, Y.; Zhang, Z.; Hadjichristidis, N. Organocatalytic Synthesis of Polysulfonamides with Well-Defined Linear and Brush Architectures from a Designed/Synthesized Bis(N-sulfonyl aziridine). Macromolecules 2021, 54, 8164–8172. [Google Scholar] [CrossRef]
- Xu, J.; Hadjichristidis, N. Well-Defined Poly(Ester Amide)-Based Homo- and Block Copolymers by One-Pot Organocatalytic Anionic Ring-Opening Copolymerization of N-Sulfonyl Aziridines and Cyclic Anhydrides. Angew. Chem. Int. Ed. 2021, 60, 6949–6954. [Google Scholar] [CrossRef]
- Reisman, L.; Rowe, E.A.; Jefcoat, J.A.; Rupar, P.A. Activated Monomer Polymerization of an N-Sulfonylazetidine. ACS Macro Lett. 2020, 9, 334–338. [Google Scholar] [CrossRef]
- Rowe, E.A.; Reisman, L.; Jefcoat, J.A.; Rupar, P.A. Comparison of the Anionic Ring-Opening Polymerizations of N-(Alkylsulfonyl)azetidines. Macromolecules 2019, 52, 8032–8039. [Google Scholar] [CrossRef]
- Jung, S.; Kang, S.; Kuwabara, J.; Yoon, H.J. Aziridine-based polyaddition, post-modification, and crosslinking: Can aziridine rival epoxide in polymer chemistry? Polym. Chem. 2019, 10, 4506–4512. [Google Scholar] [CrossRef]
- Rieger, E.; Gleede, T.; Manhart, A.; Lamla, M.; Wurm, F.R. Microwave-Assisted Desulfonylation of Polysulfonamides toward Polypropylenimine. ACS Macro Lett. 2018, 7, 598–603. [Google Scholar] [CrossRef]
- Subhas Bose, D.; Kiran Kumar, K.; Narsimha Reddy, A.V. A New Protocol for Selective Deprotection of N-tert -Butoxycarbonyl Protective Group (t -Boc) with Sn(OTf)2. Synth. Commun. 2003, 33, 445–450. [Google Scholar] [CrossRef]
- Wang, G.; Li, C.; Li, J.; Jia, X. Catalyst-free water-mediated N-Boc deprotection. Tetrahedron Lett. 2009, 50, 1438–1440. [Google Scholar] [CrossRef]
- Zinelaabidine, C.; Souad, O.; Zoubir, J.; Malika, B.; Nour-Eddine, A. A simple and efficient green method for the deprotection of N-Boc in various structurally diverse amines under water-mediated catalyst-free conditions. Int. J. Chem. 2012, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Eis, M.J.; Ganem, B. BF3-etherate promoted alkylation of aziridines with organocopper reagents: A new synthesis of amines. Tetrahedron Lett. 1985, 26, 1153–1156. [Google Scholar] [CrossRef]
- Cheng, C.-C. Cleavage of the Pt–S bond of thiolated terpyridine–platinum(II) complexes by copper(II) and zinc(II) ions in phosphate buffer. Chem. Commun. 1998, 253–254. [Google Scholar] [CrossRef]
- Gianatassio, R.; Kadish, D. Direct Alkylation of 1-Azabicyclo[1.1.0]butanes. Org. Lett. 2019, 21, 2060–2063. [Google Scholar] [CrossRef]
- Laha, J.K.; Sharma, S.; Dayal, N. Palladium-Catalyzed Regio- and Chemo selective Reactions of 2-Bromobenzyl Bromides: Expanding the Scope for the Synthesis of Biaryls Fused to a Seven-Membered Sultam. Eur. J. Org. Chem. 2015, 2015, 7885–7891. [Google Scholar] [CrossRef]
- Chantarasriwong, O.; Jiangchareon, B.; Putra, C.K.; Suwankrua, W.; Chavasiri, W. NBS and Br3CCOCBr3 as highly efficient catalysts for the chemoselective N-tert-butyloxycarbonylation of amines. Tetrahedron Lett. 2016, 57, 4807–4811. [Google Scholar] [CrossRef]
- Giri, C.; Sisk, S.E.; Reisman, L.; Kammakakam, I.; Bara, J.E.; West, K.N.; Rabideau, B.D.; Rupar, P.A. Anionic Ring-Opening Polymerizations of N-Sulfonylaziridines in Ionic Liquids. Macromolecules 2022, 55, 623–629. [Google Scholar] [CrossRef]

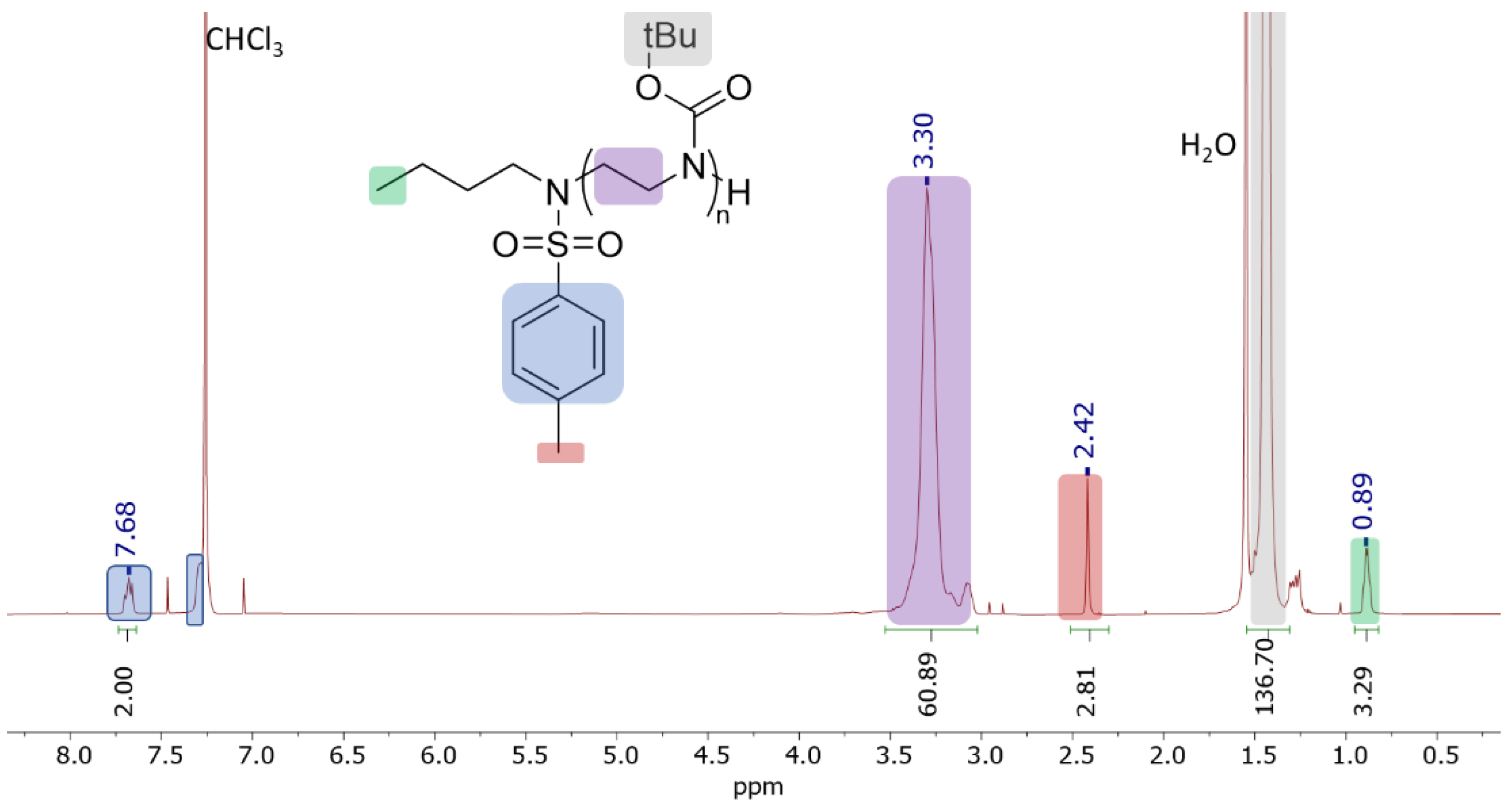

| [BocAz]:[BuN(K)Ts] | Mn(NMR) kDa 1 | Mn(GPC) kDa (Ð) 2 | Isolated Yield % |
|---|---|---|---|
| 20:1 | 2.4 kDa | 1.16 kDa (2.62) | 50 |
| 40:1 | 5.0 kDa | 1.42 kDa (2.78) | 76 |
| 80:1 | 7.2 kDa | 3.84 kDa (3.06) | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, C.; Chang, J.-Y.; Mbarushimana, P.C.; Rupar, P.A. The Anionic Polymerization of a tert-Butyl-Carboxylate-Activated Aziridine. Polymers 2022, 14, 3253. https://doi.org/10.3390/polym14163253
Giri C, Chang J-Y, Mbarushimana PC, Rupar PA. The Anionic Polymerization of a tert-Butyl-Carboxylate-Activated Aziridine. Polymers. 2022; 14(16):3253. https://doi.org/10.3390/polym14163253
Chicago/Turabian StyleGiri, Chandan, Jen-Yu Chang, Pierre Canisius Mbarushimana, and Paul A. Rupar. 2022. "The Anionic Polymerization of a tert-Butyl-Carboxylate-Activated Aziridine" Polymers 14, no. 16: 3253. https://doi.org/10.3390/polym14163253
APA StyleGiri, C., Chang, J.-Y., Mbarushimana, P. C., & Rupar, P. A. (2022). The Anionic Polymerization of a tert-Butyl-Carboxylate-Activated Aziridine. Polymers, 14(16), 3253. https://doi.org/10.3390/polym14163253







