Preparation and Tribological Properties of Bismaleimide Matrix Composites Reinforced with Covalent Organic Framework Coated Graphene Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene Oxide
2.3. Preparation of G/COFs
2.4. Preparation of G/COFs/BMI
2.5. Characterization
3. Results and Discussion
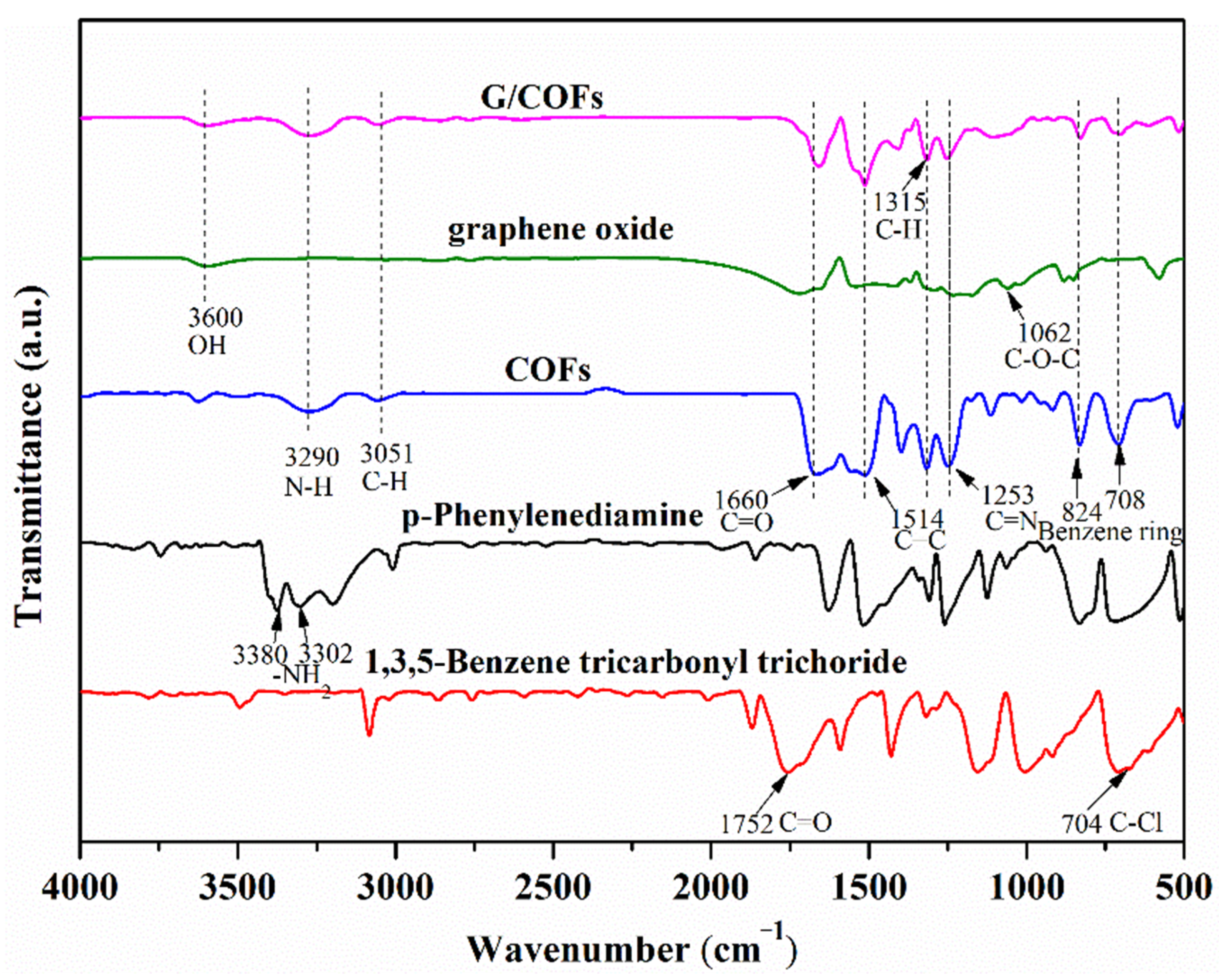
3.1. Structural Analysis of G/COFs
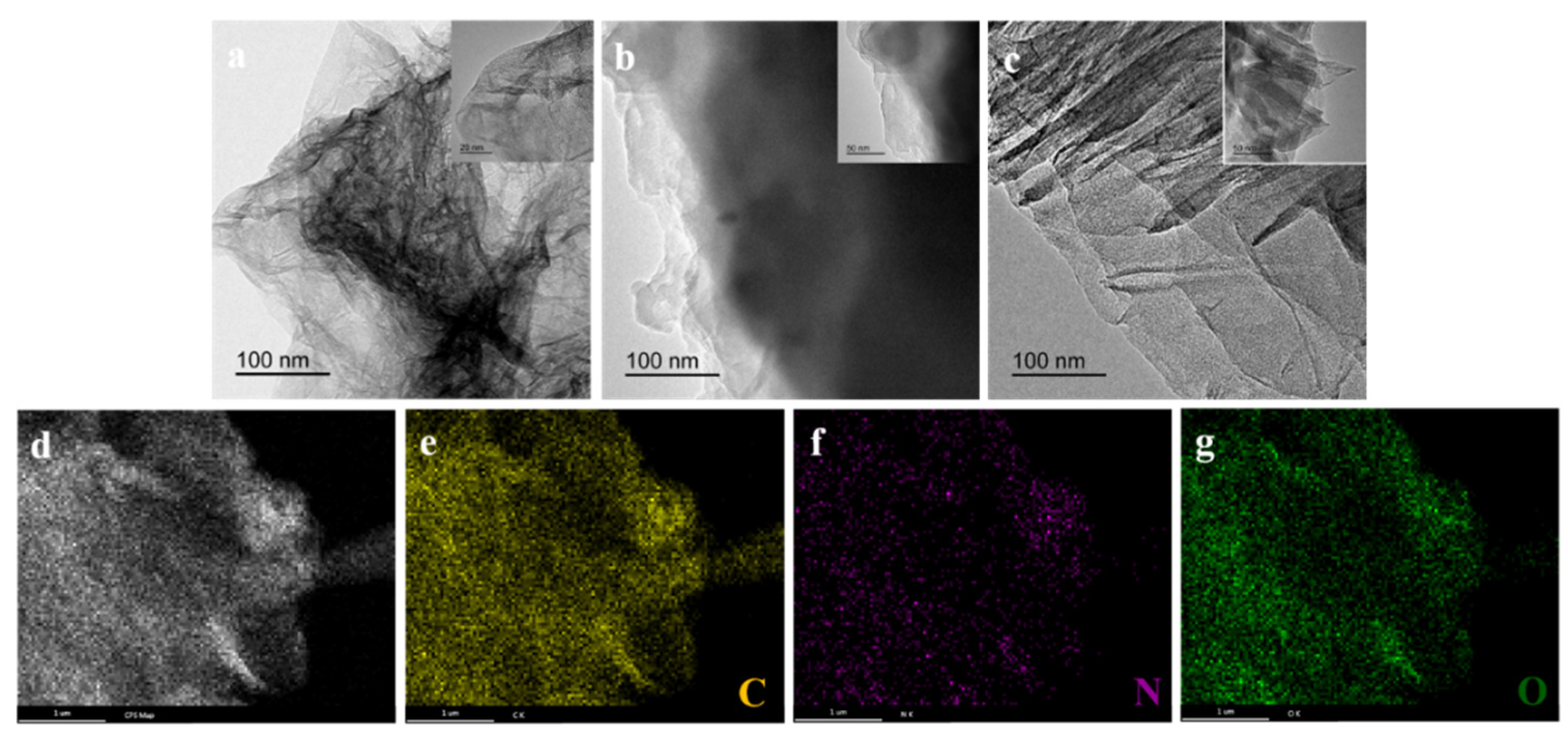
3.2. Microstructure of G/COFs Hybrid
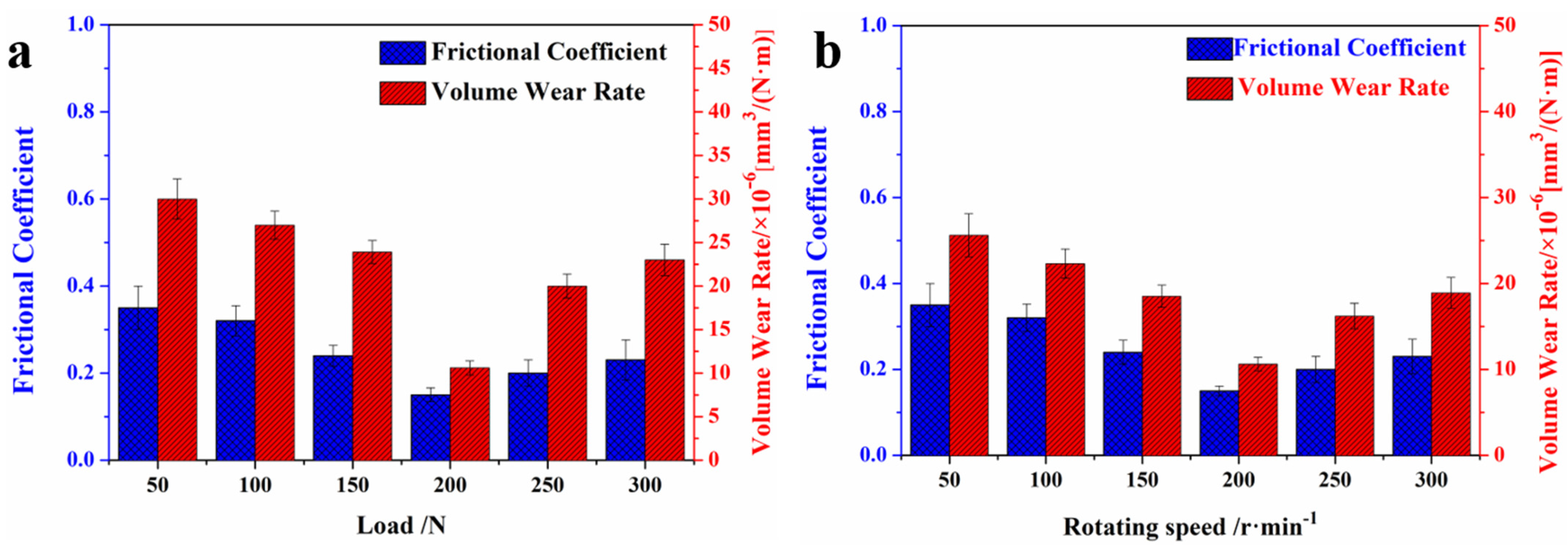
3.3. Tribological Properties of G/COFs/BMI Composites
3.4. Friction and Wear Mechanism Analysis
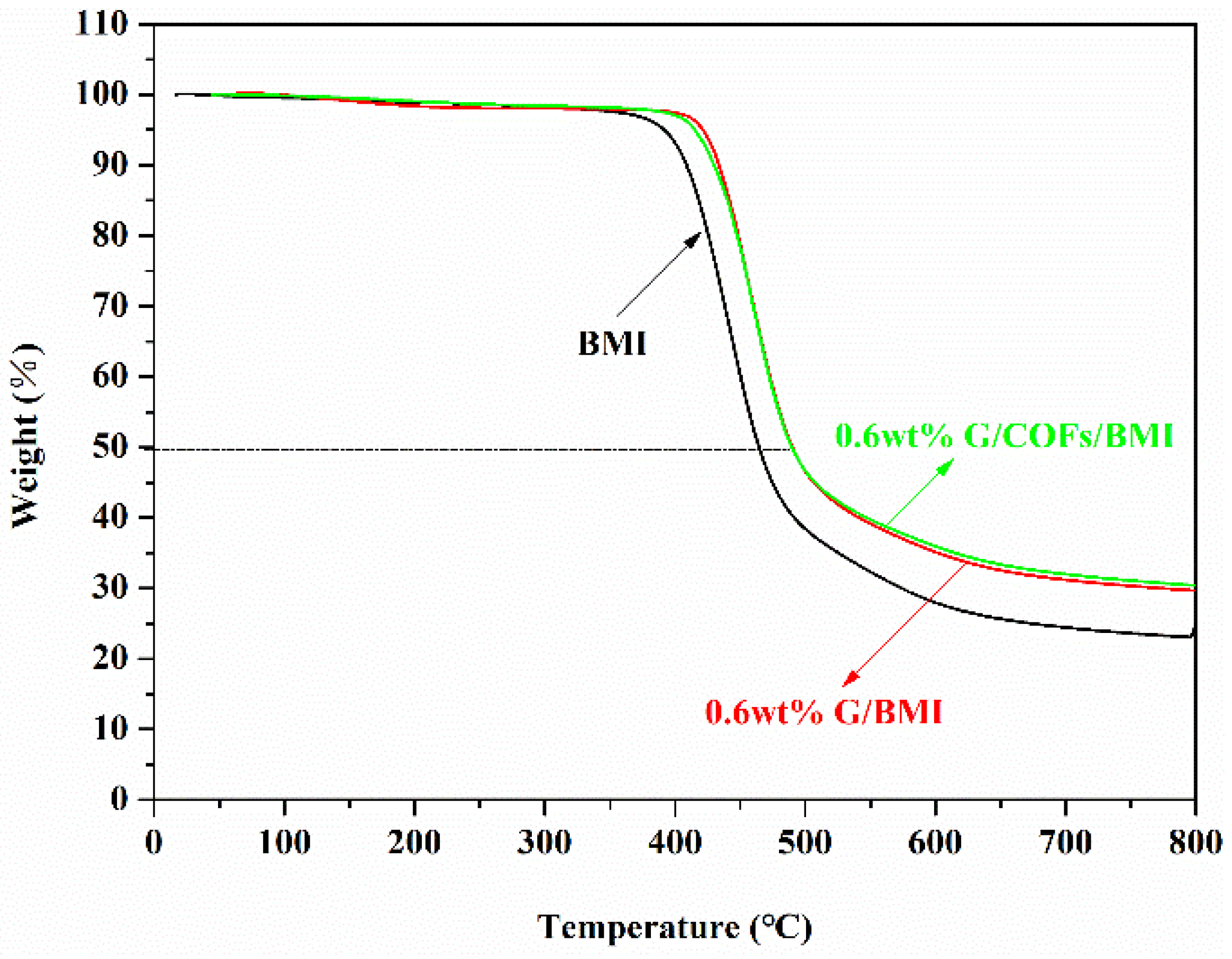
3.5. Thermal Stability of G/COFs/BMI Composites
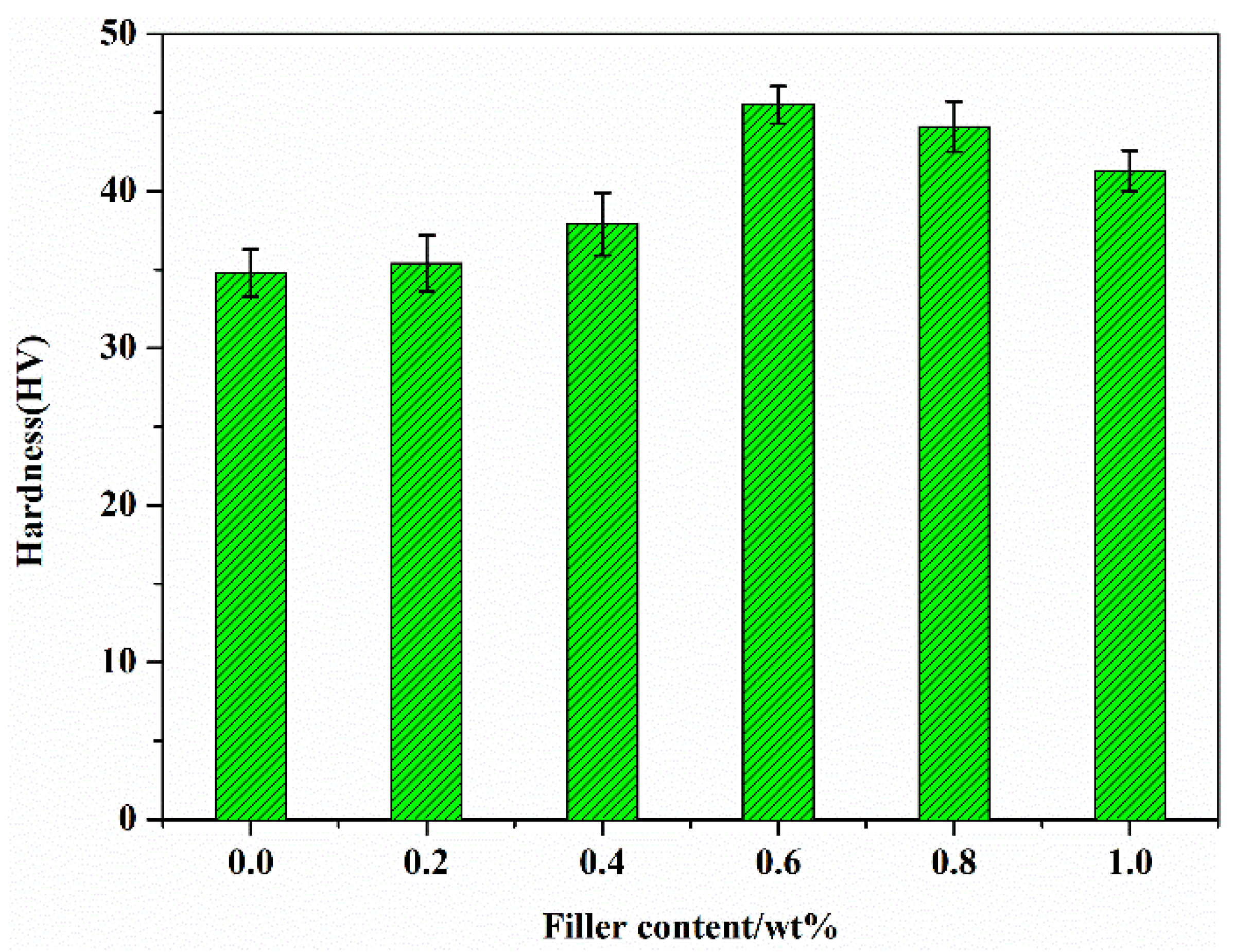
3.6. Hardness Performance of G/COFs/BMI Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Qiu, S.; Chu, F.K.; Xu, Z.M.; Hu, Y. An integrated intumescent flame retardant of bismaleimide from novel maleimide-functionalized triazine-rich polyphosphazene microspheres. Chem. Eng. J. 2022, 45, 138083. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhou, Y.J.; Cai, L.F.; Xuan, L.X.; Wu, X.; Ma, X.Y. Synthesis of eugenol-functionalized polyhedral oligomer silsesquioxane for low-k bismaleimide resin combined with excellent mechanical and thermal properties as well as its composite reinforced by silicon fiber. Chem. Eng. J. 2022, 439, 135740. [Google Scholar] [CrossRef]
- Jopen, M.; Degen, P.; Henzler, S.; Grabe, B.; Hiller, W.; Weberskirch, R. Polyurea thickened lubricating grease—The effect of degree of polymerization on rheological and tribological properties. Polymers 2022, 14, 795. [Google Scholar] [CrossRef]
- Panin, S.V.; Alexenko, V.O.; Buslovich, D.G. High performance polymer composites: A role of transfer films in ensuring tribological properties—A review. Polymers 2022, 14, 975. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Klausen, L.H.; Li, Q.; Dong, M.D. Friction behaviors of two-dimensional materials at the nanoscale. Mater. Today Phys. 2022, 27, 100771. [Google Scholar] [CrossRef]
- Li, J.F.; Li, J.J.; Yi, S.; Wang, K.Q. Boundary slip of oil molecules at MoS2 homojunctions governing superlubricity. ACS Appl. Mater. Interfaces 2022, 14, 8644–8653. [Google Scholar] [CrossRef]
- Serles, P.; Hamidinejad, M.; Demingos, P.G.; Ma, L.; Barri, N.; Taylor, H.; Singh, C.V.; Park, C.B.; Filleter, T. Friction of Ti3C2Tx MXenes. Nano Lett. 2022, 22, 3356–3363. [Google Scholar] [CrossRef]
- Guo, Y.B.; Zhou, X.L.; Lee, K.J.; Yoon, H.C.; Xu, Q.; Wang, D.G. Recent development in friction of 2D materials: From mechanisms to applications. Nanotechnology 2021, 32, 312002. [Google Scholar] [CrossRef]
- Roy, A.; Mu, L.W.; Shi, Y.J. Tribological properties of polyimide-graphene composite coatings at elevated temperatures. Prog. Org. Coat. 2020, 142, 105602. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y.C. Nano-tribological behavior of graphene nanoplatelet–reinforced magnesium matrix nanocomposites. J. Magnes. Alloys 2021, 9, 895–909. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, D.; Ye, W.; Li, S.; Huang, Z.B.; Ma, B.; Wang, H.F. Mechanical and tribological behaviors of metallic glass/graphene film with a laminated structure. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106851. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, J.H.; Zhao, Y.F. Improving tribological performance of fluoroether rubber composites by ionic liquid modified graphene. Compos. Sci. Technol. 2019, 170, 109–115. [Google Scholar] [CrossRef]
- Río, J.M.L.; López, E.R.; García, F.; Fernández, J. Tribological synergies among chemical-modified graphene oxide nanomaterials and a phosphonium ionic liquid as additives of a biolubricant. J. Mol. Liq. 2021, 336, 116885. [Google Scholar]
- Li, X.P.; Gan, C.L.; Han, Z.Y.; Yan, H.; Chen, D.L.; Li, W.; Li, H.; Fan, X.Q.; Li, D.S.; Zhu, M.H. High dispersivity and excellent tribological performance of titanate coupling agent modified graphene oxide in hydraulic oil. Carbon 2020, 165, 238–250. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y.F.; Lin, Y.; Yan, H.X.; Zhang, W.B.; Yan, B.; Ma, J.Z. Enhanced mechanical and tribological properties of gra-phene/bismaleimide composites by using reduced graphene oxide with non-covalent functionalization. Compos. Part B Eng. 2019, 165, 491–499. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Lin, Y.; Xue, X.; Yuan, Q.Y.; Zhang, W.B.; Bao, Y.; Ma, J.Z. Tribological properties of bismaleimide-based self-lubricating composite enhanced by MoS2 quantum dots/graphene hybrid. Compos. Commun. 2021, 28, 100922. [Google Scholar] [CrossRef]
- Nobile, M.R.; Raimondo, M.; Naddeo, C.; Guadagno, L. Rheological and morphological properties of non-covalently function-alized graphene-based structural epoxy resins with intrinsic electrical conductivity and thermal stability. Nanomaterials 2020, 10, 1310. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Xu, G.Y.; Barnes, M.; Li, Y.L.; Tseng, C.P.; Zhang, Z.Q.; Zhang, J.J.; Zhu, Y.F.; Khalil, S.; Rahman, M.M.; et al. Covalent organic frameworks for batteries. Adv. Funct. Mater. 2021, 31, 2100505. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, l1585. [Google Scholar] [CrossRef]
- Song, Y.P.; Sun, Q.; Aguila, B.; Ma, S.Q. Covalent organic frameworks: Opportunities of covalent organic frameworks for ad-vanced applications. Adv. Sci. 2019, 6, 1970011. [Google Scholar] [CrossRef]
- Wen, P.; Zhang, C.Y.; Yang, Z.G.; Dong, R.; Wang, D.M.; Fan, M.J.; Wang, J.Q. Triazine-based covalent-organic frameworks: A novel lubricant additive with excellent tribological performances. Tribol. Int. 2017, 111, 57–65. [Google Scholar] [CrossRef]
- Wen, P.; Lei, Y.Z.; Li, W.Q.; Fan, M.J. Synergy between covalent organic frameworks and surfactants to promote water-based lubrication and corrosion resistance. ACS Appl. Nano Mater. 2020, 3, 1400–1411. [Google Scholar] [CrossRef]
- Wen, P.; Lei, Y.Z.; Yan, Q.Q.; Han, Y.Y.; Fan, M.J. Multilayer tribofilm: An unique structure to strengthen interface tribological behaviors. ACS Appl. Mater. Interfaces 2021, 13, 11524–11534. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ji, W.Y.; Ding, X.S.; Han, B.H. Exfoliated covalent organic framework nanosheets. J. Mater. Chem. A 2021, 9, 7336–7365. [Google Scholar] [CrossRef]
- Wen, P.; Lei, Y.Z.; Li, W.Q.; Fan, M.J. Two-dimension layered nanomaterial as lubricant additives: Covalent organic frameworks beyond oxide graphene and reduced oxide graphene. Tribol. Int. 2020, 143, 106051. [Google Scholar] [CrossRef]
- Zhang, T.T.; Liu, S.; Zhang, X.Z.; Gao, J.D.; Yu, H.; Ye, Q.; Liu, S.J.; Liu, W.M. Fabrication of two-dimensional functional covalent organic frameworksviathe thiol-ene “click “reaction as lubricant additives for antiwear and friction reduction. ACS Appl. Mater. Interfaces 2021, 13, 36213–36220. [Google Scholar] [CrossRef]
- Shinde, D.B.; Aiyappa, H.B.; Bhadra, M.; Biswal, B.P.; Wadge, P.; Kandambeth, S.; Garai, B.; Kundu, T.; Kurungot, S.; Banerjee, R. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 2016, 4, 2682–2690. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.Y.; Zhang, C.Y.; Yu, M.Y.; Ren, J.F.; Wang, W.; Chen, S.G. Exploring the corrosion resistance of epoxy coated steel by integrating mechanochemical synthesized 2D covalent organic framework. Prog. Org. Coat. 2021, 157, 106299. [Google Scholar] [CrossRef]
- Wang, X.L.; Ma, R.Y.; Hao, L.; Wu, Q.H.; Wang, C.; Wang, Z. Mechanochemical synthesis of covalent organic framework for the efficient extraction of benzoylurea insecticides. J. Chromatog. A 2018, 1551, 1–9. [Google Scholar] [CrossRef]
- Liang, L.; Chen, J.; Chen, X.W.; Wang, J.H.; Qiu, H.D. In-situ synthesis of GO/COFs composite to enhance the adsorption per-formance of organic pollutants in water. Environ. Sci. Nano 2022, 9, 554–567. [Google Scholar] [CrossRef]
- Wang, Q.M.; Wang, D.N.; Liao, Q.B.; Ke, C.; Zhang, Y.Y.; Han, Q.W.; Zhang, Y.F.; Jiang, D.C.; Xi, K. Preparation of functional material layers of TT-COFs with built-in formyl groups for efficient dyes removal. J. Colloid Interf. Sci. 2022, 612, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.K.; Wang, Y.L.; Wang, J.; Men, X.H.; Zhang, Z.Z.; Singh, V. Synergistic effects of COF and GO on high flux oil/water separation performance of superhydrophobic composites. Sep. Purif. Technol. 2021, 276, 119268. [Google Scholar] [CrossRef]
- Yao, M.Y.; Guo, C.F.; Geng, Q.H.; Zhang, Y.F.; Zhao, X.; Xin Zhao, X.; Wan, Y. Construction of anthraquinone-containing co-valent organic frameworks/graphene hybrid films for a flexible high-performance microsupercapacitor. Ind. Eng. Chem. Res. 2022, 61, 7480–7488. [Google Scholar] [CrossRef]
- Liu, C.; Yin, Q.; Zhang, W.B.; Bao, Y.; Li, P.P.; Hao, L.F. Tribological properties of graphene-modified with ionic liquids and carbon quantum dots/bismaleimide composites. Carbon 2021, 183, 504–514. [Google Scholar] [CrossRef]
- Wu, S.; Ding, N.; Jiang, P.W.; Wu, L.; Feng, Q.L.; Zhao, L.B.; Wang, Y.B.; Su, Q.; Zhang, H.; Yang, Q.L. A two-dimensional am-ide-linked covalent organic framework anchored Pd catalyst for Suzuki-Miyaura coupling reaction in the aqueous phase at room temperature. Tetrahedron Lett. 2020, 61, 152656. [Google Scholar] [CrossRef]
- Yin, C.C.; Fang, S.Y.; Shi, X.S.; Zhang, Z.; Wang, Y. Pressure-modulated synthesis of self-repairing covalent organic frameworks (COFs) for high-flux nanofiltration. J. Membr. Sci. 2021, 618, 118727. [Google Scholar] [CrossRef]
- Bychko, I.; Abakumov, A.; Didenko, O.; Chen, M.Y.; Tang, J.G.; Strizhak, P. Differences in the structure and functionalities of graphene oxide and reduced graphene oxide obtained from graphite with various degrees of graphitization. J. Phys. Chem. Solids 2022, 164, 110614. [Google Scholar] [CrossRef]
- Garcia, J.L.; Miyao, T.; Inukai, J.; Tongol, B.J.V. Graphitic carbon nitride on reduced graphene oxide prepared via semi-closed pyrolysis as electrocatalyst for oxygen reduction reaction. Mater. Chem. Phys. 2022, 288, 126415. [Google Scholar] [CrossRef]
- Liu, Y.; Shin, D.G.; Xu, S.; Kim, C.L.; Kim, D.E. Understanding of the lubrication mechanism of reduced graphene oxide coating via dual in-situ monitoring of the chemical and topographic structural evolution. Carbon 2021, 173, 941–952. [Google Scholar] [CrossRef]
- Chouhan, A.; Kumari, S.; Sarkar, T.K.; Rawat, S.S.; Khatri, O.P. Graphene-based aqueous lubricants: Dispersion stability to the enhancement of tribological properties. ACS Appl. Mater. Interfaces 2020, 12, 51785–51796. [Google Scholar] [CrossRef]
- Li, L.H.; Yang, L.; Zou, R.; Lan, J.W.; Shang, J.J.; Dou, B.J.; Liu, H.Y.; Lin, S.J. Facile and scalable preparation of ZIF-67 decorated cotton fibers as recoverable and efficient adsorbents for removal of malachite green. J. Leather Sci. Eng. 2021, 33, 28. [Google Scholar] [CrossRef]
- Lv, H.Z.; Zhao, X.L.; Niu, H.Y.; He, S.J.; Tang, Z.; Wu, F.C.; Giesy, J.P. Ball milling synthesis of covalent organic framework as a highly active photocatalyst for degradation of organic contaminants. J. Hazard. Mater. 2019, 369, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Weare, B.L.; Lodge, R.W.; Zyk, N.; Weilhard, A.; Housley, C.L.; Strutyński, K.; Melle-Franco, M.; Mateo-Alonso, A.; Khlobystov, A.N. Imaging and analysis of covalent organic framework crystallites on a carbon surface: A nanocrystalline scaly COF/nanotube hybrid. Nanoscale 2021, 13, 6834–6845. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Sharma, S.; Mukherjee, B.; Singh, P.; Islam, A.; Keshri, A.K. Microstructural, mechanical and marine water tribological properties of plasma-sprayed graphene nanoplatelets reinforced Al2O3-40 wt% TiO2` coating. J. Eur. Ceram. Soc. 2022, 42, 2892–2904. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.B.; Zhang, X.Y.; Chen, X.; Zhang, J.F.; Jing, L.; Wu, Y.X.; Yu, S.W. Tribological properties and self-lubrication mechanism of in-situ grown graphene reinforced nickel matrix composites in ambient air. Wear 2022, 496, 204308. [Google Scholar] [CrossRef]
- Lee, P.C.; Kim, S.Y.; Ko, Y.K.; Ha, J.U.; Jeoung, S.K.; Shin, D.; Kim, J.H.; Kim, M. Tribological properties of polyamide 46/graphene nanocomposites. Polymers 2022, 14, 1139. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of nanofillers on tribological properties of polymer nanocomposites: A review on recent development. Polymers 2021, 13, 2867. [Google Scholar]
- Zhang, J.; Gao, X.; Xu, Q.; Ma, T.B.; Hu, Y.Z.; Luo, J.B. Atomistic insights into friction and wear mechanisms of graphene oxide. Appl. Surf. Sci. 2021, 546, 149130. [Google Scholar] [CrossRef]
- Guo, L.L.; Yan, H.X.; Chen, Z.Y.; Lv, Q.; Bai, T.; Zhang, Y.B. Graphene oxide grafted by hyperbranched polysiloxane to enhance mechanical and frictional properties of epoxy resin. SN Appl. Sci. 2020, 2, 473. [Google Scholar] [CrossRef]
- Chen, X.B.; Lu, S.L.; Sun, C.F.; Song, Z.B.; Jian Kang, J.; Ya Cao, Y. Exploring impacts of hyper-branched polyester surface modification of graphene oxide on the mechanical performances of acrylonitrile-butadiene-styrene. Polymers 2021, 13, 2614. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Du, Y.F.; Zhu, C.H.; Guo, L.C.; Lu, Y.; Yu, J.H.; Parkin, I.P.; Zhao, J.H.; Guo, D.M. Unprecedented enhancement of wear resistance for epoxy-resin graphene composites. Nanoscale 2021, 13, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- Eayal Awwad, K.Y.; Yousif, B.F.; Fallahnezhad, K.; Saleh, K.; Zeng, X.S. Influence of graphene nanoplatelets on mechanical properties and adhesive wear performance of epoxy-based composite. Friction 2020, 9, 856–875. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.T.; Zhang, J.L.; Zhang, Y.L.; Gu, J.W. Lightweight and robust rGO/sugarcane derived hybrid carbon foams with outstanding EMI shielding performance. J. Mater. Sci. Technol. 2020, 52, 119–126. [Google Scholar] [CrossRef]
- Yehia, H.M.; Abu-Oqail, A.; Elmaghraby, M.A.; Elkady, O.A. Microstructure, hardness, and tribology properties of the (Cu/MoS2)/graphene nanocomposite via the electroless deposition and powder metallurgy technique. J. Compos. Mater. 2020, 54, 3435–3446. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yan, H.X.; Xu, P.L.; Guo, L.L.; Yang, K.M.; Liu, R.; Feng, W.X. A novel POSS-containing polyimide: Synthesis and its composite coating with graphene-like MoS2 for outstanding tribological performance. Prog. Org. Coat. 2021, 151, 106013. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Xue, X.; Yuan, Q.; Lin, Y.; Bao, Y.; He, Y.; Zhang, W. Preparation and Tribological Properties of Bismaleimide Matrix Composites Reinforced with Covalent Organic Framework Coated Graphene Nanosheets. Polymers 2022, 14, 3289. https://doi.org/10.3390/polym14163289
Liu C, Xue X, Yuan Q, Lin Y, Bao Y, He Y, Zhang W. Preparation and Tribological Properties of Bismaleimide Matrix Composites Reinforced with Covalent Organic Framework Coated Graphene Nanosheets. Polymers. 2022; 14(16):3289. https://doi.org/10.3390/polym14163289
Chicago/Turabian StyleLiu, Chao, Xin Xue, Qiming Yuan, Yang Lin, Yan Bao, Yinkun He, and Wenbo Zhang. 2022. "Preparation and Tribological Properties of Bismaleimide Matrix Composites Reinforced with Covalent Organic Framework Coated Graphene Nanosheets" Polymers 14, no. 16: 3289. https://doi.org/10.3390/polym14163289





