Effects of the Diamine Chain End Functionalized Liquid Butadiene Rubber as a Processing Aid on the Properties of Carbon-Black-Filled Rubber Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polymerization
2.1.2. Compounding
2.2. Measurements
2.2.1. Gel Permeation Chromatography (GPC)
2.2.2. Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Polymer Viscosity
2.2.5. Payne Effect
2.2.6. Mooney Viscosity
2.2.7. Cure Characteristics
2.2.8. Solvent Extraction and Vulcanizate Structure
2.2.9. Mechanical Properties
2.2.10. Abrasion Resistance
2.2.11. Dynamic Viscoelastic Properties
2.3. Synthesis of Liquid Butadiene Rubbers
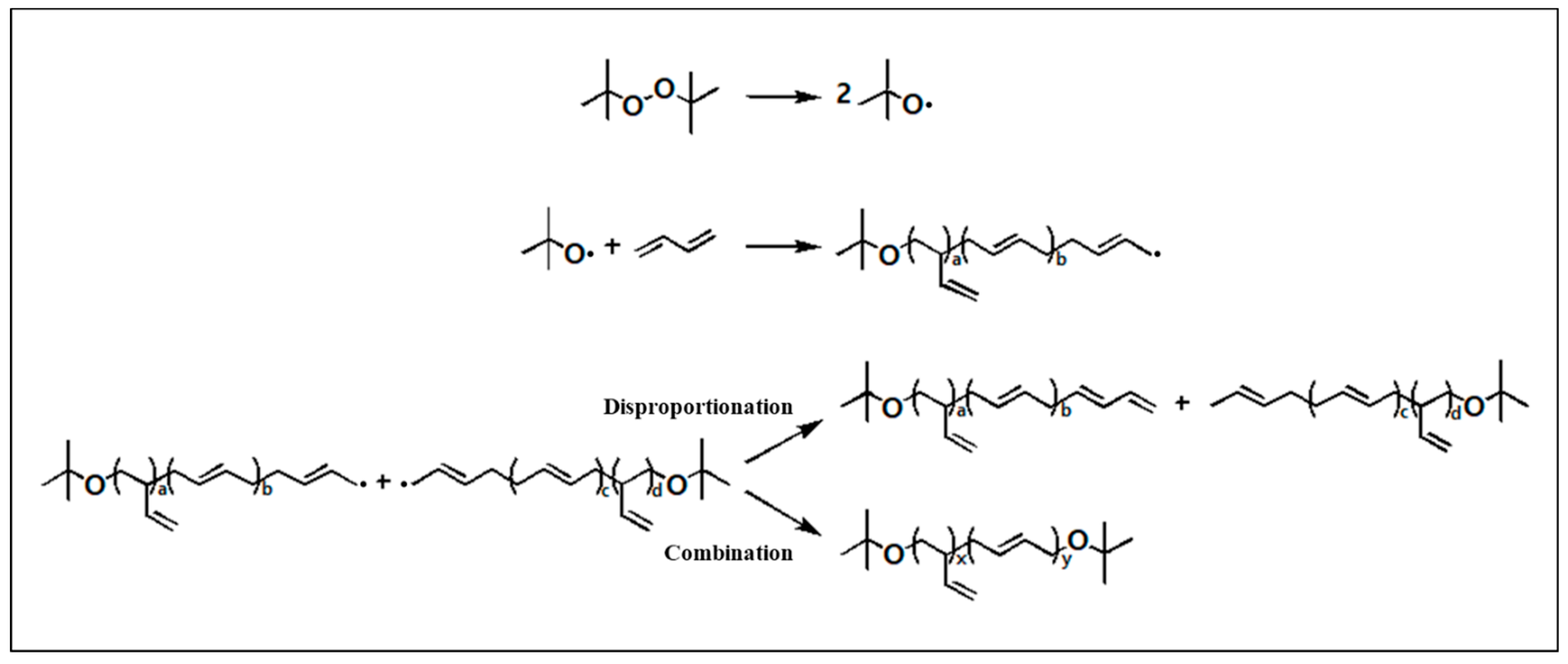
Radical Polymerization
2.4. Manufacture of Rubber/Carbon Black Compounds and Vulcanizates
3. Results and Discussion
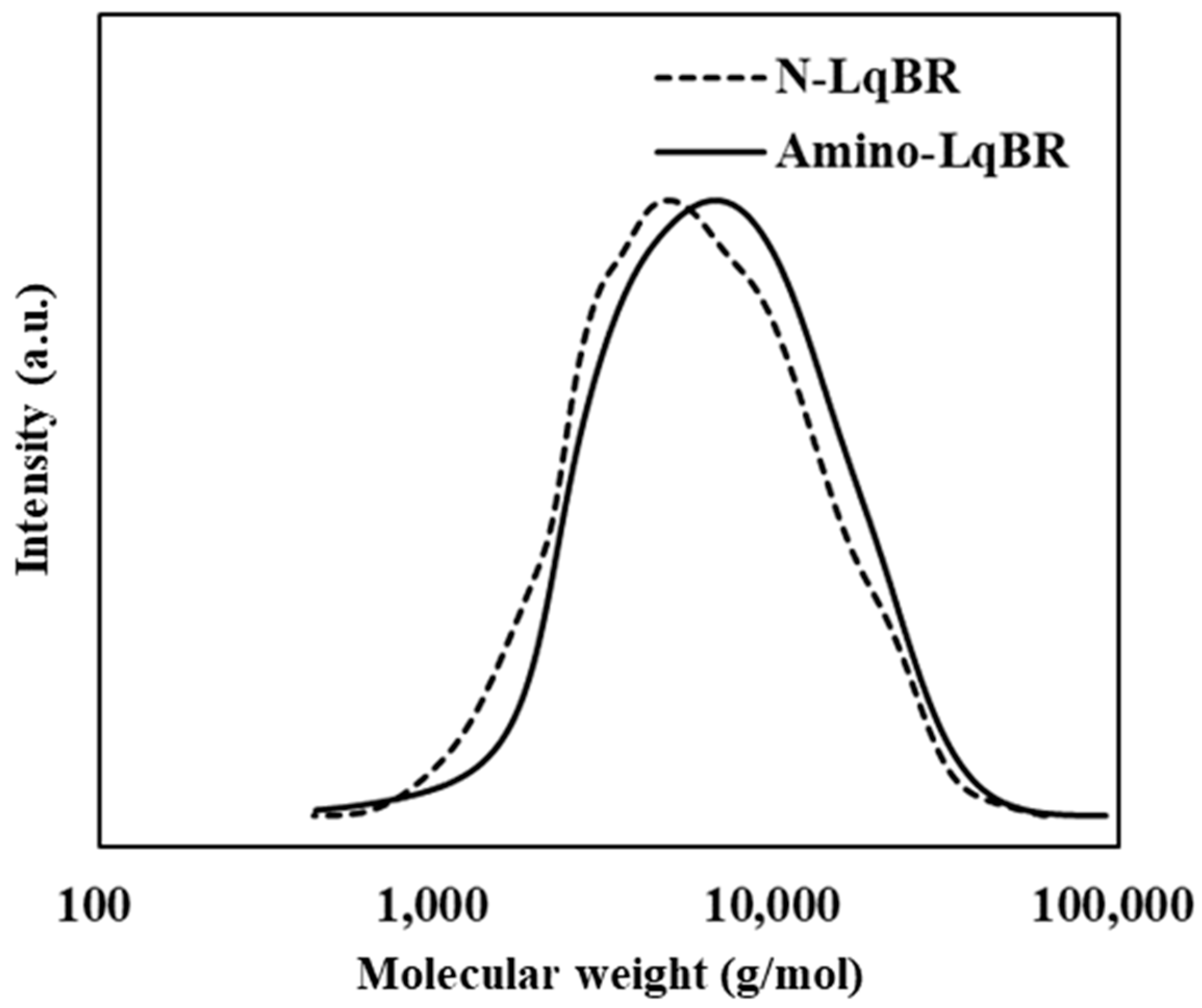
3.1. Synthesis of LqBR
3.2. Payne Effect
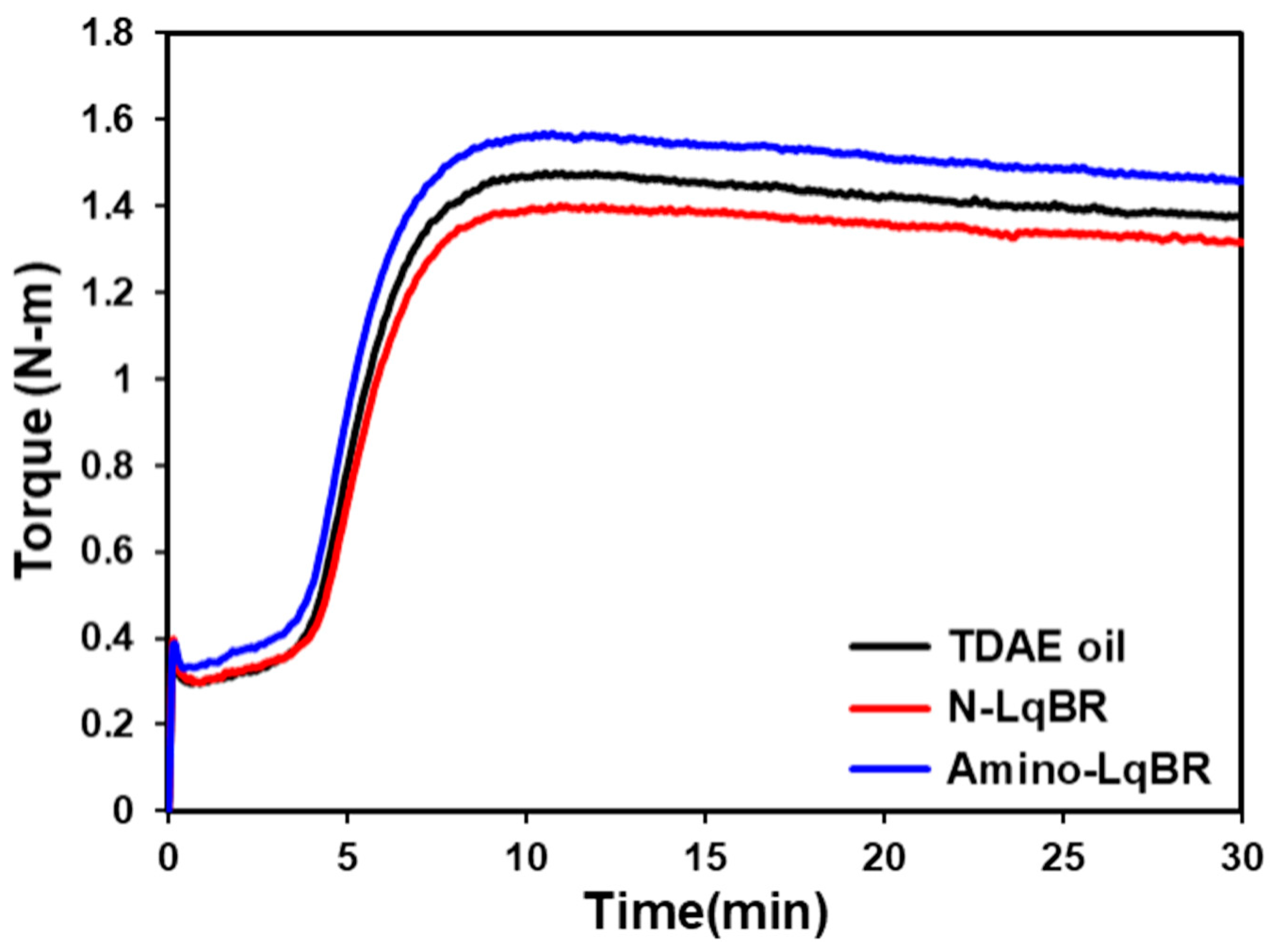
3.3. Mooney Viscosity and Cure Characteristics of the Compounds
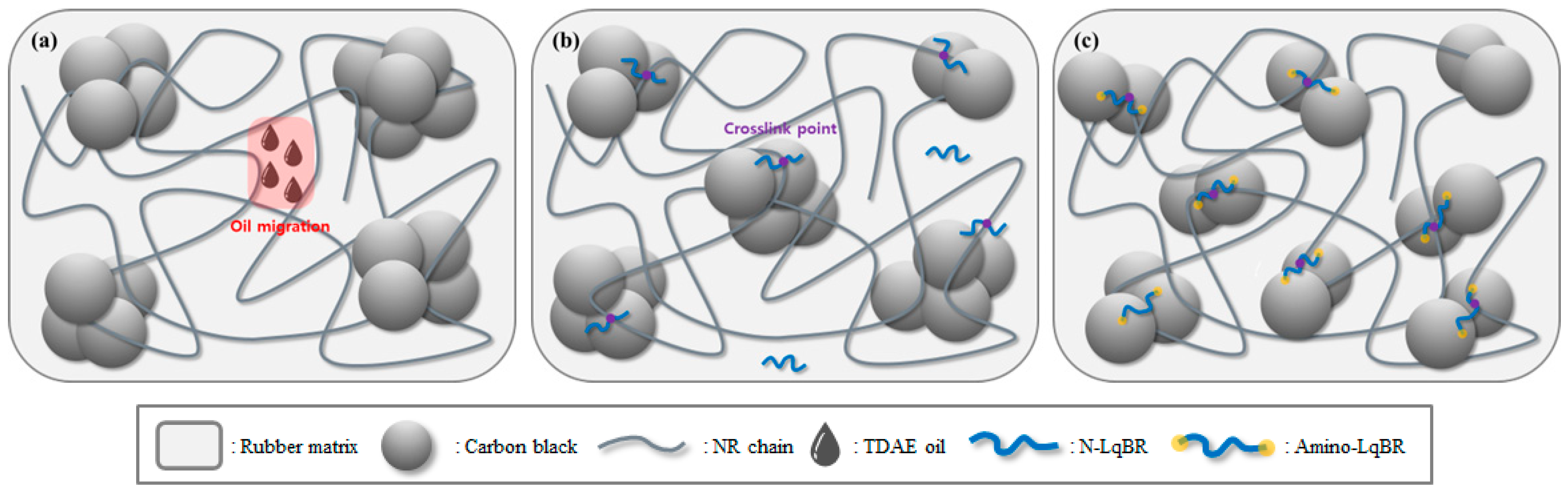
3.4. Solvent Extraction and Crosslink Density
3.5. Mechanical Properties and DIN Abrasion Loss
3.6. Dynamic Viscoelastic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodgers, B. Tire Engineering: An Introduction; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Han, S.; Kim, W.S.; Mun, D.Y.; Ahn, B.; Kim, W. Effect of coupling agents on the vulcanizate structure of carbon black filled natural rubber. Compos. Interfaces 2020, 27, 355–370. [Google Scholar] [CrossRef]
- Xu, Z.; Song, Y.; Zheng, Q. Payne effect of carbon black filled natural rubber compounds and their carbon black gels. Polymer 2019, 185, 121953. [Google Scholar] [CrossRef]
- Farida, E.; Bukit, N.; Ginting, E.M.; Bukit, B.F. The effect of carbon black composition in natural rubber compound. Case Stud. Therm. Eng. 2019, 16, 100566. [Google Scholar] [CrossRef]
- Omnès, B.; Thuillier, S.; Pilvin, P.; Grohens, Y.; Gillet, S. Effective properties of carbon black filled natural rubber: Experiments and modeling. Compos. A Appl. Sci. Manuf. 2008, 39, 1141–1149. [Google Scholar] [CrossRef]
- Ezzoddin, S.; Abbasian, A.; Aman-Alikhani, M.; Ganjali, S.T. The influence of non-carcinogenic petroleum-based process oils on tire compounds’ performance. Iran. Polym. J. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Sökmen, S.; Oßwald, K.; Reincke, K.; Ilisch, S. Influence of treated distillate aromatic extract (TDAE) content and addition time on rubber-filler interactions in silica filled SBR/BR blends. Polymers 2021, 13, 698. [Google Scholar] [CrossRef]
- Corman, B.G.; Deviney, M.L., Jr.; Whittington, L.E. The migration of extender oil in natural and synthetic rubber. IV. Effect of saturates geometry and carbon black type on diffusion rates. Rubber Chem. Technol. 1970, 43, 1349–1358. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, B.; Kim, K.; Lee, J.; Kim, I.J.; Kim, W. Effects of molecular weight of functionalized liquid butadiene rubber as a processing aid on the properties of SSBR/silica compounds. Polymers 2021, 13, 850. [Google Scholar] [CrossRef]
- Iz, M.; Kim, D.; Hwang, K.; Kim, W.; Ryu, G.; Song, S.; Kim, W. The effects of liquid butadiene rubber and resins as processing aids on the physical properties of SSBR/silica compounds. Elastom. Compos. 2020, 55, 289–299. [Google Scholar]
- Gruendken, M. Liquid rubber for safer and faster tires. In Proceedings of the Tire Technology EXPO 2018, Hannover, Germany, 20–22 February 2018. [Google Scholar]
- Ikeda, K. Bio liquid polymer for winter tires. In Proceedings of the Tire Technology EXPO 2018, Hanover, Germany, 20–22 February 2018. [Google Scholar]
- Sierra, V.P.; Wagemann, J.; Van De Pol, C.; Kendziorra, N.; Herzog, K.; Recker, C.; Mueller, N. Rubber Blend with Improved Rolling Resistance Behavior. U.S. Patent 9,080,042, 14 July 2015. [Google Scholar]
- Kitamura, T.; Lawson, D.F.; Morita, K.; Ozawa, Y. Anionic Polymerization Initiators and Reduced Hysteresis Products Therefrom. U.S. Patent 5,393,721, 28 February 1995. [Google Scholar]
- Cray Valley, Technical Data Sheet, Recon. Available online: http://www.crayvalley.com/docs/tds/ricon-603-.pdf?sfvrsn=2 (accessed on 9 April 2018).
- Evonik. Less Fuel and Lower CO2 Emissions with POLYVEST ST Tires. Available online: https://coatings.evonik.com/en/less-fuel-andlower-CO2-emissions-with-polyvest-st-tires-100233.html (accessed on 13 February 2017).
- Herpich, R.; Fruh, T.; Heiliger, L.; Schilling, K. Silica Gel-Containing Rubber Compounds with Organosilicon Compounds as Compounding Agent. U.S. Patent 6,593,418, 15 July 2003. [Google Scholar]
- Takuya, H.; Tochiro, M. Tire Tread Rubber Composition. J.P. Patent 2,005,146,115, 14 November 2003. [Google Scholar]
- Satoyuki, S.; Chikashi, Y. Rubber Composition Containing Compound Having Organosilicon Function Group through Urethane Bond at Terminal. J.P. Patent 2,005,350,603, 22 December 2005. [Google Scholar]
- Kim, D.; Yeom, G.; Joo, H.; Ahn, B.; Paik, H.J.; Jeon, H.; Kim, W. Effect of the functional group position in functionalized liquid butadiene rubbers used as processing aids on the properties of silica-filled rubber compounds. Polymers 2021, 13, 2698. [Google Scholar] [CrossRef]
- Iyama, H.; Ozturk, O.; Sumitomo Chemical Co., Ltd. Performance improvement of natural rubber/carbon black composites by novel coupling agents. Sumitomo Kagaku 2016, 2016, 1–9. [Google Scholar]
- He, S.J.; Wang, Y.Q.; Xi, M.M.; Lin, J.; Xue, Y.; Zhang, L.Q. Prevention of oxide aging acceleration by nano-dispersed clay in styrene-butadiene rubber matrix. Polym. Degrad. Stab. 2013, 98, 1773–1779. [Google Scholar] [CrossRef]
- Jansinak, S.; Markpin, T.; Wimolmala, E.; Mahathanabodee, S.; Sombatsompop, N. Tribological properties of carbon nanotube as co-reinforcing additive in carbon black/acrylonitrile butadiene rubber composites for hydraulic seal applications. J. Reinf. Plast. Compos. 2018, 37, 1255–1266. [Google Scholar] [CrossRef]
- Seo, J.; Kim, D.; Park, H.; Seo, K. Research on CR/Nylon 6 cord rubber sleeve of rubber air spring. Elastomers Compos. 2014, 49, 293–304. [Google Scholar] [CrossRef]
- Yurovska, I.; Gaudet, G. Using furnace blacks in hose compounds. Rubber & Plastics News, 7 April 2008; 18–22. [Google Scholar]
- Dwivedi, C.; Manjare, S.; Rajan, S.K. Recycling of waste tire by pyrolysis to recover carbon black: Alternative & environment-friendly reinforcing filler for natural rubber compounds. Compos. Part B Eng. 2020, 200, 108346. [Google Scholar]
- Ismawi, D.H.A.; Zaeimoedin, T.Z.; Saad, C.S.M. Recovered carbon black (rCB) from waste tyres: Effect on mechanical properties of rubber compound. In Proceedings of the Conference Malaysian Science and Technology Congress (MSTC 2010), Kuala Lumpur, Malaysia, 9–11 November 2010; pp. 1–11. [Google Scholar]
- Moulin, L.; Da Silva, S.; Bounaceur, A.; Herblot, M.; Soudais, Y. Assessment of recovered carbon black obtained by waste tires steam water thermolysis: An industrial application. Waste Biomass Valoriz. 2017, 8, 2757–2770. [Google Scholar] [CrossRef]
- Ramier, J.; Gauthier, C.; Chazeau, L.; Stelandre, L.; Guy, L. Payne effect in silica-filled styrene–butadiene rubber: Influence of surface treatment. J. Polym. Sci. B Polym. Phys. 2007, 45, 286–298. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, N.; Lim, S.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.J.; Kim, W. Influence of the silanes on the crosslink density and crosslink structure of silica-filled solution styrene butadiene rubber compounds. Compos. Interfaces 2017, 24, 711–727. [Google Scholar] [CrossRef]
- Boonstra, B.B.; Taylor, G.L. Swelling of filled rubber vulcanizates. Rubber Chem. Technol. 1965, 38, 943–960. [Google Scholar] [CrossRef]
- Verbruggen, M.A.L.; Van Der Does, L.; Noordermeer, J.W.; Van Duin, M.; Manuel, H.J. Mechanisms involved in the recycling of NR and EPDM. Rubber Chem. Technol. 1999, 72, 731–740. [Google Scholar] [CrossRef]
- Ahn, B.; Kim, D.; Kim, K.; Kim, I.J.; Kim, H.J.; Kang, C.H.; Lee, J.Y.; Kim, W. Effect of the functional group of silanes on the modification of silica surface and the physical properties of solution styrene-butadiene rubber/silica composites. Compos. Interfaces 2019, 26, 585–596. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.J.; Kim, W. The effect of accelerator contents on the vulcanizate structures of SSBR/silica vulcanizates. Compos. Interfaces 2017, 24, 563–577. [Google Scholar] [CrossRef]
- Han, S.; Gu, B.; Kim, S.; Kim, S.; Mun, D.; Morita, K.; Kim, D.; Kim, W. Effect of sulfur variation on the vulcanizate structure of silica-filled styrene-butadiene rubber compounds with a sulfide–silane coupling agent. Polymers 2020, 12, 2815. [Google Scholar] [CrossRef] [PubMed]
- Shefer, A.; Grodzinsky, A.J.; Prime, K.L.; Busnel, J.P. Free-radical telomerization of tert-butyl acrylate in the presence of bis (4-aminophenyl) disulfide as a useful route to amino-terminated telomers of poly (acrylic acid). Macromolecule 1993, 26, 2240–2245. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Boston, MA, USA, 2014; pp. 262–263. [Google Scholar]
- Rothfuss, N.E.; Petters, M.D. Influence of functional groups on the viscosity of organic aerosol. Environ. Sci. Technol. 2017, 51, 271–279. [Google Scholar] [CrossRef]
- Jayalakshmy, M.S.; Mishra, R.K. Applications of carbon-based nanofiller-incorporated rubber composites in the fields of tire engineering, flexible electronics and EMI shielding. In Carbon-Based Nanofillers and Their Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 441–472. [Google Scholar]
- Wang, X.; Wu, L.; Xiao, T.; Yu, H.; Li, H.; Yang, J. Preparation and application of carbon black-filled rubber composite modified with a multi-functional silane coupling agent. Int. Polym. Process. 2022, 37, 15–24. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, I.S.; Woo, C.S. Influence of TESPT content on crosslink types and rheological behaviors of natural rubber compounds reinforced with silica. J. Appl. Polym. Sci. 2007, 106, 2753–2758. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. B 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, D.; Song, S.; Hwang, K.; Kim, W. Effect of the epoxide contents of liquid isoprene rubber as a processing aid on the properties of silica-filled natural rubber compounds. Polymers 2021, 13, 3026. [Google Scholar] [CrossRef]
- Halasa, A.F.; Prentis, J.; Hsu, B.; Jasiunas, C. High vinyl high styrene solution SBR. Polymer 2005, 46, 4166–4174. [Google Scholar] [CrossRef]
- Isitman, N.A.H.; Thielen, G.M.V. Rubber Composition and Pneumatic Tire. E.U. Patent 3450490A1, 6 August 2018. [Google Scholar]
- Wang, M.J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Wang, M.J.; Kutsovsky, Y.; Zhang, P.; Murphy, L.J.; Laube, S.; Mahmud, K. New generation carbon-silica dual phase filler part I. Characterization and application to passenger tire. Rubber Chem. Technol. 2002, 75, 247–263. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, P.; Mahmud, K. Carbon—silica dual phase filler, a new generation reinforcing agent for rubber: Part IX. Application to truck tire tread compound. Rubber Chem. Technol. 2001, 74, 124–137. [Google Scholar] [CrossRef]







| Organic Compounds | N-LqBR | Amino-LqBR | |||
|---|---|---|---|---|---|
| g | mol | g | mol | ||
| Initiator | DTBPO | 5.849 | 0.04 | 0.487 | 0.0033 |
| Chain transfer agent | BAPD | 0 | 0 | 49.674 | 0.20 |
| Monomer | 1,3-butadiene | 72 | 1.33 | 180 | 3.33 |
| Solvent | THF | 180 | 2.50 | 180 | 2.50 |
| Sample Code | TDAE Oil | N-LqBR | Amino-LqBR |
|---|---|---|---|
| NR | 100 | 100 | 100 |
| Carbon black (N134) | 35/55 | 35/55 | 35/55 |
| TDAE oil | 5 | - | - |
| N-LqBR | - | 5 | - |
| Amino-LqBR | - | - | 5 |
| Zinc oxide | 4 | 4 | 4 |
| Stearic acid | 3 | 3 | 3 |
| 6PPD | 2 | 2 | 2 |
| TMQ | 1 | 1 | 1 |
| Sulfur | 1.3 | 1.3 | 1.3 |
| TBBS | 1.1 | 1.1 | 1.1 |
| PVI | 0.3 | 0.3 | 0.3 |
| Time, min | RPM | Action | |
|---|---|---|---|
| Carbon black masterbatch (CMB) mixing | 00:00–01:30 | 20 | NR mastication (initial temperature: 80 °C) |
| 01:30–02:30 | 40 | Add carbon black 1/2, processing aid 1/2 | |
| 02:30–03:30 | 40 | Add carbon black 1/2, processing aid 1/2 | |
| 03:30–05:30 | 50 | Add ZnO, stearic acid, 6PPD, TMQ and dump (dump temperature: 135–140 °C) | |
| Final masterbatch (FMB) mixing | 00:00–00:30 | 20 | Add CMB (initial temperature: 60 °C) |
| 00:30–02:30 | 40 | Add sulfur, TBBS, PVI and dump (dump temperature: 80–90 °C) |
| Polymer | Unit | N-LqBR | Amino-LqBR |
|---|---|---|---|
| Sample Mn | g/mol | 3900 | 4300 |
| Polydispersity index (PDI) | - | 1.66 | 1.71 |
| Vinyl content (% in BD) | - | 20 | 20 |
| Tg | °C | −88 | −81 |
| Functionality (NH2/chain) | - | 0 | 2.24 |
| Compound | Unit | TDAE Oil | N-LqBR | Amino-LqBR |
|---|---|---|---|---|
| G′ (at a 0.28% strain) | MPa | 1.66 | 1.58 | 1.49 |
| G′ (at a 40.04% strain) | MPa | 0.28 | 0.30 | 0.32 |
| △G′ (at a 0.28–40.04% strain) | MPa | 1.38 | 1.28 | 1.17 |
| Compound | Unit | TDAE Oil | N-LqBR | Amino-LqBR |
|---|---|---|---|---|
| Mooney viscosity (ML1+4 at 100 °C, FMB) | MU | 73 | 73 | 80 |
| Tmin | N-m | 0.30 | 0.30 | 0.33 |
| Tmax | N-m | 1.48 | 1.40 | 1.57 |
| ΔTorque | N-m | 1.18 | 1.10 | 1.24 |
| t10 | min:s | 3:53 | 4:00 | 3:40 |
| t90 | min:s | 7:17 | 7:27 | 7:11 |
| Compound | Unit | TDAE Oil | N-LqBR | Amino-LqBR |
|---|---|---|---|---|
| Weight loss after the extraction | % | 7.06 | 6.38 | 5.22 |
| Weight loss after the extraction in 5 phr of oil and LqBR | % | 100 | 77 | 37 |
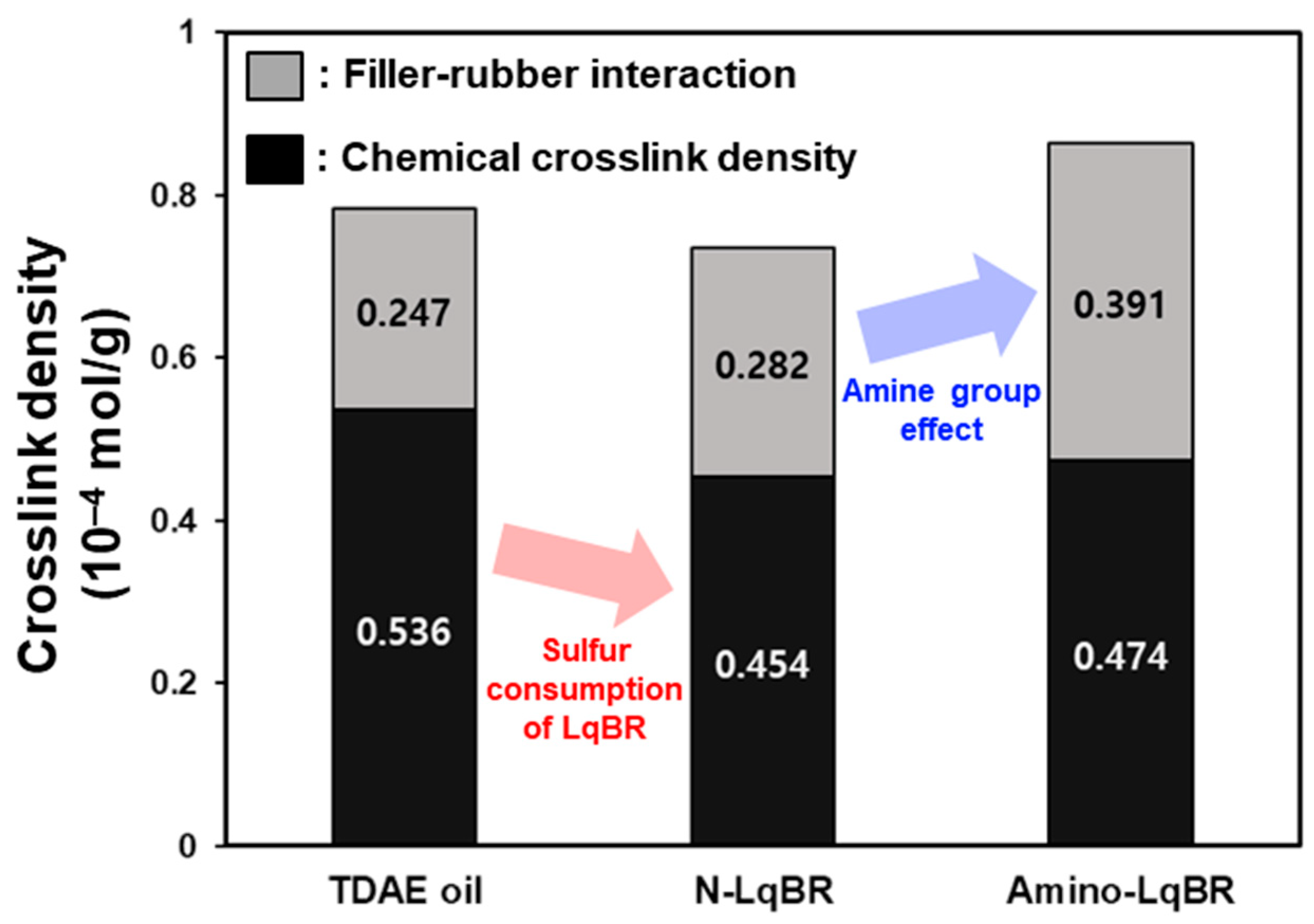
| Total crosslink density | 10−4 mol/g | 0.783 | 0.736 | 0.865 |
| Chemical crosslink density | 10−4 mol/g | 0.536 | 0.454 | 0.474 |
| Filler–rubber interaction | 10−4 mol/g | 0.247 | 0.282 | 0.391 |
| Compound | Unit | TDAE Oil | N-LqBR | Amino-LqBR |
|---|---|---|---|---|
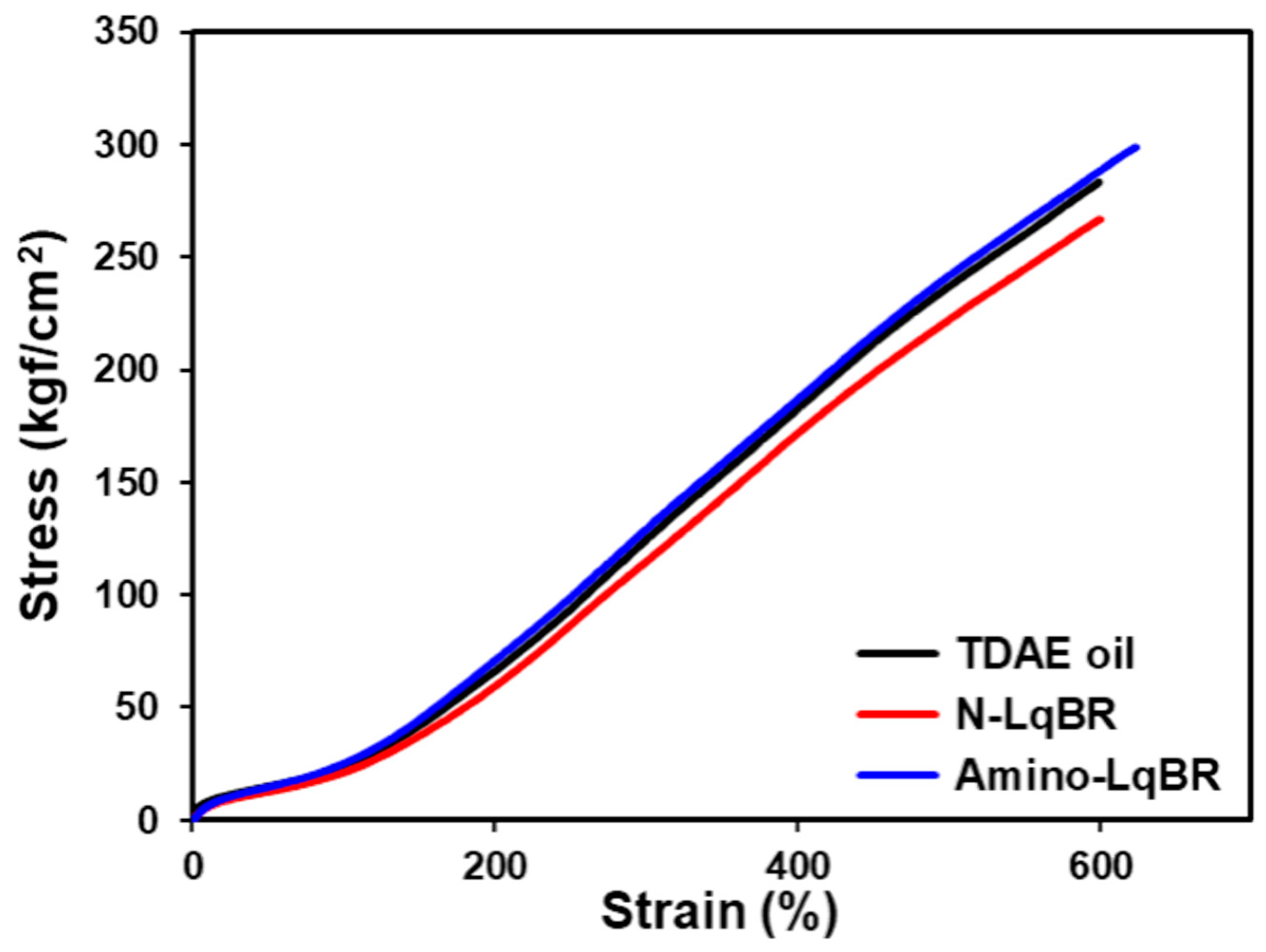
| M100 | kgf/cm2 | 24 | 21 | 25 |
| M300 | kgf/cm2 | 124 | 115 | 129 |
| Elongation at break | % | 599 | 600 | 624 |
| Tensile strength | kgf/cm2 | 283 | 267 | 299 |
| DIN abrasion loss | mg | 88 | 84 | 80 |
| Compound | Unit | TDAE Oil | N-LqBR | Amino-LqBR |
|---|---|---|---|---|
| Number of free chain ends | - | N/A | 2 | 0 |
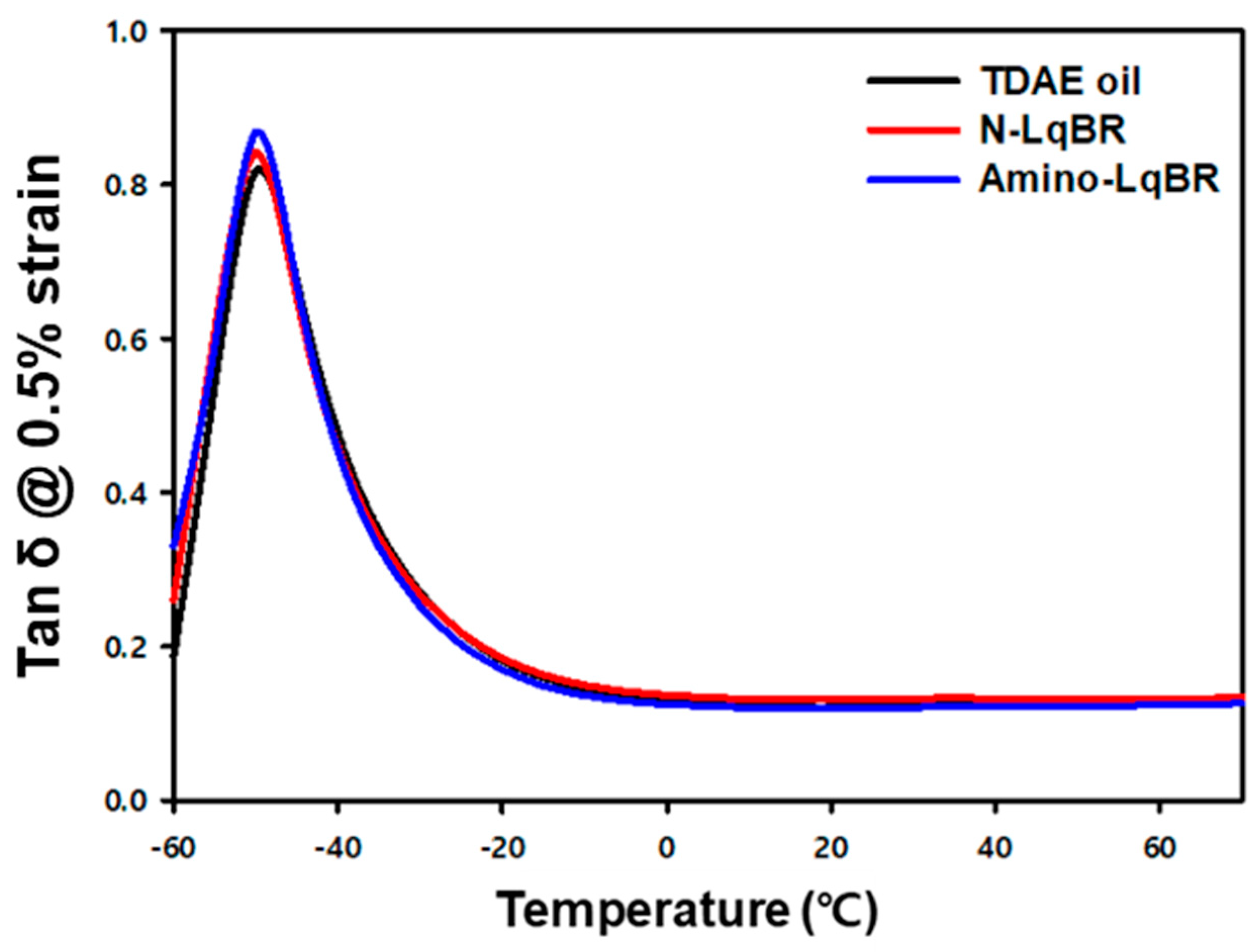
| Tg | °C | −49.2 | −50.1 | −49.7 |
| Tan δ at Tg | - | 0.822 | 0.848 | 0.867 |
| Tan δ at 60 °C (0.5% strain, temperature sweep) | - | 0.124 | 0.132 | 0.124 |
| Tan δ at 60 °C (5% strain, strain sweep) | - | 0.230 | 0.225 | 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Yeom, G.; Kim, D.; Ryu, G.; Hwang, K.; Ahn, B.; Choi, H.; Paik, H.-J.; Chung, S.; Kim, W. Effects of the Diamine Chain End Functionalized Liquid Butadiene Rubber as a Processing Aid on the Properties of Carbon-Black-Filled Rubber Compounds. Polymers 2022, 14, 3343. https://doi.org/10.3390/polym14163343
Song S, Yeom G, Kim D, Ryu G, Hwang K, Ahn B, Choi H, Paik H-J, Chung S, Kim W. Effects of the Diamine Chain End Functionalized Liquid Butadiene Rubber as a Processing Aid on the Properties of Carbon-Black-Filled Rubber Compounds. Polymers. 2022; 14(16):3343. https://doi.org/10.3390/polym14163343
Chicago/Turabian StyleSong, Sanghoon, Gyeongdong Yeom, Donghyuk Kim, Gyeongchan Ryu, Kiwon Hwang, Byungkyu Ahn, Haeun Choi, Hyun-Jong Paik, Sungwook Chung, and Wonho Kim. 2022. "Effects of the Diamine Chain End Functionalized Liquid Butadiene Rubber as a Processing Aid on the Properties of Carbon-Black-Filled Rubber Compounds" Polymers 14, no. 16: 3343. https://doi.org/10.3390/polym14163343
APA StyleSong, S., Yeom, G., Kim, D., Ryu, G., Hwang, K., Ahn, B., Choi, H., Paik, H.-J., Chung, S., & Kim, W. (2022). Effects of the Diamine Chain End Functionalized Liquid Butadiene Rubber as a Processing Aid on the Properties of Carbon-Black-Filled Rubber Compounds. Polymers, 14(16), 3343. https://doi.org/10.3390/polym14163343








