Polymer Composite-Based Materials with Photocatalytic Applications in Wastewater Organic Pollutant Removal: A Mini Review
Abstract
:1. Introduction
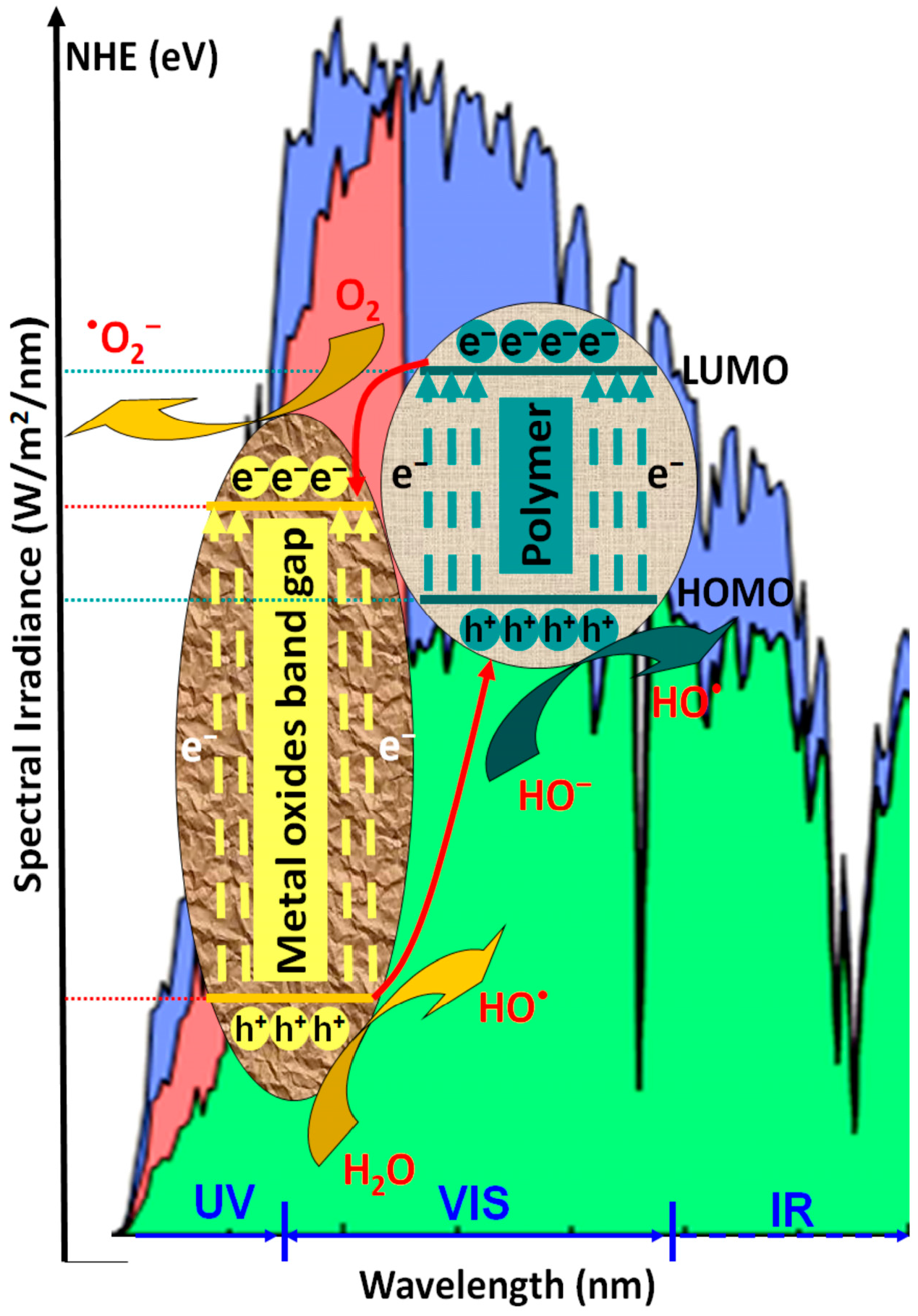
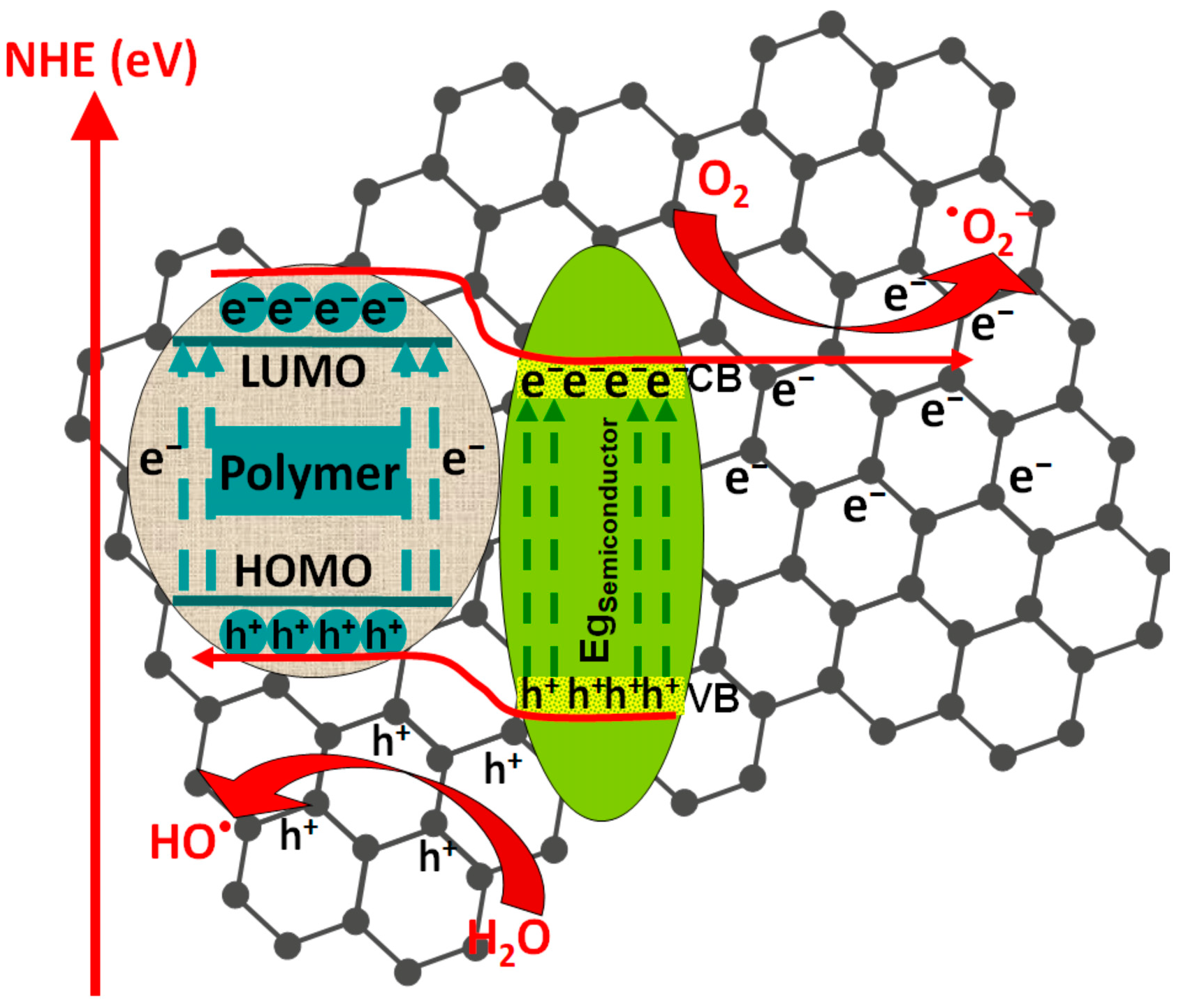
2. Photocatalytic Mechanism of Polymeric Composites
3. Polymer Composite Photocatalytic Materials
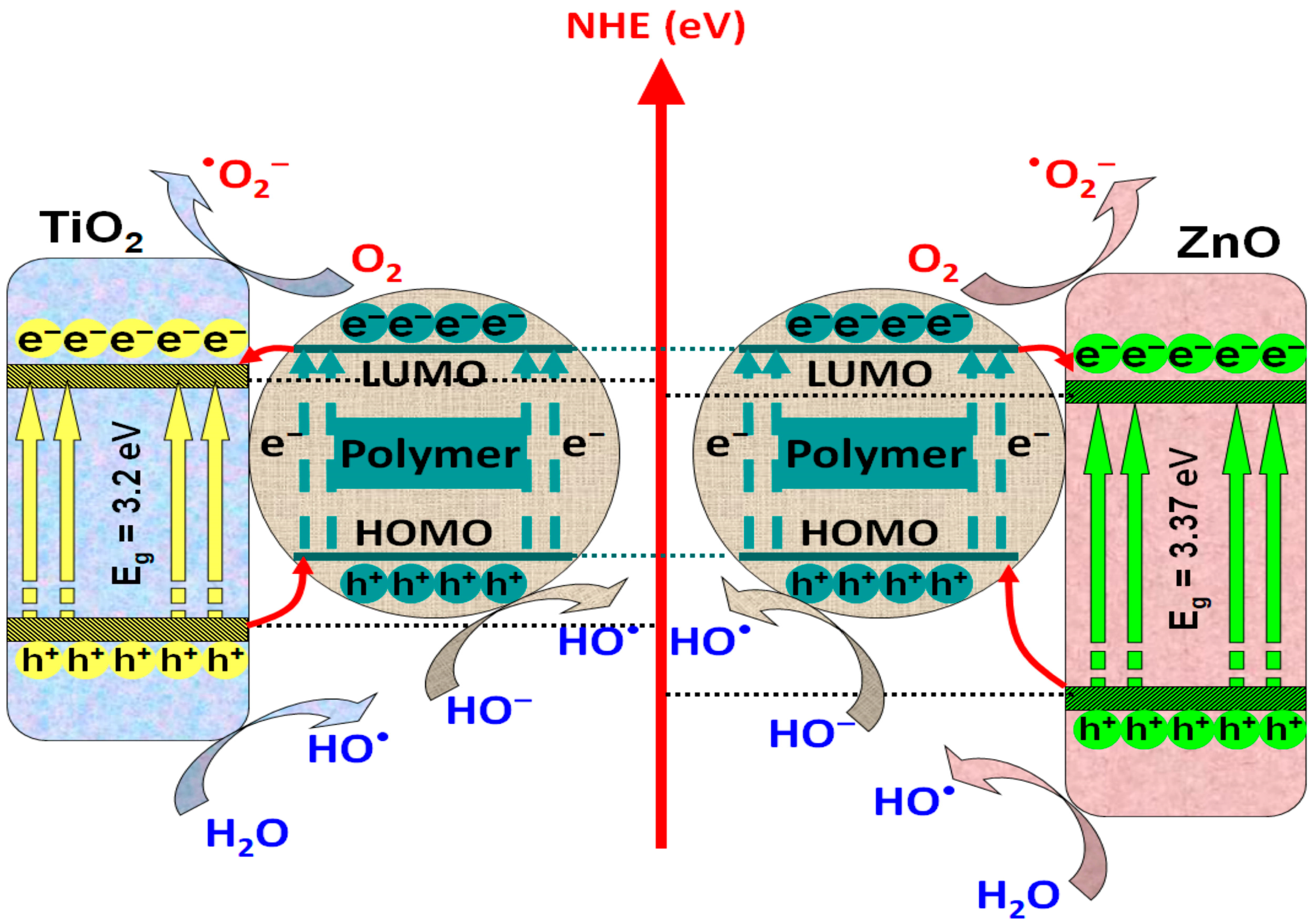
3.1. Polymer/TiO2- or ZnO-Based Composites
3.2. Other Polymer Composites Based Materials
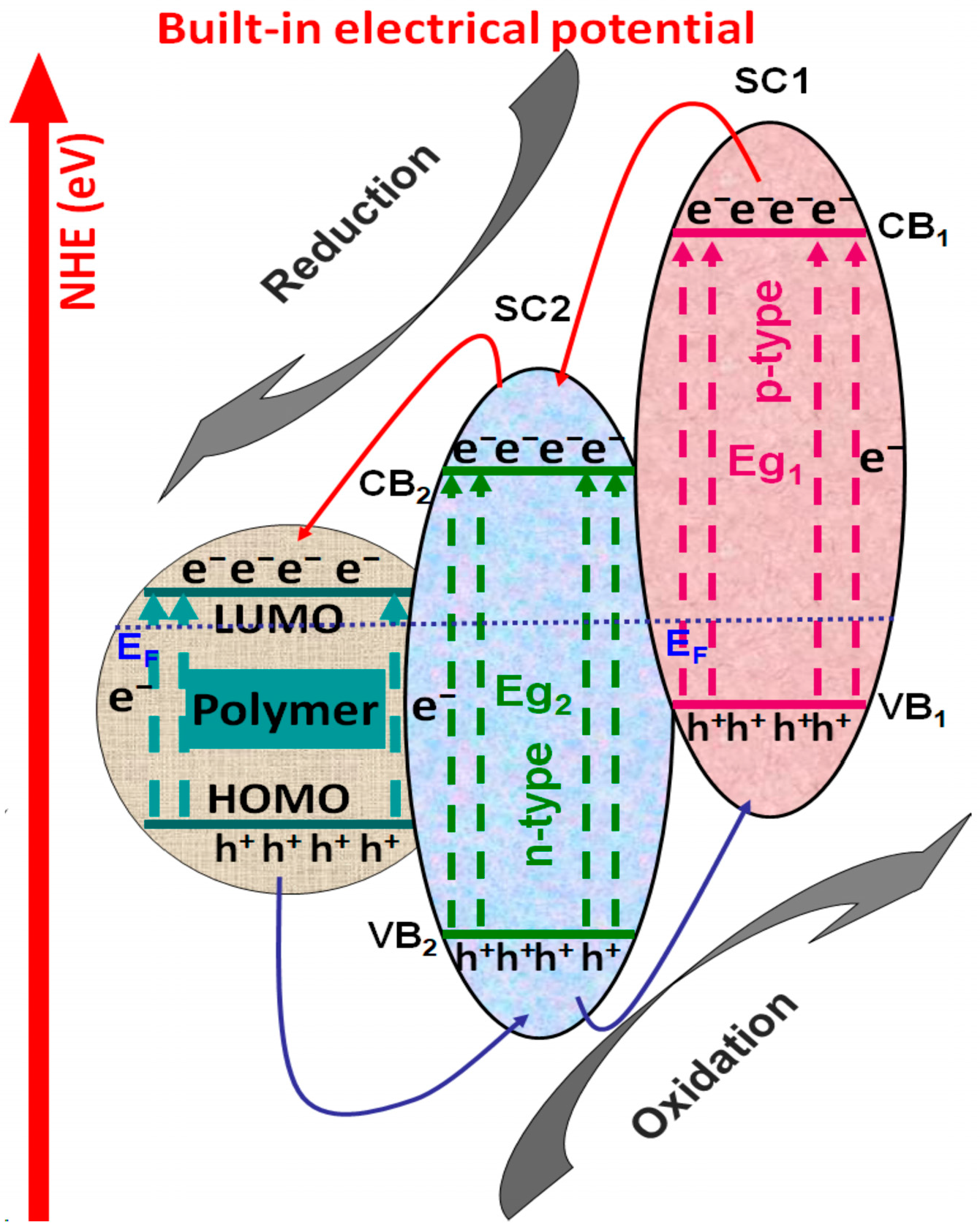
3.2.1. Polymer/Tandem Structure-Based Materials
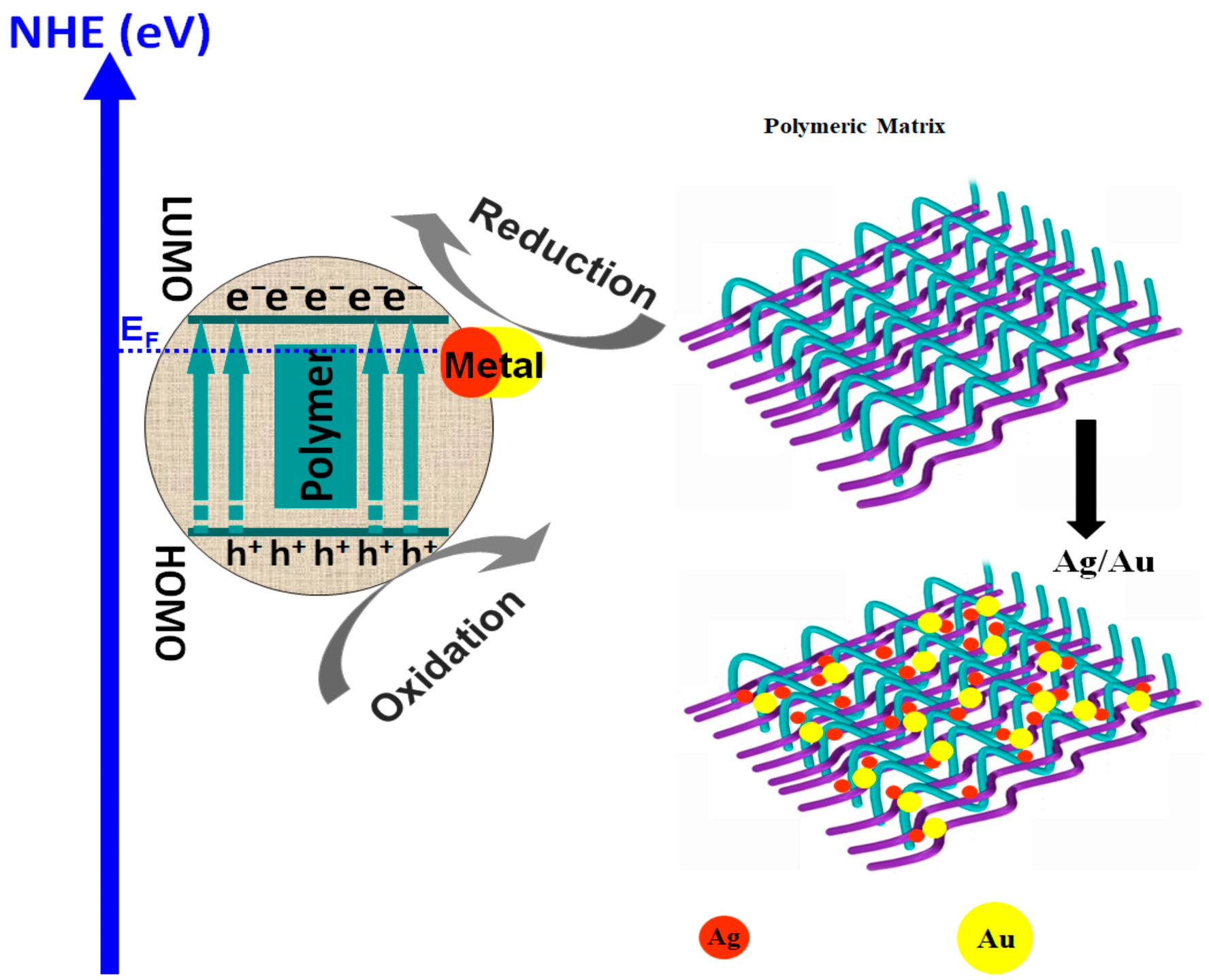
3.2.2. Polymer/Metal-Based Materials
3.2.3. Other Examples of Polymer Composite-Based Materials
4. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajlaxmi; Gupta, N.; Behere, R.P.; Layek, R.K.; Kuila, B.K. Polymer na–nocomposite membranes and their application for flow catalysis and photocatalytic degradation of organic pollutants. Mater. Today Chem. 2021, 22, 100600. [Google Scholar] [CrossRef]
- Zia, J.; Riaz, U. Photocatalytic degradation of water pollutants using conducting polymer-based nanohybrids: A review on recent trends and future prospects. J. Mol. Liq. 2021, 340, 117162. [Google Scholar] [CrossRef]
- Pandey, B.; Singh, P.; Kumar, V. Photocatalytic-sorption processes for the removal of pollutants from wastewater using polymer metal oxide nanocomposites and associated environmental risks. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100596. [Google Scholar] [CrossRef]
- Ahmad, N.; Anae, J.; Khan, M.Z.; Sabir, S.; Yang, X.J.; Thakur, V.K.; Campo, P.; Coulon, F. Visible light-conducting polymer nanocomposites as efficient photocatalysts for the treatment of organic pollutants in wastewater. J. Environ. Manag. 2021, 295, 113362. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhu, X.; Yao, D. The oil pollution and nitric oxide photocatalytic degradation evaluation of composite nanomaterials for asphalt pavement. Constr. Build. Mater. 2022, 314, 125497. [Google Scholar] [CrossRef]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Goh, P.S. Advances of nanomaterials for air pollution remediation and their impacts on the environment. Chemosphere 2022, 287, 132083. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Zhang, L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019, 662, 414–421. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Santosh, S.S.; Wang, M.H. Unraveling the hazardous impact of diverse contaminants in the marine environment: Detection and remedial approach through nanomaterials and nano-biosensors. J. Hazard. Mater. 2022, 433, 128720. [Google Scholar] [CrossRef]
- Goh, P.S.; Kang, H.S.; Higgins, D. Nanomaterials for microplastic remediation from aquatic environment: Why nano matters? Chemosphere 2022, 299, 134418. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Wang, X. MXenes as emerging nanomaterials in water purification and environmental remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef]
- Ochiai, T.; Aoki, D.; Saito, H.; Akutsu, Y.; Nagata, M. Analysis of Adsorption and Decomposition of Odour and Tar Components in Tobacco Smoke on Non-Woven Fabric-Supported Photocatalysts. Catalysts 2020, 10, 304. [Google Scholar] [CrossRef]
- Enesca, A.; Duta, A.; Schoonman, J. Influence of tantalum dopant ions (Ta5+) on the efficiency of the tungsten trioxide photoelectrode. Phys. Status Solidi A 2008, 205, 2038–2041. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Ambrožová, N.; Kočí, K. Photocatalytic Reduction of Carbon Dioxide on TiO2 Heterojunction Photocatalysts—A Review. Materials 2022, 15, 967. [Google Scholar] [CrossRef]
- Sciscenko, I.; Mestre, S.; Climent, J.; Valero, F.; Escudero-Oñate, C.; Oller, I.; Arques, A. Magnetic Photocatalyst for Wastewater Tertiary Treatment at Pilot Plant Scale: Disinfection and Enrofloxacin Abatement. Water 2021, 13, 329. [Google Scholar] [CrossRef]
- Shafiqul, I.M.; Deep, R.; Lin, J.; Yoshida, T.; Fujita, Y. The Role of Nitrogen Dopants in ZnO Nanoparticle-Based Light Emitting Diodes. Nanomaterials 2022, 12, 358. [Google Scholar] [CrossRef]
- Alsharif, A.; Smith, N.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydroisomerisation of α-Pinene and Limonene to p-Cymene over Silica-Supported ZnO in the Gas Phase. Catalysts 2021, 11, 1245. [Google Scholar] [CrossRef]
- Huang, P.-H.; Zhang, Z.-X.; Hsu, C.-H.; Wu, W.-Y.; Huang, C.-J.; Lien, S.-Y. Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition. Materials 2021, 14, 690. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Platonov, V.; Garshev, A.; Rumyantseva, M. Low Temperature HCHO Detection by SnO2/TiO2@Au and SnO2/TiO2@Pt: Understanding by In-Situ DRIFT Spectroscopy. Nanomaterials 2021, 11, 2049. [Google Scholar] [CrossRef]
- Bridges, L.; Mohamed, R.A.M.; Khan, N.A.; Brusseau, M.L.; Carroll, K.C. Comparison of Manganese Dioxide and Permanganate as Amendments with Persulfate for Aqueous 1,4-Dioxane Oxidation. Water 2020, 12, 3061. [Google Scholar] [CrossRef]
- Dong, Y.; Su, C.; Liu, K.; Wang, H.; Zheng, Z.; Zhao, W.; Lu, S. The Catalytic Oxidation of Formaldehyde by FeOx-MnO2-CeO2 Catalyst: Effect of Iron Modification. Catalysts 2021, 11, 555. [Google Scholar] [CrossRef]
- Hessien, M.; Taha, A.; Dana, E. Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites. Materials 2022, 15, 2291. [Google Scholar] [CrossRef]
- Cao, C.; Yu, L.; Jin, H. Hydrogen production by supercritical water gasification of lignin over CuO–ZnO catalyst synthesized with different methods. Int. J. Hydrogen Energy 2022, 47, 8716–8728. [Google Scholar] [CrossRef]
- Mahy, J.G.; Tsaffo Mbognou, M.H.; Léonard, C.; Fagel, N.; Woumfo, E.D.; Lambert, S.D. Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water. Catalysts 2022, 12, 148. [Google Scholar] [CrossRef]
- Jing, Y.; Yin, H.; Yu, S. Fabrication of Pt doped TiO2–ZnO@ZIF-8 core@shell photocatalyst with enhanced activity for phenol degradation. Environ. Res. 2022, 203, 111819. [Google Scholar] [CrossRef]
- Peng, F.; Sun, Y.; Yu, W.; Lu, Y.; Hao, J.; Cong, R.; Shi, J.; Ge, M.; Dai, N. Gas Sensing Performance and Mechanism of CuO(p)-WO3(n) Composites to H2S Gas. Nanomaterials 2020, 10, 1162. [Google Scholar] [CrossRef]
- Patil, A.M.; An, X.; Guan, G. Fabrication of three-dimensionally heterostructured rGO/WO3·0.5H2O@Cu2S electrodes for high-energy solid-state pouch-type asymmetric supercapacitor. Chem. Eng. J. 2020, 403, 126411. [Google Scholar] [CrossRef]
- Ramesh, K.; Gnanavel, B.; Shkir, M. Enhanced visible light photocatalytic degradation of bisphenol A (BPA) by reduced graphene oxide (RGO)–metal oxide (TiO2, ZnO and WO3) based nanocomposites. Diam. Relat. Mater. 2021, 118, 108514. [Google Scholar] [CrossRef]
- Quy, V.H.V.; Kim, J.H.; Ahn, K.S. Enhanced electrocatalytic activity of electrodeposited F-doped SnO2/Cu2S electrodes for quantum dot-sensitized solar cells. J. Power Sources 2016, 316, 53–59. [Google Scholar]
- Enesca, A.; Duta, A. Tailoring WO3 thin layers using spray pyrolysis technique. Phys. Status Solidi C 2008, 5, 3499–3502. [Google Scholar] [CrossRef]
- Chen, H.; Lei, Y.; Zheng, Z. Using a CdS under-layer to suppress charge carrier recombination at the Ag2S/FTO interface. J. Alloys Compd. 2021, 879, 160348. [Google Scholar] [CrossRef]
- Madjet, M.E.; Berdiyorov, G.R.; Hamoudi, H. Nonradiative relaxation of charge carriers at molecule-metal interfaces: Nonadiabatic molecular dynamics study. Surf. Interfaces 2022, 30, 101830. [Google Scholar] [CrossRef]
- Govea-Alcaide, E.; Rodríguez-Milanés, J.; Jardim, R.F. Transport of charge carriers across the normal-superconducting interfaces in Bi1.65Pb0.35Sr2Ca2Cu3O10+δ nanoceramics. Ceram. Int. 2021, 47, 13039–13099. [Google Scholar] [CrossRef]
- Ravichandran, J.; Karthikeyan, B.S.; Samal, A. Investigation of a derived adverse outcome pathway (AOP) network for endocrine-mediated perturbations. Sci. Total Environ. 2022, 826, 154112. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Arslan, M.; El-Din, H.G. Combined solar activated sulfate radical-based advanced oxidation processes (SR-AOPs) and biofiltration for the remediation of dissolved organics in oil sands produced water. Chem. Eng. J. 2022, 433, 134579. [Google Scholar] [CrossRef]
- Wang, N.; He, L.; Bu, Y. The potential environmental behavior and risks of TBECH transformation initiated by reactive oxygen species in natural waters and AOPs. Ecotoxicol. Environ. Saf. 2021, 228, 113055. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, Y.; Yin, R. Concentration-dependent chloride effect on radical distribution and micropollutant degradation in the sulfate radical-based AOPs. J. Hazard. Mater. 2022, 430, 128450. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour. Ind. 2021, 26, 100160. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Zhang, L. AOPs-based remediation of petroleum hydrocarbons-contaminated soils: Efficiency, influencing factors and environmental impacts. Chemosphere 2020, 246, 125726. [Google Scholar] [CrossRef]
- Esfahani, K.N.; Pérez-Moya, M.; Graells, M. A Hybrid Model Coupling Advanced Oxidation Processes (AOP) and Conventional Bio-processes for the Removal of Recalcitrant Contaminants in Wastewaters. Comput. Aided Chem. Eng. 2021, 50, 883–889. [Google Scholar]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Chen, C.; Feng, H.; Deng, Y. Re-evaluation of sulfate radical based–advanced oxidation processes (SR-AOPs) for treatment of raw municipal landfill leachate. Water Res. 2019, 153, 100–107. [Google Scholar] [CrossRef]
- Zgheib, E.; Gao, W.; Bois, F.Y. Application of three approaches for quantitative AOP development to renal toxicity. Comput. Toxicol. 2019, 11, 13. [Google Scholar] [CrossRef]
- Cako, E.; Gunasekaran, K.D.; Boczkaj, G. Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs). Water Resour. Ind. 2020, 24, 100134. [Google Scholar] [CrossRef]
- Miklos, D.B.; Wang, W.L.; Hübner, U. Comparison of UV-AOPs (UV/H2O2, UV/PDS and UV/Chlorine) for TOrC removal from municipal wastewater effluent and optical surrogate model evaluation. Chem. Eng. J. 2019, 362, 537–547. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Fernandes, J.R.; Lucas, M.S. Wireless UV-A LEDs-driven AOP in the treatment of agro-industrial wastewaters. Environ. Res. 2021, 200, 111430. [Google Scholar] [CrossRef]
- Cako, E.; Soltani, R.D.C.; Boczkaj, G. Desulfurization of raw naphtha cuts using hybrid systems based on acoustic cavitation and advanced oxidation processes (AOPs). Chem. Eng. J. 2022, 439, 135354. [Google Scholar] [CrossRef]
- Kolle, S.N.; Landsiedel, R.; Natsch, A. Replacing the refinement for skin sensitization testing: Considerations to the implementation of adverse outcome pathway (AOP)-based defined approaches (DA) in OECD guidelines. Regul. Toxicol. Pharmacol. 2020, 115, 104713. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Jiang, W. A two-step process coupling photocatalysis with adsorption to treat tetracycline—Copper(II) hybrid wastewaters. J. Water Proc. Eng. 2022, 47, 102710. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, X.; Luo, H. Efficient removal of Cr(VI) by a 3D Z-scheme TiO2-ZnxCd1-xS graphene aerogel via synergy of adsorption and photocatalysis under visible light. J. Environ. Sci. 2023, 124, 360–370. [Google Scholar] [CrossRef]
- Fatima, H.; Azhar, M.R.; Shao, Z. Rational design of ZnO-zeolite imidazole hybrid nanoparticles with reduced charge recombination for enhanced photocatalysis. J. Colloid Interface Sci. 2022, 614, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yang, Q. One-pot synthesis of mesosilica/nano covalent organic polymer composites and their synergistic effect in photocatalysis. Chin. J. Catal. 2021, 42, 1821–1830. [Google Scholar] [CrossRef]
- Moradeeya, P.G.; Kumar, M.A.; Basha, S. Conductive polymer layered semiconductor for degradation of triclopyr acid and 2,4-dichlorophenoxyacetic acid from aqueous stream using coalesce adsorption-photocatalysis technique. Chemosphere 2022, 298, 134360. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Yuan, H.; Luo, K.; Li, J.; Hu, W.; Pan, Z.; Xu, M.; Xu, S.; Levchenko, I.; et al. Comparative study of photocatalysis and gas sensing of ZnO/Ag nanocomposites synthesized by one- and two-step polymer-network gel processes. J. Alloys Compd. 2021, 868, 158723. [Google Scholar] [CrossRef]
- Mansurov, R.R.; Zverev, V.S.; Safronov, A.P. Dynamics of diffusion-limited photocatalytic degradation of dye by polymeric hydrogel with embedded TiO2 nanoparticles. J. Catal. 2022, 406, 9–18. [Google Scholar] [CrossRef]
- Uricchio, A.; Nadal, E.; Plujat, B.; Plantard, G.; Massines, F.; Fanelli, F. Low-temperature atmospheric pressure plasma deposition of TiO2-based nanocomposite coatings on open-cell polymer foams for photocatalytic water treatment. Appl. Surf. Sci. 2021, 561, 150014. [Google Scholar] [CrossRef]
- Justh, N.; Mikula, G.J.; Bakos, L.P.; Nagy, B.; Laszlo, K.; Parditka, B.; Erdelyi, Z.; Takats, V.; Mizsei, J.; Szilagyi, I.M. Photocatalytic properties of TiO2@polymer and TiO2@carbon aerogel composites prepared by atomic layer deposition. Carbon 2019, 147, 476–482. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Zhou, X.; He, X.; Zhou, M.; Jia, K.; Liu, X. Design of polymer composite-based porous membrane for in-situ photocatalytic degradation of adsorbed organic dyes. J. Phys. Chem. Solids 2021, 154, 110094. [Google Scholar] [CrossRef]
- Chen, X.; Nie, Q.; Shao, Y.; Wang, Z.; Cai, Z. TiO2 nanoparticles functionalized borneol-based polymer films with enhanced photocatalytic and antibacterial performances. Environ. Technol. Innov. 2021, 21, 101304. [Google Scholar] [CrossRef]
- Lin, L.H.; Liu, H.J.; Hwang, J.J.; Chen, K.M.; Chao, J.C. Photocatalytic effects and surface morphologies of modified silicone–TiO2 polymer composites. Mater. Chem. Phys. 2011, 127, 248–252. [Google Scholar] [CrossRef]
- Tekin, D.; Birhan, D.; Kiziltas, H. Thermal, photocatalytic, and antibacterial properties of calcinated nano-TiO2/polymer composites. Mater. Chem. Phys. 2020, 251, 123067. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, L.; Yang, H.; Song, Y.; Dan, Y. Structure and Photocatalytic Activity of Polythiophene/TiO2 Composite Particles Prepared by Photoinduced Polymerization. Chin. J. Catal. 2011, 32, 536–545. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Preparation of titanium dioxide modified biomass polymer microspheres for photocatalytic degradation of rhodamine-B dye and tetracycline. J. Taiwan Inst. Chem. Eng. 2021, 122, 157–167. [Google Scholar] [CrossRef]
- Neghi, N.; Kumar, M.; Burkhalov, D. Synthesis and application of stable, reusable TiO2 polymeric composites for photocatalytic removal of metronidazole: Removal kinetics and density functional analysis. Chem. Eng. J. 2019, 359, 963–975. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Yang, L.; Cao, S.; Yang, H.; Zhang, J.; Jiang, L.; Dan, Y.; LeRendu, P.; Nguyen, T.P. Photocatalytic activity of TiO2 based composite films by porous conjugated polymer coating of nanoparticles. Mater. Sci. Semicond. Process. 2016, 42, 54–57. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.C.; Chiang, L.Y.; Hsieh, C.T.; Liu, S.H.; Juang, R.S. Immobilization of TiO2 and TiO2-GO hybrids onto the surface of acrylic acid-grafted polymeric membranes for pollutant removal: Analysis of photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104422. [Google Scholar] [CrossRef]
- Gomes, J.; Alves, B.M.P.; Ferreira, P.; Martins, R.C. Immobilization of TiO2 onto a polymeric support for photocatalytic oxidation of a paraben’s mixture. J. Water Proc. Eng. 2022, 46, 102458. [Google Scholar] [CrossRef]
- Singh, A.R.; Dhumal, P.S.; Bhakare, M.A.; Lokhande, K.D.; Bondarde, M.P.; Some, S. In-situ synthesis of metal oxide and polymer decorated activated carbon-based photocatalyst for organic pollutants degradation. Sep. Purif. Technol. 2022, 286, 120380. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Panigrahi, P.; Kumaar, S.; Nagarajan, G.S. Effect of conducting polymer on photoluminescence quenching of green synthesized ZnO thin film and its photocatalytic properties. Nano-Struct. Nano-Objects 2020, 22, 100446. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Yang, J.; Hu, C.; Shang, J.; Zhai, H. In situ construction of conjugated polymer P3HT coupled hierarchical ZnO composite with Z-scheme enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2018, 106, 19–27. [Google Scholar] [CrossRef]
- Podasca, V.E.; Damaceanu, M.D. ZnO-Ag based polymer composites as photocatalysts for highly efficient visible-light degradation of Methyl Orange. J. Photochem. Photobiol. A Chem. 2021, 406, 113003. [Google Scholar] [CrossRef]
- Kongseng, P.; Amornpitoksuk, P.; Chantarak, S. Development of multifunctional hydrogel composite based on poly(vinylalcohol-g-acrylamide) for removal and photocatalytic degradation of organic dyes. React. Funct. Polym. 2022, 172, 105207. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Aziz, M.; Nasir, M.U. Effects of oxidants on the in-situ polymerization of aniline to form Cu2O/ZnO/PANI composite photocatalyst. Mater. Today Proc. 2021, 46, 2030–2035. [Google Scholar]
- Ma, A.; Wei, Y.; Zhou, Z.; Xu, W.; Ren, F.; Ma, H.; Wang, J. Preparation Bi2S3eTiO2 heterojunction/polymer fiber composites and its photocatalytic degradation of methylene blue under Xe lamp irradiation. Polym. Degrad. Stab. 2012, 97, 125–131. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, D.; Wang, J.; Yang, Z. In situ growth of photocatalytic Ag-decorated β-Bi2O3/Bi2O2.7 heterostructure film on PVC polymer matrices with self-cleaning and antibacterial properties. Chem. Eng. J. 2022, 429, 131058. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.; Gu, P.; Zhang, T.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Conjugate Polymer-clothed TiO2@V2O5 nanobelts and their enhanced visible light photocatalytic performance in water remediation. J. Colloid Interface Sci. 2020, 578, 402–411. [Google Scholar] [CrossRef]
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: Superior photocatalytic activity for degradation of methylene blue. Mater. Chem. Phys. 2021, 269, 124734. [Google Scholar] [CrossRef]
- Djellabi, R.; Ali, J.; Zhao, X.; Saber, A.N.; Yang, B. CuO NPs incorporated into electron-rich TCTA@PVP photoactive polymer for the photocatalytic oxidation of dyes and bacteria inactivation. J. Water Proc. Eng. 2020, 36, 101238. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Ma, T.; Zhao, X. Seed-mediated growth of Au core–Pd shell-structured nanoparticles on a polymer nanofibrous mat as a highly active photocatalytic composite material. Compos. Commun. 2020, 21, 100406. [Google Scholar] [CrossRef]
- Jin, X.; Wu, Y.; Lin, Z.; Liang, D.; Wang, F.; Zheng, X.; Liu, H.; Lv, W.; Liu, G. Plasmonic Ag nanoparticles decorated copper-phenylacetylide polymer for visible-light-driven photocatalytic reduction of Cr(VI) and degradation of PPCPs: Performance, kinetics, and mechanism. J. Hazard. Mater. 2022, 425, 127599. [Google Scholar] [CrossRef]
- Han, H.; Fu, M.; Li, Y.; Guan, W.; Lu, P.; Hu, X. In-situ polymerization for PPy/g-C3N4 composites with enhanced visible light photocatalytic performance. Chin. J. Catal. 2018, 39, 831–840. [Google Scholar] [CrossRef]
- Chaitanya, B.H.; Pawan, K.K.; Priyesh, V.M. Probing the real-time photocatalytic activity of CdS QDs sensitized conducting polymers: Featured PTh, PPy and PANI. Vacuum 2018, 155, 159–168. [Google Scholar]
- Park, Y.; Numan, A.; Ponomarev, N.; Iqbal, J.; Khalid, M. Enhanced photocatalytic performance of PANI-rGO-MnO2 ternary composite for degradation of organic contaminants under visible light. J. Environ. Chem. Eng. 2021, 9, 106006. [Google Scholar] [CrossRef]
- Biswas, R.D.; Cho, K.Y.; Na, J.D.; Oh, W.C. Highly electro-conductive graphene-decorated PANI-BiVO4 polymersemiconductor nanocomposite with outstanding photocatalytic performance. Mater. Sci. Eng. B 2019, 251, 114469. [Google Scholar] [CrossRef]
- Mittal, H.; Kumar, A.; Khanuja, M. In-situ oxidative polymerization of aniline on hydrothermally synthesized MoSe2 for enhanced photocatalytic degradation of organic dyes. J. Saudi Chem. Soc. 2019, 23, 836–845. [Google Scholar] [CrossRef]
- Oh, W.C.; Fatema, K.N.; Liu, Y.; Lim, C.S.; Cho, K.Y.; Jung, C.H.; Biswas, R.D. Sonochemical synthesis of quaternary LaNiSbWO4-G-PANI polymer nanocomposite for photocatalytic degradation of Safranin-O and gallic acid under visible light irradiation. J. Photochem. Photobiol. A Chem. 2020, 394, 112484. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Vaulot, C.; Michelin, L.; Dumur, F.; Airoudj, A.; Morlet-Savary, F.; Raveau, B.; Bousselmi, L.; Lalevee, J. New hybrid perovskites/polymer composites for the photodegradation of organic dyes. Eur. Polym. J. 2021, 157, 110641. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Zhang, Y.; Xu, G.; Tu, Y.; Jia, X.; Wu, D.; Xie, X.; Liu, Y. Insights into the photocatalytic activation of peroxymonosulfate by visible light over BiOBr-cyclodextrin polymer complexes for efficient degradation of dye pollutants in water. Environ. Res. 2022, 207, 112160. [Google Scholar] [CrossRef]
- Hojamberdieva, M.; Kadirova, Z.C.; Daminova, S.S.; Yubuta, K.; Razavi-Khosroshahi, H.; Sharipov, K.T.; Miyauchi, M.; Teshima, K.; Hasegawa, M. Amorphous Fe2O3 nanoparticles embedded into hypercrosslinked porous polymeric matrix for designing an easily separable and recyclable photocatalytic system. Appl. Surf. Sci. 2019, 466, 837–846. [Google Scholar] [CrossRef]
- Xie, R.; Fan, J.; Fang, K.; Chen, W.; Song, Y.; Pan, Y.; Li, Y.; Liu, J. Hierarchical Bi2MoO6 microsphere photocatalysts modified with polypyrrole conjugated polymer for efficient decontamination of organic pollutants. Chemosphere 2022, 286, 131541. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Y.; Zhao, K.; Hu, J.; Liu, H.; Hu, Y. Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer. Chin. J. Chem. Eng. 2022, 41, 488–496. [Google Scholar] [CrossRef]
- Ghali, M.; Benltifa, M.; Brahmi, C.; Elbassi, L.; Dumur, F.; Simonnet-Jegat, C.; Bousselmi, L.; Lalevee, J. LED and solar photodecomposition of erythrosine B and rose Bengal using H3PMo12O40/polymer photocatalyst. Eur. Polym. J. 2021, 159, 110743. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Ghali, M.; Dumur, F.; Simonnet-Jegat, C.; Valerie, M.; Morlet-Savary, F.; Bousselmi, L.; Lalevee, J. Performance improvement of the photocatalytic process for the degradation of pharmaceutical compounds using new POM/polymer photocatalysts. J. Environ. Chem. Eng. 2021, 9, 106015. [Google Scholar] [CrossRef]
- Meng, P.; Heng, H.; Sun, Y.; Liu, X. In situ polymerization synthesis of Z-scheme tungsten trioxide/polyimide photocatalyst with enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2018, 428, 1130–1140. [Google Scholar] [CrossRef]

| Composite Composition | Pollutant | Photocatalytic Properties | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Polymer (s) | Second Material | Molecule | Conc. | Radiation Type | Radiation Intensity | Exposure Time | Catalyst Dosage | Removal Efficiency | |
| Polyacrylamide | TiO2 | methyl orange | 1.0 mg/L | UV | 3 W | 300 min | np * | 95% | [57] |
| Polyurethane | TiO2 | methyl orange | 10 mg/L | UV | 38 W/m2 | 50 min | 0.046 g/3.6 mL | 100% | [58] |
| Resorcinol-formaldehyde | TiO2 | methyl orange | 13 mg/L | UV | 36 W | 240 min | 1 mg/3 mL | 55% | [59] |
| Polyethylene glycol | TiO2 | methylene blue | 20 mg/L | UV | 300 W | 240 min | 3 g/100 mL | 100% | [60] |
| Polytrifluorochloro ethylene | TiO2 | methylene blue | 10 mg/L | UV | 0.5 mW/cm2 | 270 min | np | 100% | [61] |
| PEG2000-silicone | TiO2 | methylene blue | 3.2 mg/L | UV | 20 W | 120 min | np | 40% | [62] |
| Polyvinyl alcohol | TiO2 | acid black | 20 mg/L | UV | 44 W/m2 | 120 min | 50 mg/400 mL | 55.4% | [63] |
| Polyethylene glycol | 62.8% | ||||||||
| Polythiophene | TiO2 | rhodamine B | 40 mg/L | UV | 10 W | 180 min | 300 mg/300 mL | 76% | [64] |
| Vis | 320 W | 600 min | 98% | ||||||
| Poly-phenylpropenes trans-anethole and N-phenylmaleimide | TiO2 | rhodamine B | 10 mg/L | UV | 600 W | 90 min | 20 mg/20 mL | 95% | [65] |
| tetracycline | 97% | ||||||||
| Chitosan Polyvinyl alcohol–chitosan | TiO2 | metronidazole | 10 mg/L | UV | 32 W | 120 min | 0.3 g/L | 100% | [66] |
| Polyvinyl alcohol | TiO2 | phenol | 10 mg/L | Vis | 500 W | 360 min | np | 67.5% | [67] |
| Poly(vinylidene fluoride) | TiO2/GO | phenol | 50 mg/L | UV | 100 W | 180 min | np | 65% | [68] |
| Polydimethylsiloxane | TiO2 | methylparaben | 1 mg/L | Sun Radiation | np | 120 min | 140 mg/L | 50% | [69] |
| ethylparaben | 52% | ||||||||
| propylparaben | 55% | ||||||||
| Polypyrrole | ZnO | methylene blue | 50 mg/L | UV | 100 W | 20 min | 50 mg/50 mL | 98.12% | [70] |
| Polypyrrole | ZnO | rhodamine B | 5 mg/L | Vis | 150 W | 300 min | np | 65% | [71] |
| Poly(3-hexylthiophene-2,5-diyl) | ZnO | rhodamine B | 0.01 mg/L | Vis | 300 W | 80 min | 20 mg/100 mL | 99% | [72] |
| Poly(propylene glycol)-dimethacrylate–methacryloyloxyethyl-N,N-dimethyl-3-(trimethoxysilyl)-propane | ZnO | methyl orange | 16.35 mg/L | Vis | 4.9 mW/cm2 | 250 min | 1 g/50 mL | 56.12% | [73] |
| ZnO-Ag | 95% | ||||||||
| Composite Composition | Pollutant | Photocatalytic Properties | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Polymer (s) | Second Material | Molecule | Conc. | Radiation Type | Radiation Intensity | Exposure Time | Catalyst Dosage | Removal Efficiency | |
| Poly(vinyl alcohol-g-acrylamide) | ZnO/SiO2 | methylene blue | 5 mg/L | UV | 18 W | 960 min | 0.1 g/20 mL | 86% | [74] |
| crystal violet | 77% | ||||||||
| congo red | 70% | ||||||||
| Polyaniline | Cu2O/ZnO | congo red | 30 mg/L | UV | np * | 30 min | 100 mg/100 mL | 100% | [75] |
| Polysulphone–styrene maleic anhydride copolymer | Bi2S3/TiO2 | methylene blue | 20.26 mg/L | UV–vis | 350 W | 180 min | np | 95.32% | [76] |
| Polyvinyl chloride | Ag-decorated Bi2O3/Bi2O2.7 | rhodamine B | 12 mg/L | Vis | 5 W | 150 min | np | 97% | [77] |
| Polypyrrole | TiO2/V2O5 | tetracycline | 50 mg/L | Vis | 300 W | 120 min | 30 mg/50 mL | 98% | [78] |
| doxycycline | 96% | ||||||||
| oxytetracycline | 85% | ||||||||
| ofloxacin | 37% | ||||||||
| rhodamine B | 100% | ||||||||
| TX-SCH2COOH-DO | Au/Ag | methylene blue | 10 mg/L | UV | 100 W | 90 min | 5 mg/5 mL | 72.5% | [79] |
| Tris(4-carbazoyl-9-ylphenyl) amine/polyvinylpyrrolidone | Cu | methylene blue | np | Vis | 300 W | 90 min | 20 mg/15 mL | 80% | [80] |
| CuO | 90% | ||||||||
| Polyvinyl alcohol | Au/Pd | styrene | 20 mg/L | Vis | 50 mW/cm2 | 60 min | np | 100% | [81] |
| Phenylacetylide | Ag/Cu | norfloxacin | 10 mg/L | Vis | 9 W | 40 min | 10 mg/50 mL | 100% | [82] |
| diclofenac | 64.3% | ||||||||
| bisphenol A | 47.6% | ||||||||
| naproxen | 70.5% | ||||||||
| sulfisoxazole | 42% | ||||||||
| Polypyrrole | g-C3N4 | methylene blue | 10 mg/L | Vis | 12 W | 120 min | 0.05 g/50 mL | 99% | [83] |
| Polypyrrole | CdS | methylene blue | 10 mg/L | Vis | 75,000–90,000 Lux | 300 min | 25 mg/50 mL | 77% | [84] |
| Polythiophene | 71% | ||||||||
| Polyaniline | 61% | ||||||||
| Polyaniline | rGO/MnO2 | methylene blue | 5 mg/L | Vis | 150 W | 120 min | 10 mg/50 mL | 90% | [85] |
| Polyaniline | BiVO4/GO | rhodamine B | 4.8 mg/L | Vis | 500 W | 180 min | 0.1 g/100 mL | 62% | [86] |
| methylene blue | 3.2 mg/L | 73% | |||||||
| safrarine O | 35 mg/L | 82% | |||||||
| Polyaniline | MoSe2 | methylene blue | np | Vis | 100 mW/cm2 | 120 min | 20 mg/100 mL | 65% | [87] |
| methyl orange | 150 min | 94% | |||||||
| Polyaniline | LaNiSbWO4/GO | safrarine O | 35 mg/L | Vis | 500 W | 180 min | 0.1 g/100 mL | 84% | [88] |
| gallic acid | 1.7 mg/L | 92% | |||||||
| Polyether Tetraacrylate | Nd0.9TiO3 | Acid Black | 15 mg/L | UV | 250 mW/cm2 | 30 min | np | 94% | [89] |
| LaTiO3 | 95% | ||||||||
| Cyclodextrin | BiOBr | Acid Orange 7 | 70 mg/L | Vis | 500 W | 60 min | 40 mg/40 mL | 99.2% | [90] |
| Polystyrene/divinylbenzene | Fe2O3 | methylene blue + oxalic acid | 8 mg/L + 38.7 mg/L | UV | 20 W | 120 min | 10 mg/100 mL | 98% | [91] |
| oxalic acid | 88.2 mg/L | 73.6% | |||||||
| Polypyrrole | Bi2MoO6 | methylene blue | 5 mg/L | Vis | 350 W | 80 min | 35 mg/50 mL | 93.6% | [92] |
| tetracycline | 30 mg/L | 88.3% | |||||||
| 4,7-dibromobenzo thiadiazole/4-ethynylphenyl amine | Bi2WO6 | tetracycline | 10 mg/L | Vis | 300 W | 90 min | 20 mg/100 mL | 86% | [93] |
| rhodamine B | 84% | ||||||||
| Poly(trimethyl-propane triacrylate)/bis(acyl)phosphane oxides | H3PMo12O40 | erythrosine B | 10 mg/L | UV | 0.07 W/cm2 | 120 min | np | 81% | [94] |
| rose bengal | 86% | ||||||||
| Poly(trimethyl-propane triacrylate) | H3PMo12O40 W10O32 (TH)4 | ibuprofen | 15 mg/L | UV–vis | 250 mW/cm2 | 90 min | np | 100% | [95] |
| ciprofloxacin | 75 min | 90% | |||||||
| oxytetracycline | 75 min | 86% | |||||||
| Polyimide | WO3 | imidacloprid | 20 mg/L | Vis | 225 W | 180 min | 1 g/L | 50% | [96] |
| 2.5 g/L | 73.2% | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Cazan, C. Polymer Composite-Based Materials with Photocatalytic Applications in Wastewater Organic Pollutant Removal: A Mini Review. Polymers 2022, 14, 3291. https://doi.org/10.3390/polym14163291
Enesca A, Cazan C. Polymer Composite-Based Materials with Photocatalytic Applications in Wastewater Organic Pollutant Removal: A Mini Review. Polymers. 2022; 14(16):3291. https://doi.org/10.3390/polym14163291
Chicago/Turabian StyleEnesca, Alexandru, and Cristina Cazan. 2022. "Polymer Composite-Based Materials with Photocatalytic Applications in Wastewater Organic Pollutant Removal: A Mini Review" Polymers 14, no. 16: 3291. https://doi.org/10.3390/polym14163291







