Al2O3-Modified Polymer-Derived Ceramic SiCN High-Temperature Anti-Oxidative Composite Coating Fabricated by Direct Writing
Abstract
:1. Introduction
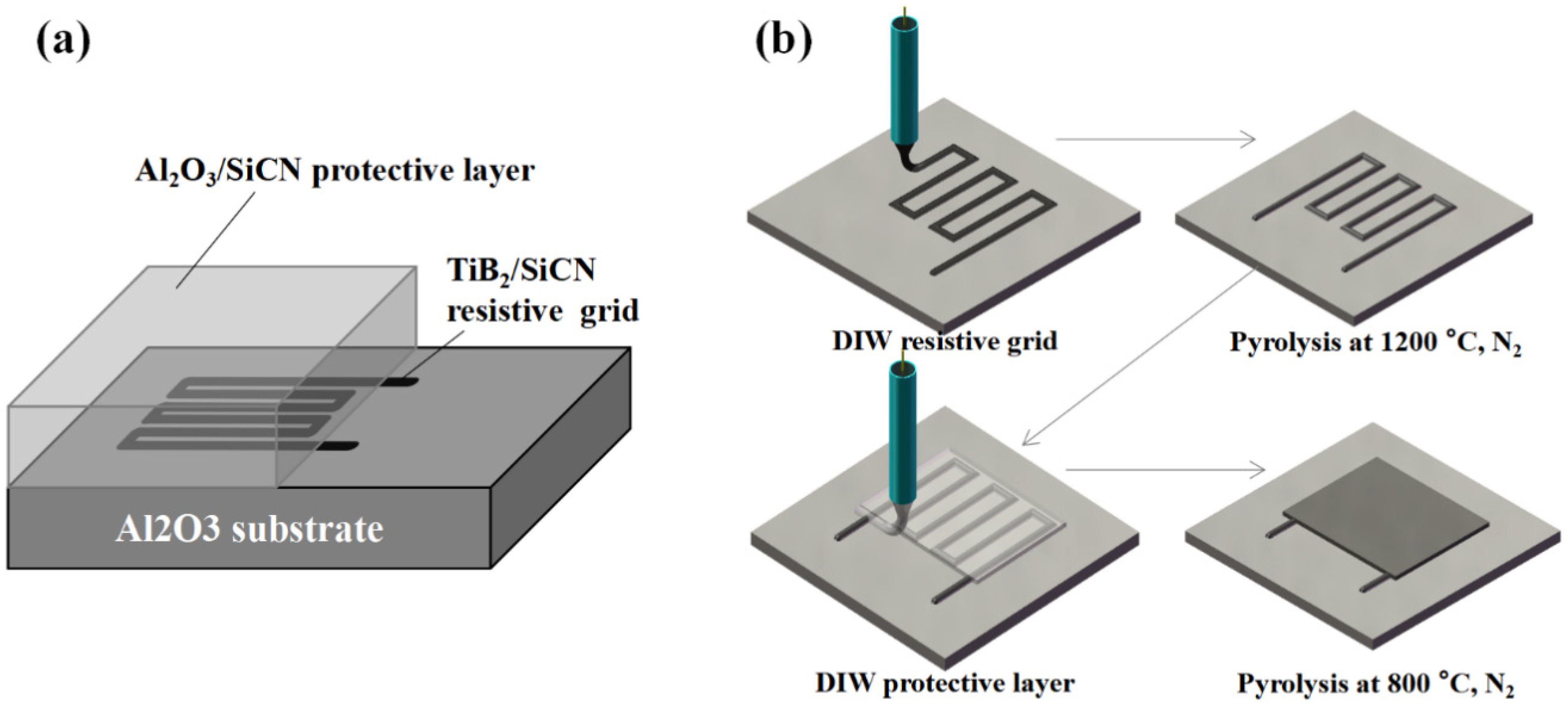
2. Materials and Methods
3. Results
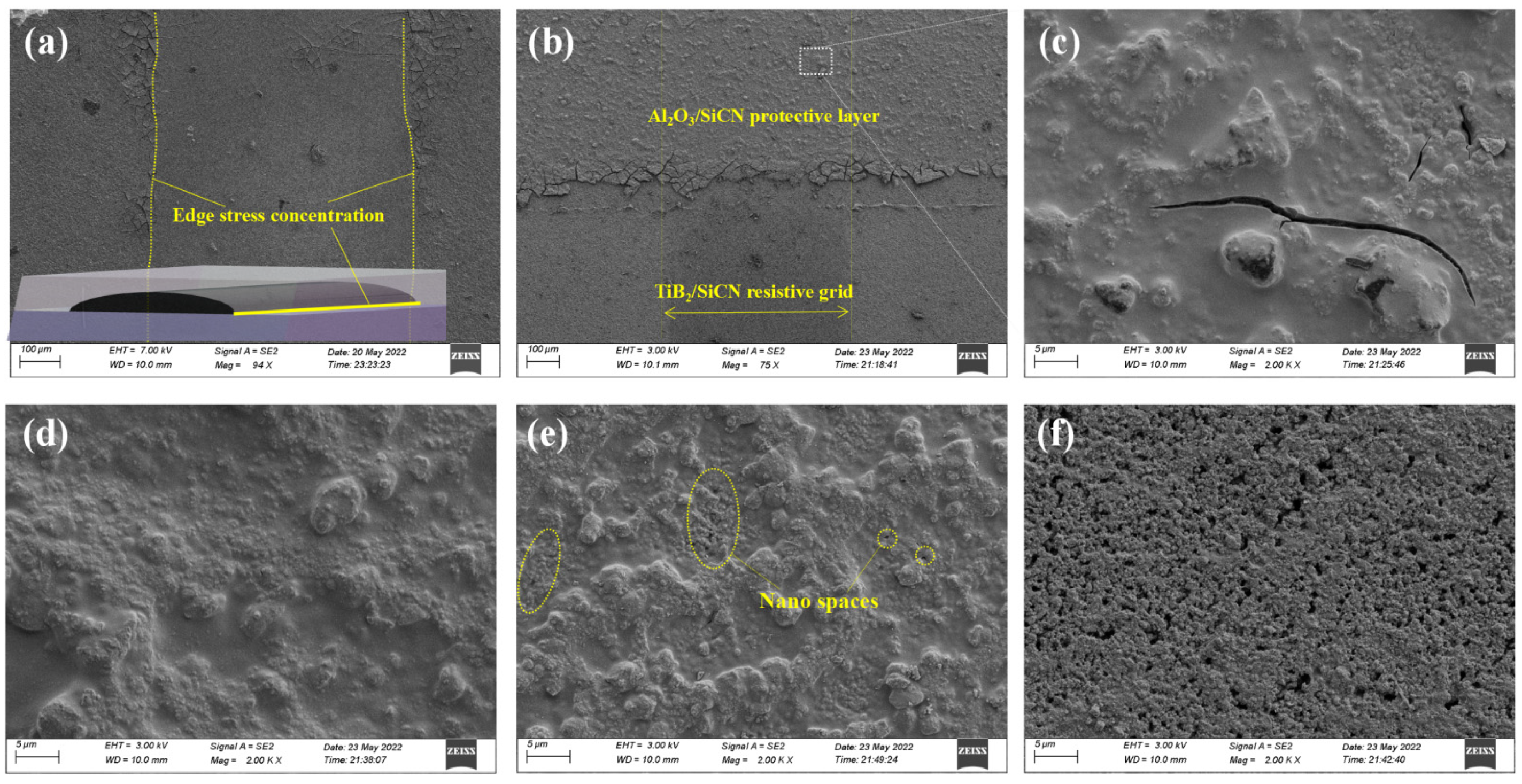
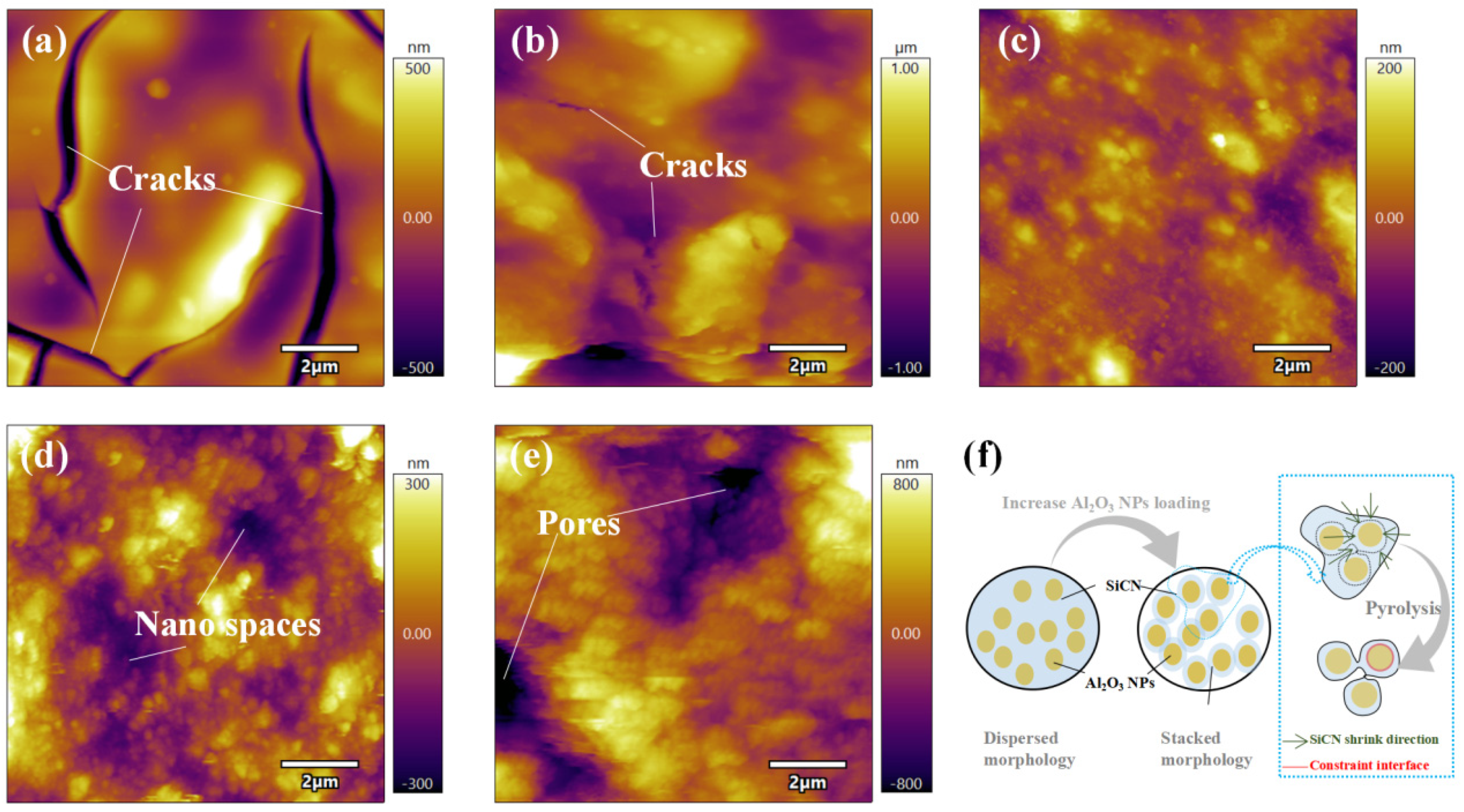
3.1. Film Morphology
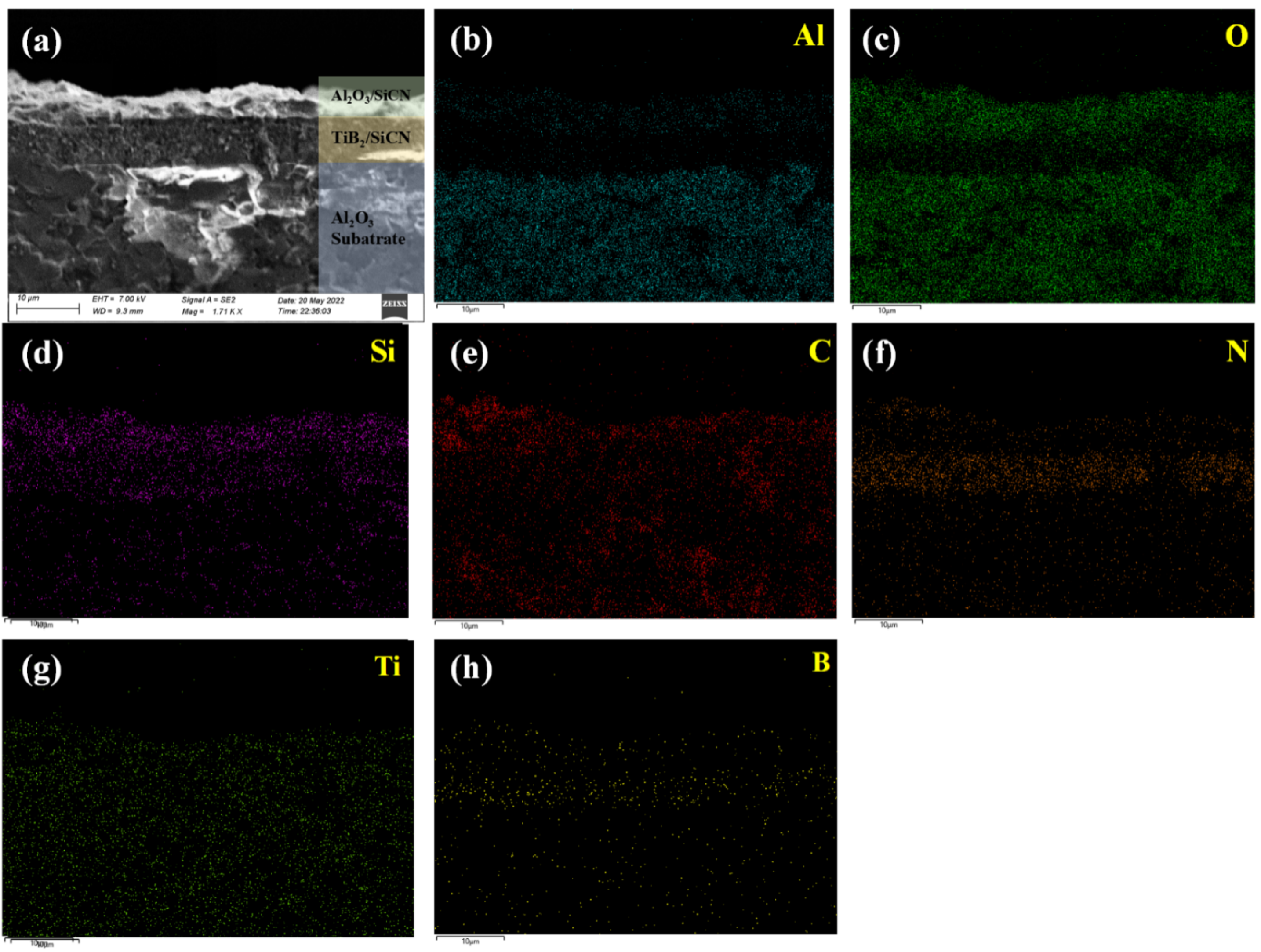
3.2. Film Composition
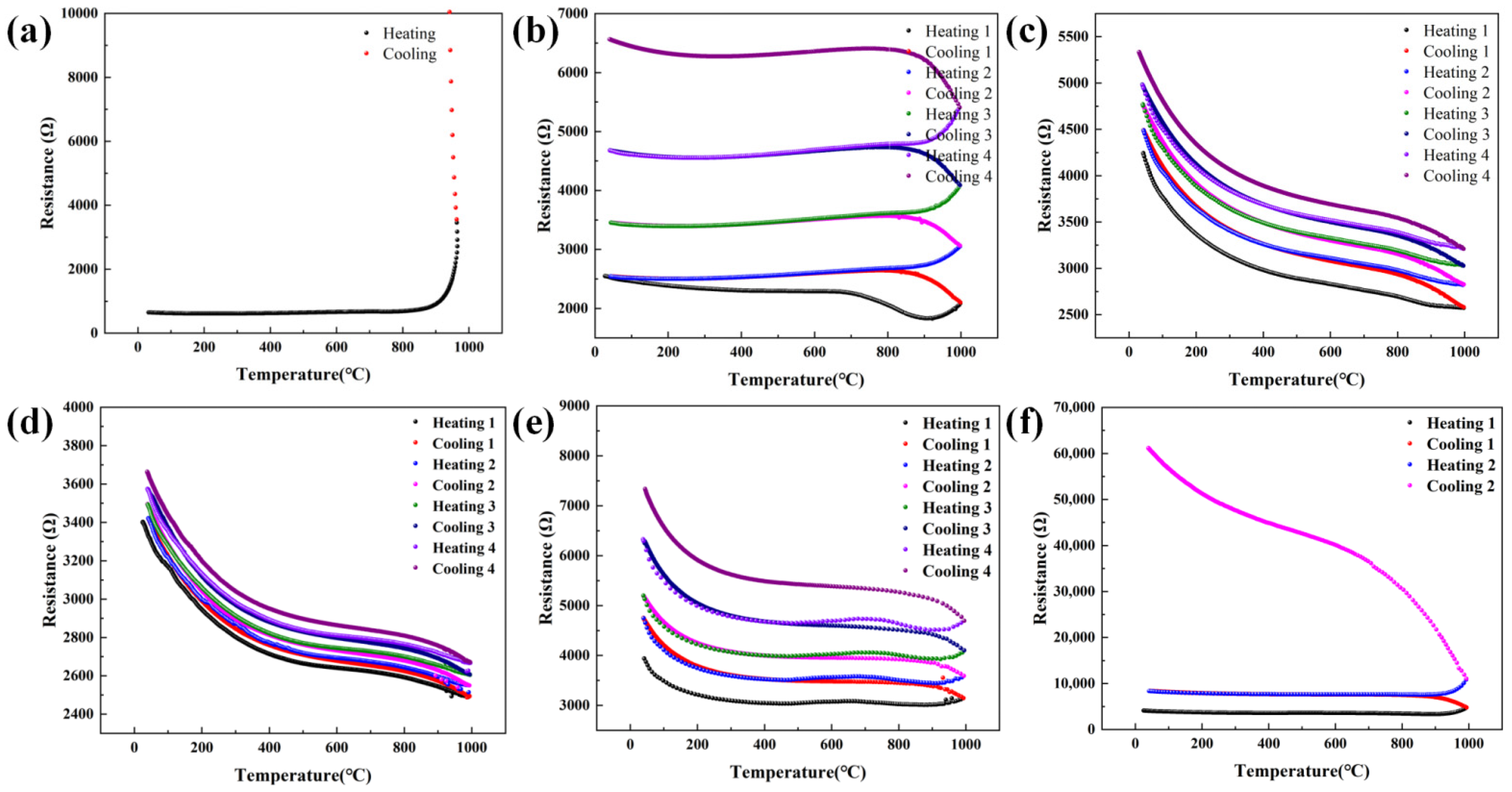
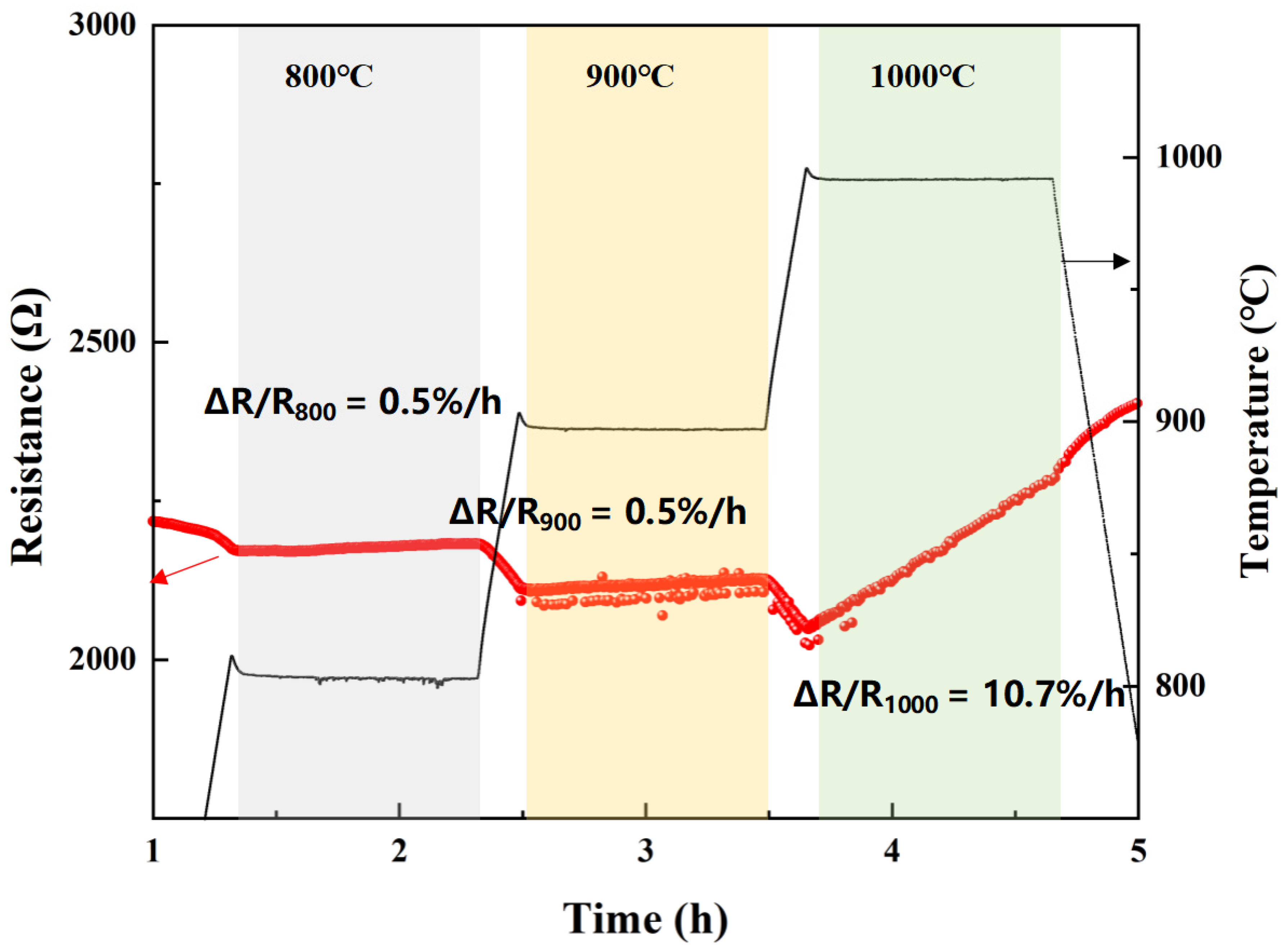
3.3. Protection Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, G.S. Characteristics of tantalum nitride thin film strain gauges for harsh environments. Sens. Actuator A-Phys. 2007, 135, 355–359. [Google Scholar] [CrossRef]
- Jin, X.H.; Ma, B.H.; Deng, J.J.; Luo, J.; Yuan, W.Z. High temperature failure modes of In2O3 thin films and improved thermal stability using Al2O3/ZrO2 protective layers. Ceram. Int. 2021, 47, 28411–28418. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, S.W.; Zhao, X.H.; Jiang, H.C.; Zhang, W.L. YSZ/Al2O3 multilayered film as insulating layer for high temperature thin film strain gauge prepared on Ni-based superalloy. Sens. Actuator A Phys. 2018, 279, 272–277. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, C.C.; Li, J.; Ding, G.F.; Duan, L. Fabrication and characterization of ITO thin film resistance temperature detector. Vacuum 2017, 140, 121–125. [Google Scholar] [CrossRef]
- Schmid, P.; Triendl, F.; Zarfl, C.; Schwarz, S.; Artner, W.; Schneider, M.; Schmid, U. Electro-mechanical properties of multilayered aluminum nitride and platinum thin films at high temperatures. Sens. Actuator A-Phys. 2019, 293, 128–135. [Google Scholar] [CrossRef]
- Wu, C.; Pan, X.C.; Lin, F.; Cui, Z.F.; He, Y.P.; Chen, G.C.; Zeng, Y.J.; Liu, X.L.; Chen, Q.N.; Sun, D.H.; et al. TiB2/SiCN Thin-Film Strain Gauges Fabricated by Direct Writing for High-Temperature Application. IEEE Sens. J. 2022, 22, 11517–11525. [Google Scholar] [CrossRef]
- Liu, H.; Mao, X.L.; Cui, J.T.; Jiang, S.W.; Zhang, W.L. Influence of a heterolayered Al2O3-ZrO2/Al2O3 ceramic protective overcoat on the high temperature performance of PdCr thin film strain gauges. Ceram. Int. 2019, 45, 16489–16495. [Google Scholar] [CrossRef]
- Wu, C.; Lin, F.; Pan, X.C.; Cui, Z.F.; He, Y.P.; Chen, G.C.; Liu, X.L.; He, G.H.; Chen, Q.N.; Sun, D.H.; et al. TiB2-Modified Polymer-Derived Ceramic SiCN Double-Layer Thin Films Fabricated by Direct Writing for High-Temperature Application. Adv. Eng. Mater. 2022, 2200228. [Google Scholar] [CrossRef]
- Zhao, X.H.; Yan, S.; Wang, H.M.; Jiang, H.C.; Zhang, W.L. Fabrication and microstructure evolution of Al2O3/ZrBN-SiCN/Al2O3 high-temperature oxidation-resistant composite coating. Ceram. Int. 2019, 45, 247–251. [Google Scholar] [CrossRef]
- Fu, X.L.; Lin, Q.Y.; Peng, Y.Q.; Liu, J.H.; Yang, X.F.; Zhu, B.P.; Ouyang, J.; Zhang, Y.; Xu, L.C.; Chen, S. High-Temperature Heat Flux Sensor Based on Tungsten-Rhenium Thin-Film Thermocouple. IEEE Sens. J. 2020, 20, 10444–10452. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Menzel, S.B.; Oswald, S.; Gemming, T. Improving the oxidation resistance of RuAl thin films with Al2O3 or SiO2 cover layers. J. Alloy. Compd. 2019, 776, 819–825. [Google Scholar] [CrossRef]
- Bakhit, B.; Palisaitis, J.; Thornberg, J.; Rosen, J.; Persson, P.O.A.; Hultman, L.; Petrov, I.; Greene, J.E.; Greczynski, G. Improving the high-temperature oxidation resistance of TiB2 thin films by alloying with Al. Acta Mater. 2020, 196, 677–689. [Google Scholar] [CrossRef]
- Ni, D.W.; Cheng, Y.; Zhang, J.P.; Liu, J.X.; Zou, J.; Chen, B.W.; Wu, H.Y.; Li, H.J.; Dong, S.M.; Han, J.C.; et al. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Yang, S.Y.; Li, H.F.; Lin, X.K.; Yao, J.Y.; Yang, Z.Q.; Zhang, C.C.; Wang, H.; Ding, G.F. Effect of Al2O3/Al bilayer protective coatings on the high-temperature stability of PdCr thin film strain gages. J. Alloy. Compd. 2018, 759, 1–7. [Google Scholar] [CrossRef]
- Li, H.Q.; Wang, Q.M.; Gong, J.; Sun, C. Interfacial reactions and oxidation behavior of Al2O3 and Al2O3/Al coatings on an orthorhombic Ti2AlNb alloy. Appl. Surf. Sci. 2011, 257, 4105–4112. [Google Scholar] [CrossRef]
- Justus, T.; Goncalves, P.; Seifert, M.; Leite, M.L.; Probst, S.M.H.; Binder, C.; Motz, G.; Klein, A.N. Oxidation Resistance and Microstructure Evaluation of a Polymer Derived Ceramic (PDC) Composite Coating Applied onto Sintered Steel. Materials 2019, 12, 914. [Google Scholar] [CrossRef]
- Gunthner, M.; Schutz, A.; Glatzel, U.; Wang, K.S.; Bordia, R.K.; Greissl, O.; Krenkel, W.; Motz, G. High performance environmental barrier coatings, Part I: Passive filler loaded SiCN system for steel. J. Eur. Ceram. Soc. 2011, 31, 3003–3010. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Lu, K.; Erb, D. Polymer derived silicon oxycarbide-based coatings. Int. Mater. Rev. 2018, 63, 139–161. [Google Scholar] [CrossRef]
- Torrey, J.D.; Bordia, R.K. Phase and microstructural evolution in polymer-derived composite systems and coatings. J. Mater. Res. 2007, 22, 1959–1966. [Google Scholar] [CrossRef]
- Wang, K.S.; Gunthner, M.; Motz, G.; Bordia, R.K. High performance environmental barrier coatings, Part II: Active filler loaded SiOC system for superalloys. J. Eur. Ceram. Soc. 2011, 31, 3011–3020. [Google Scholar] [CrossRef]
- Wen, Q.B.; Yu, Z.J.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 63. [Google Scholar] [CrossRef]
- Wu, C.; Pan, X.C.; Lin, F.; Cui, Z.F.; Li, X.; Chen, G.C.; Liu, X.L.; He, Y.P.; He, G.H.; Hai, Z.Y.; et al. High-temperature electrical properties of polymer-derived ceramic SiBCN thin films fabricated by direct writing. Ceram. Int. 2022, 48, 15293–15302. [Google Scholar] [CrossRef]
- Shuttleworth, R. The Surface Tension of Solids. Proc. Phys. Soc. Sect. A 1950, 63, 444. [Google Scholar] [CrossRef]
- Lüth, H. Morphology and Structure of Surfaces, Interfaces and Thin Films. In Solid Surfaces, Interfaces and Thin Films; Lüth, H., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 65–127. [Google Scholar]
- Gao, Y.; Yao, H.M. Homogenizing interfacial shear stress via thickness gradient. J. Mech. Phys. Solids 2019, 131, 112–124. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, M.; Kim, J. Effect of Al2O3 coverage on SiC particles for electrically insulated polymer composites with high thermal conductivity. Rsc. Adv. 2014, 4, 17015–17021. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Menzel, S.B.; Gemming, T. The influence of barrier layers (SiO2, Al2O3, W) on the phase formation and stability of RuAl thin films on LGS and CTGS substrates for surface acoustic wave technology. J. Alloy. Compd. 2016, 688, 228–240. [Google Scholar] [CrossRef]
- Zhang, X.H.; Hu, P.; Han, J.C. Structure evolution of ZrB2-SiC during the oxidation in air. J. Mater. Res. 2008, 23, 1961–1972. [Google Scholar] [CrossRef]
- Guo, X.; Feng, Y.R.; Lin, X.; Liu, Y.; Gong, H.Y.; Zhang, Y.J. The dielectric and microwave absorption properties of polymer-derived SiCN ceramics. J. Eur. Ceram. Soc. 2018, 38, 1327–1333. [Google Scholar] [CrossRef]
- Liu, H.; Mao, X.L.; Yang, Z.B.; Cui, J.T.; Jiang, S.W.; Zhang, W.L. High temperature static and dynamic strain response of PdCr thin film strain gauge prepared on Ni-based superalloy. Sens. Actuator A Phys. 2019, 298, 111571. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Pan, X.; Lin, F.; Chen, G.; Xu, L.; Zeng, Y.; He, Y.; Sun, D.; Hai, Z. Al2O3-Modified Polymer-Derived Ceramic SiCN High-Temperature Anti-Oxidative Composite Coating Fabricated by Direct Writing. Polymers 2022, 14, 3281. https://doi.org/10.3390/polym14163281
Wu C, Pan X, Lin F, Chen G, Xu L, Zeng Y, He Y, Sun D, Hai Z. Al2O3-Modified Polymer-Derived Ceramic SiCN High-Temperature Anti-Oxidative Composite Coating Fabricated by Direct Writing. Polymers. 2022; 14(16):3281. https://doi.org/10.3390/polym14163281
Chicago/Turabian StyleWu, Chao, Xiaochuan Pan, Fan Lin, Guochun Chen, Lida Xu, Yingjun Zeng, Yingping He, Daoheng Sun, and Zhenyin Hai. 2022. "Al2O3-Modified Polymer-Derived Ceramic SiCN High-Temperature Anti-Oxidative Composite Coating Fabricated by Direct Writing" Polymers 14, no. 16: 3281. https://doi.org/10.3390/polym14163281





