Characterisation of White Metakaolin-Based Geopolymers Doped with Synthetic Organic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
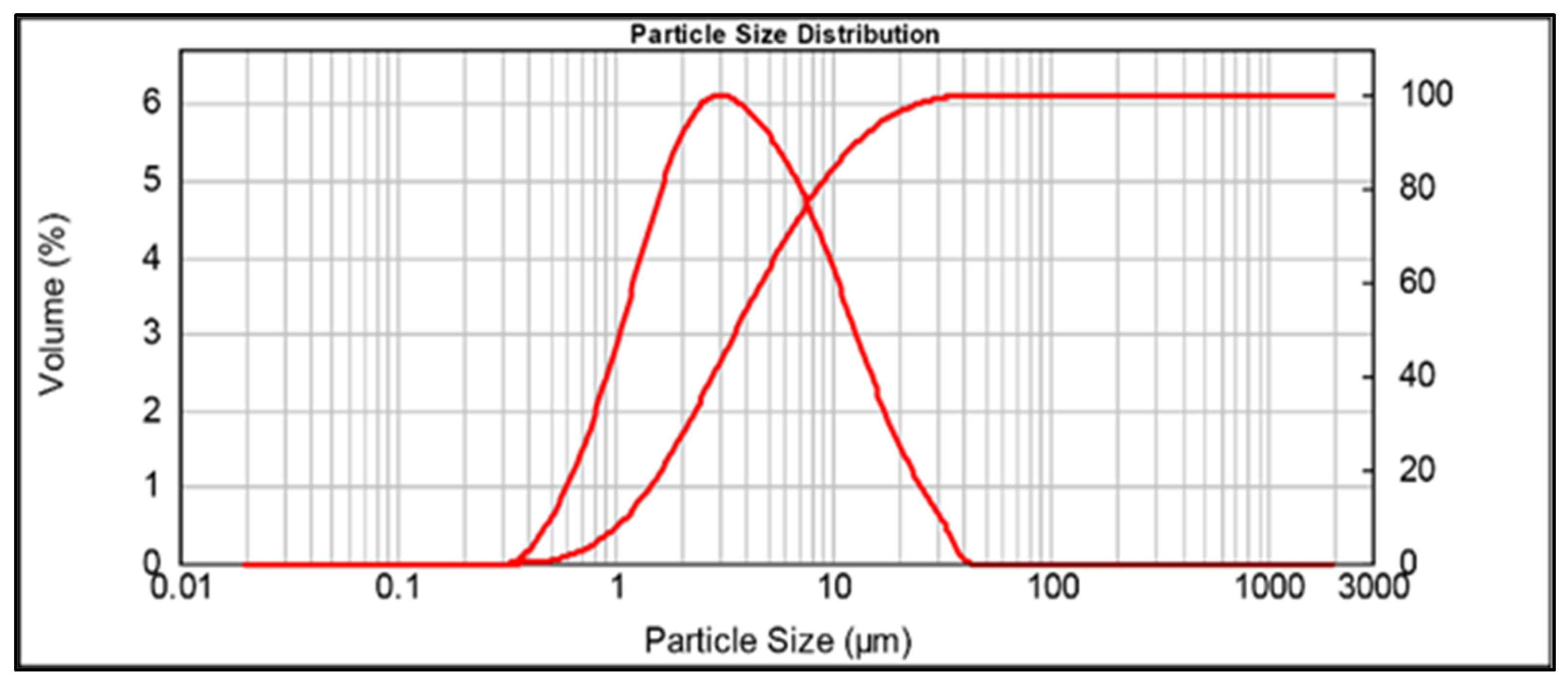
2.1.1. Metakaolin (MK)
2.1.2. Alkaline Solutions
2.1.3. Organic Dyes
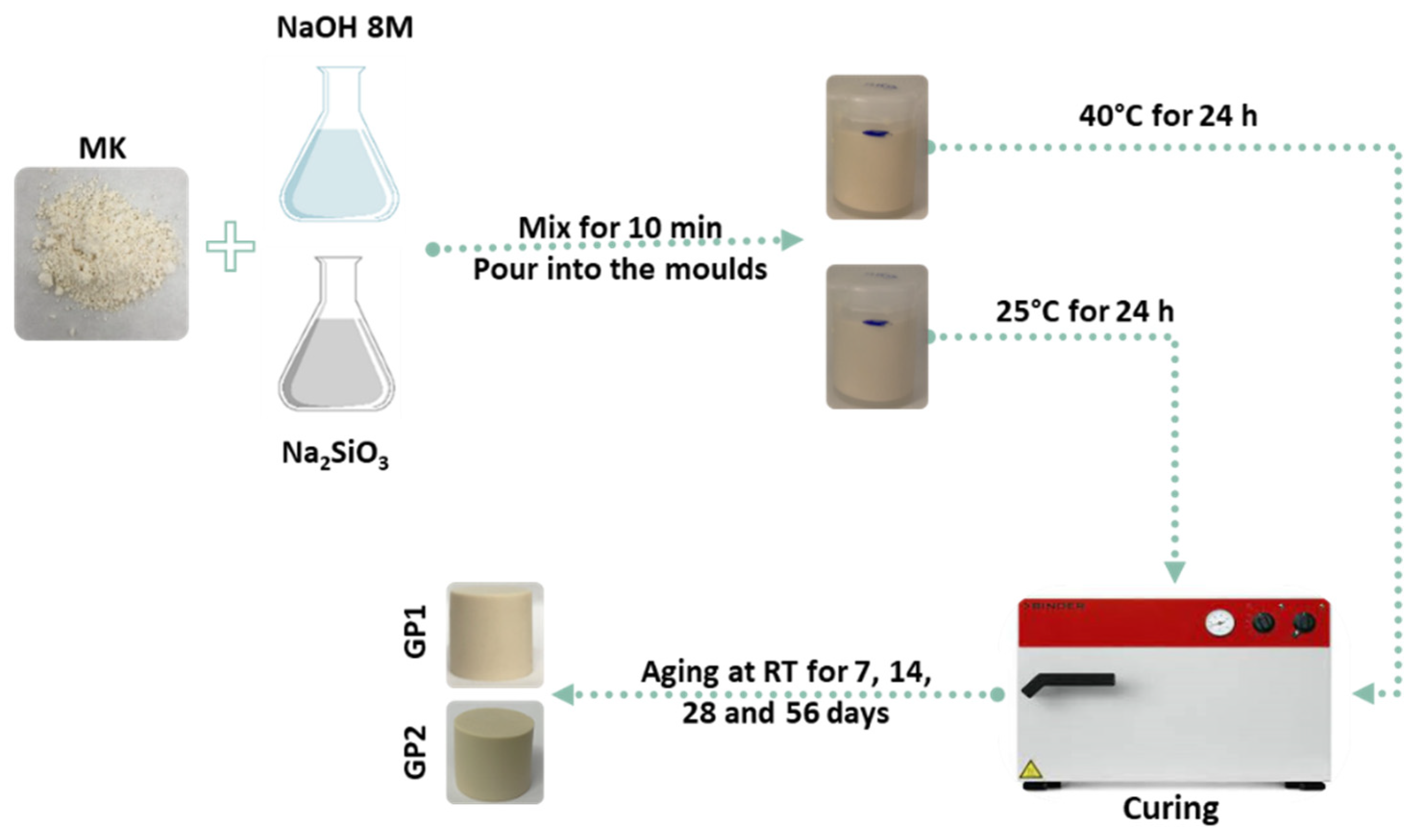
2.2. Geopolymers Synthesis: White and Coloured Samples
2.3. Geopolymer Characterisation
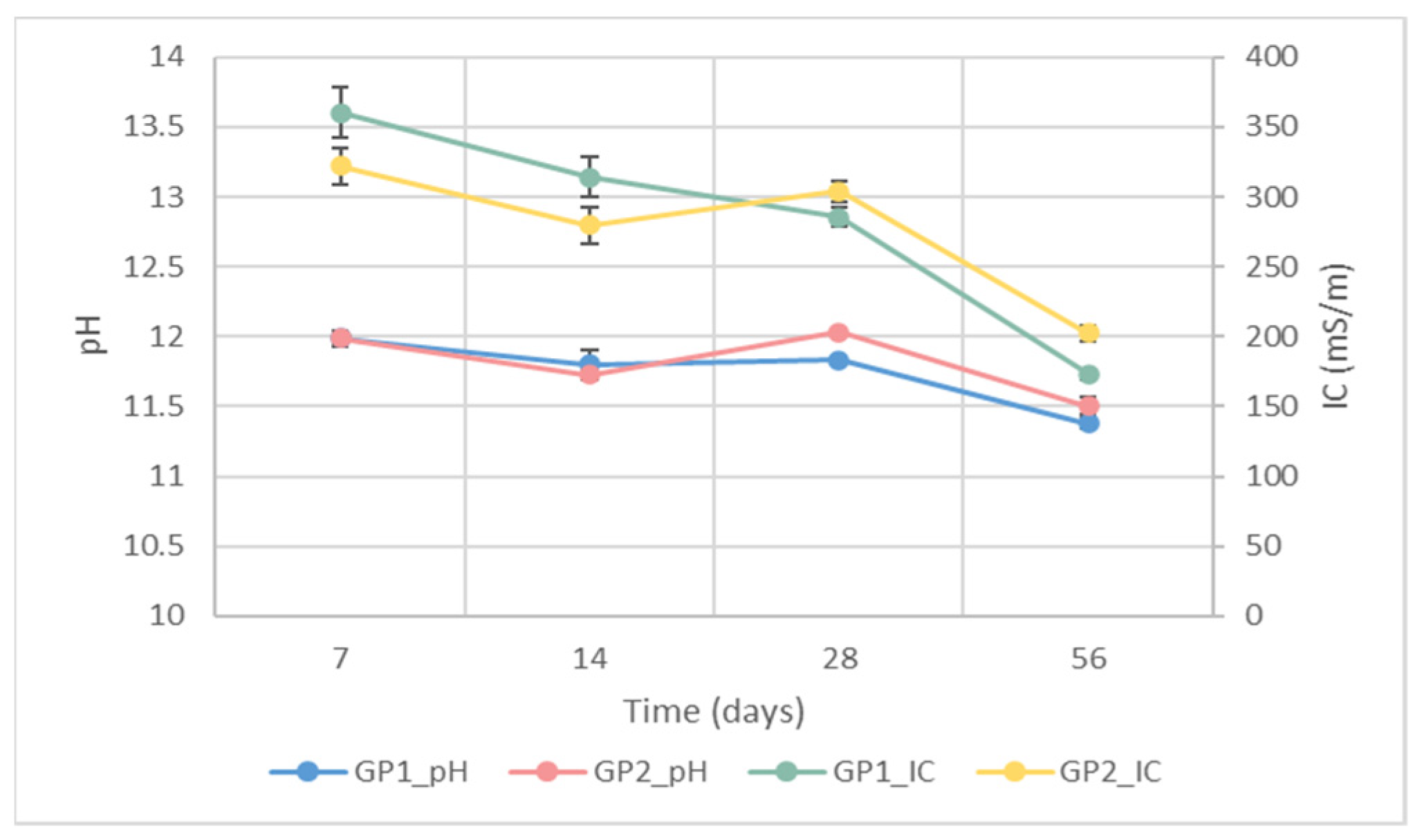
2.3.1. Ionic Conductivity and pH
2.3.2. Integrity Tests and Colour Evaluation
2.3.3. FT-IR Analysis
2.3.4. Leaching Test
3. Results and Discussion
3.1. Characterisation of Geopolymer Samples
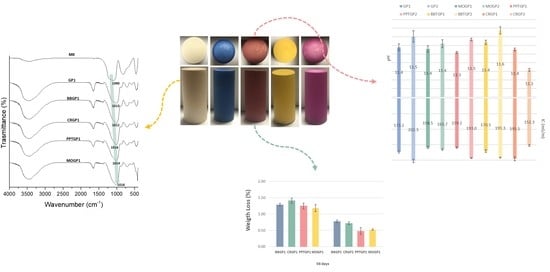
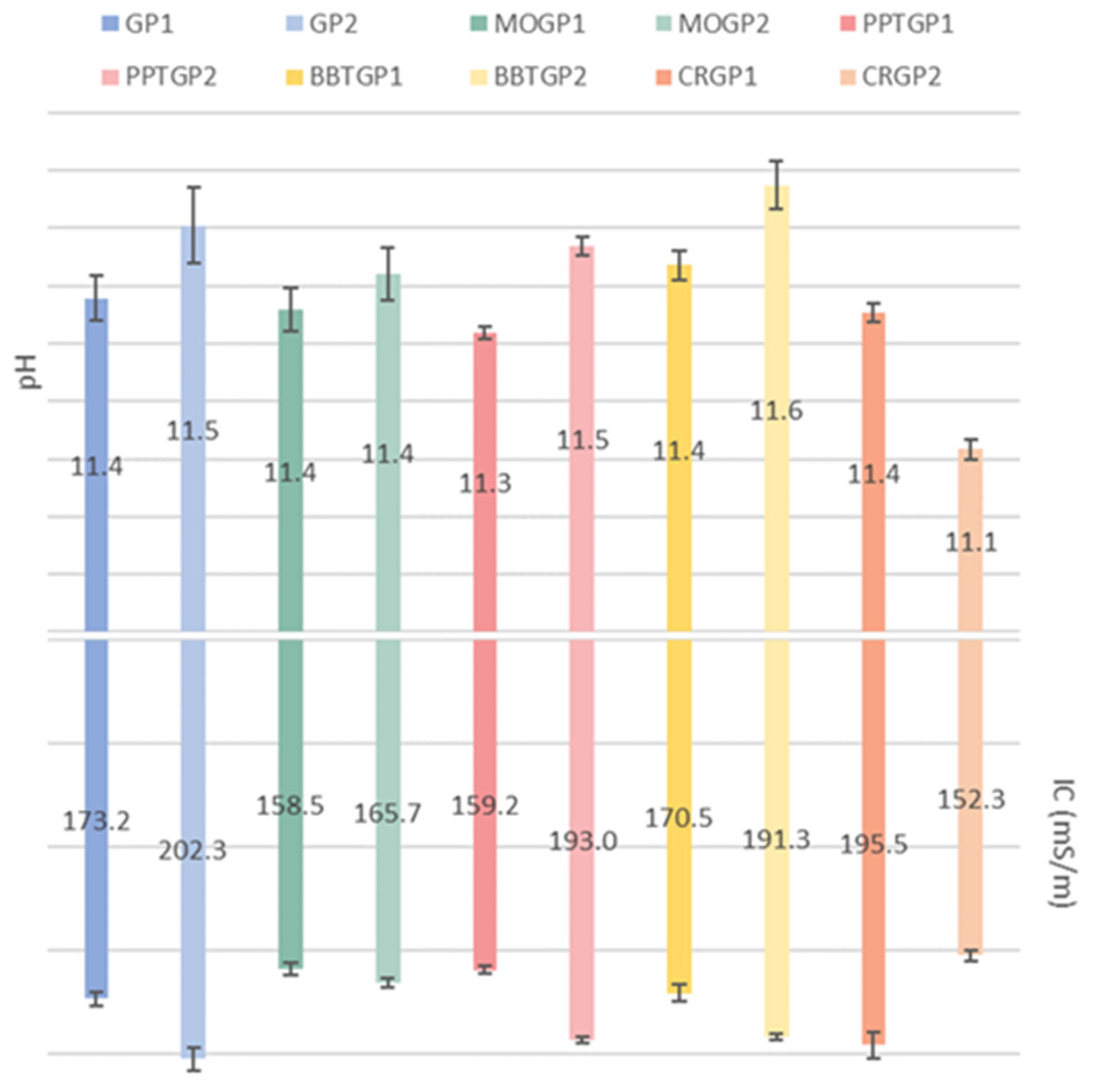
3.1.1. Ionic Conductivity and pH Measurements
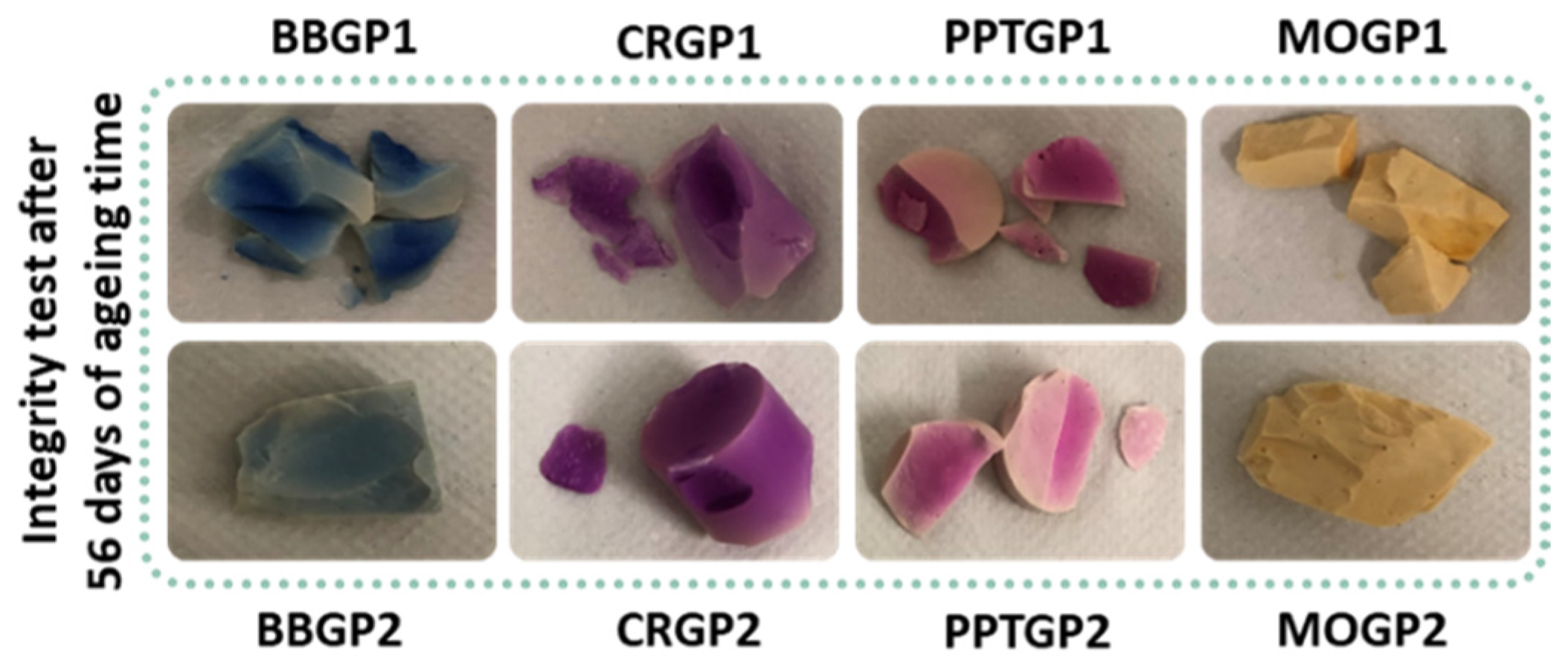
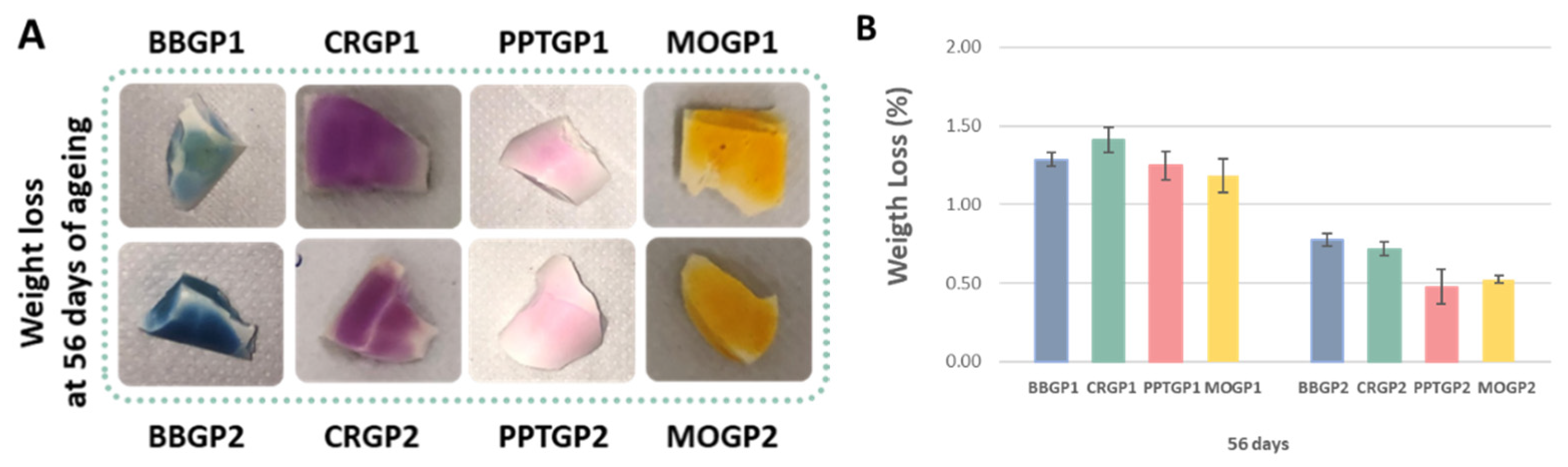
3.1.2. Integrity and Weight Loss Tests
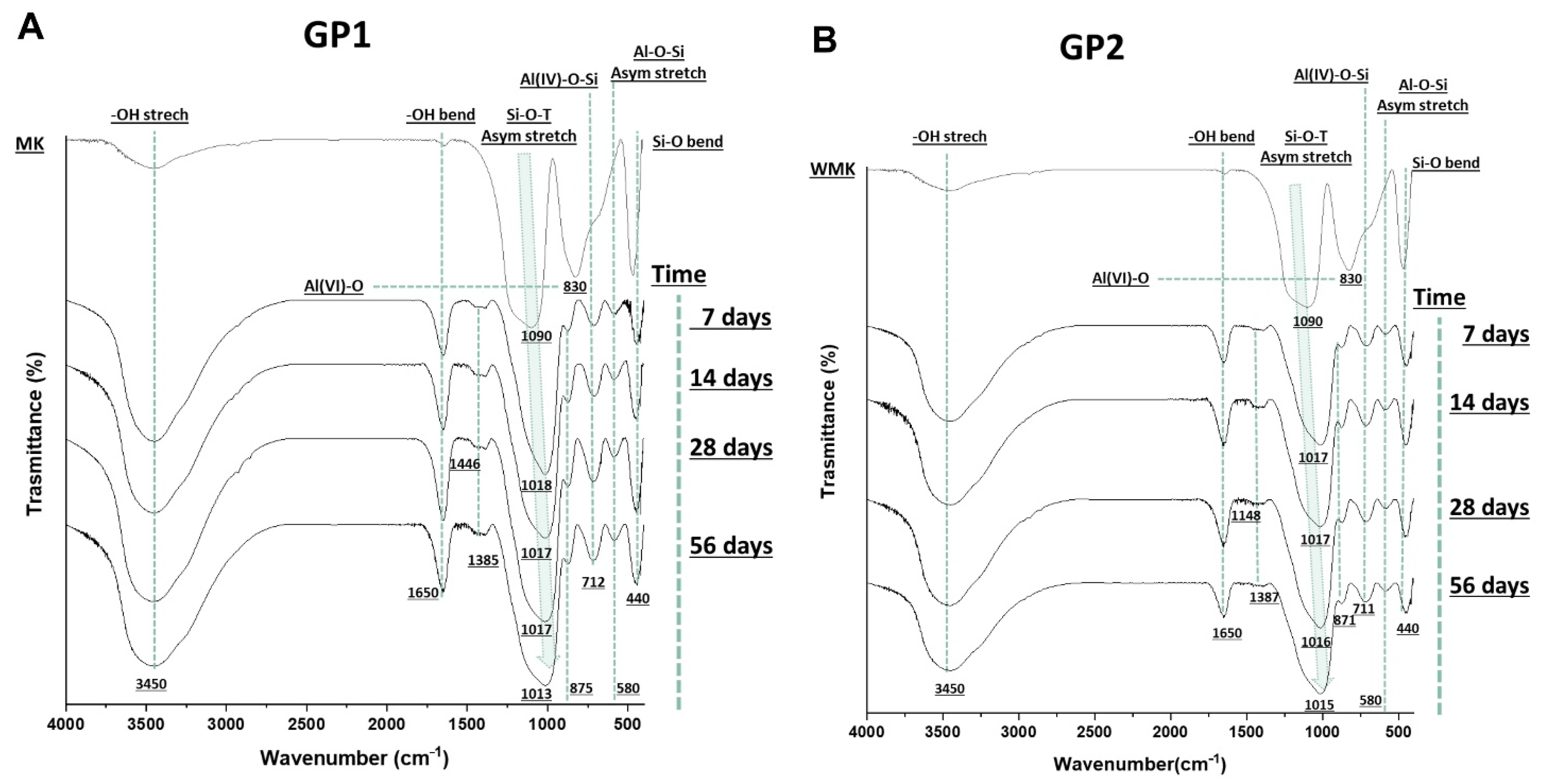
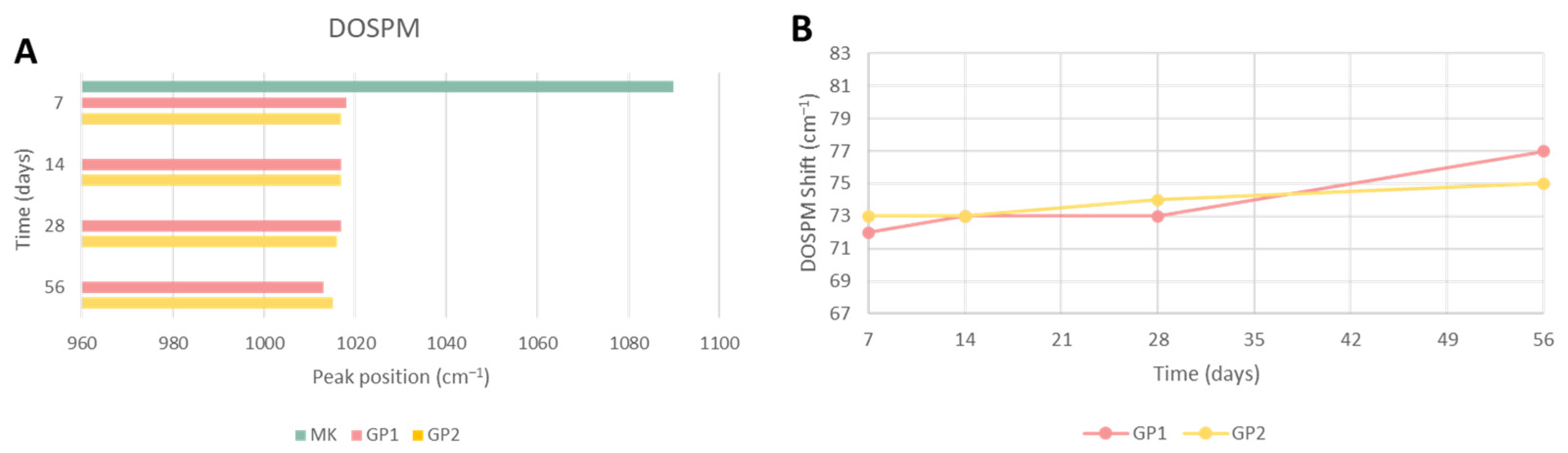
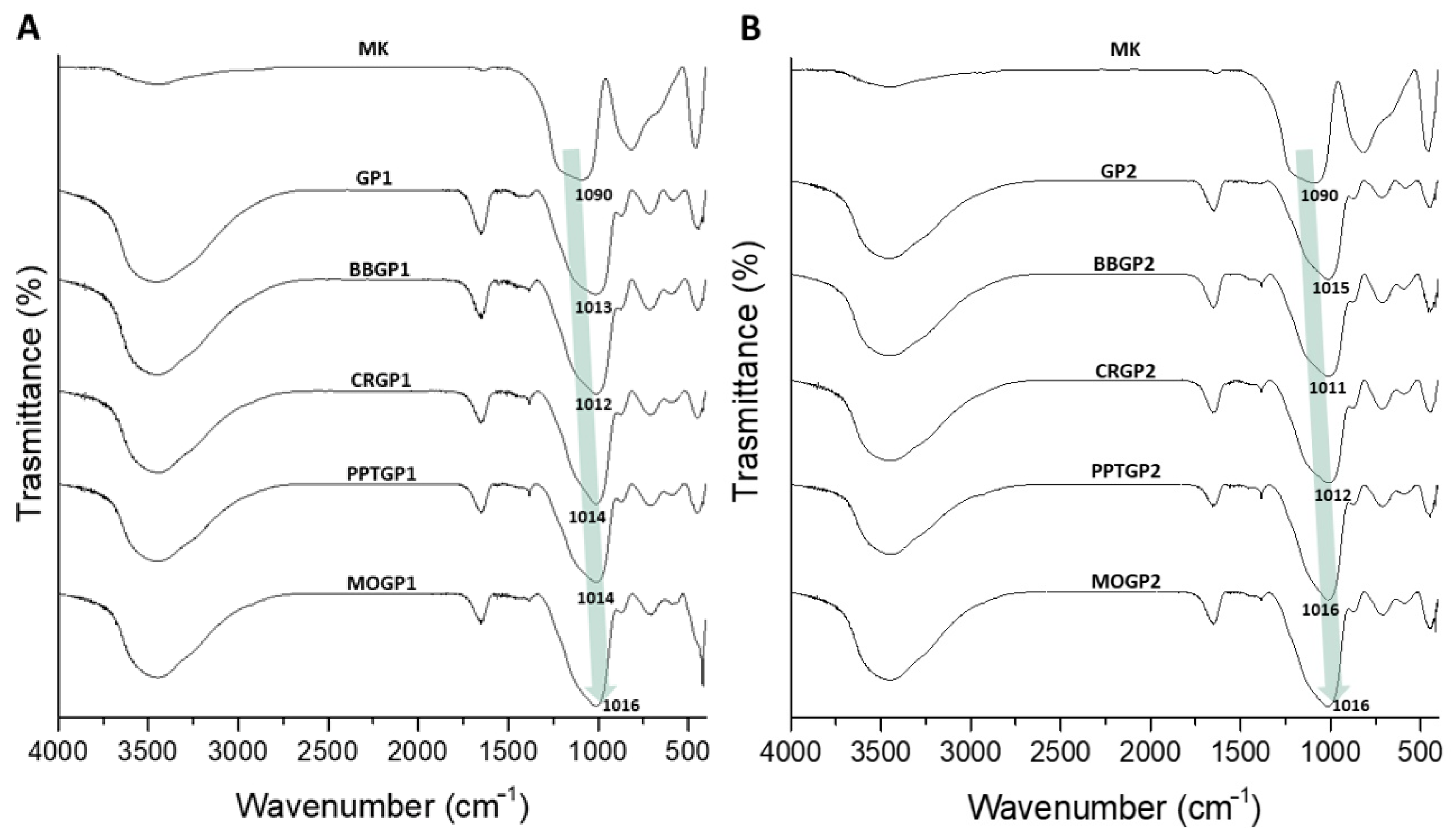
3.1.3. FT-IR Analysis
3.1.4. Leaching Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouellet-Plamondon, C.; Aranda, P.; Favier, A.; Habert, G.; van Damme, H.; Ruiz-Hitzky, E. The Maya Blue Nanostructured Material Concept Applied to Colouring Geopolymers. RSC Adv. 2015, 5, 98834–98841. [Google Scholar] [CrossRef]
- Dal Poggetto, G.; D’Angelo, A.; Catauro, M.; Barbieri, L.; Leonelli, C. Recycling of Waste Corundum Abrasive Powder in MK-Based Geopolymers. Polymers 2022, 14, 2173. [Google Scholar] [CrossRef] [PubMed]
- Sgarlata, C.; Formia, A.; Ferrari, F.; Leonelli, C. Effect of the Introduction of Reactive Fillers and Metakaolin in Waste Clay-Based Materials for Geopolymerization Processes. Molecules 2021, 26, 1325. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Turk, J.; Poggetto, G.D.; Catauro, M.; Traven, K.; Mauko Pranjić, A.; Ducman, V. Environmental and Biological Impact of Fly Ash and Metakaolin-Based Alkali-Activated Foams Obtained at 70 °C and Fired at 1000 °C. Front. Chem. 2022, 10, 845452. [Google Scholar] [CrossRef] [PubMed]
- Sgarlata, C.; Formia, A.; Siligardi, C.; Ferrari, F.; Leonelli, C. Mine Clay Washing Residues as a Source for Alkali-Activated Binders. Materials 2021, 15, 83. [Google Scholar] [CrossRef]
- Banerjee, A.; Guru Prathap Reddy, V.; Kumar Pancharathi, R.; Tadepalli, T. Colour-Stability Analysis for Estimation of Deterioration in Concrete Due to Chemical Attack. Constr. Build. Mater. 2022, 321, 126288. [Google Scholar] [CrossRef]
- Hameed, M.M.; Ali, M.A. Using of metakaolin to produce colored geopolymer concrete. J. Phys. Conf. Ser. 2021, 2114, 012018. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; O’Leary, B. Inorganic polymers (geopolymers) containing acid–base indicators as possible colour-change humidity indicators. Mater. Lett. 2009, 63, 230–232. [Google Scholar] [CrossRef]
- Candela, R.G.; Lazzara, G.; Piacente, S.; Bruno, M.; Cavallaro, G.; Badalamenti, N. Conversion of Organic Dyes into Pigments: Extraction of Flavonoids from Blackberries (Rubus ulmifolius) and Stabilization. Molecules 2021, 26, 6278. [Google Scholar] [CrossRef] [PubMed]
- EN 12457-2:2002; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/Kg for Materials with Particle Size below 4 mm (without or with Size Reduction). European Committee for Standardization-CEN: Brussels, Belgium, 2002.
- ISO 11885:2007; Water Quality. Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007.
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer Binders from Metakaolin Using Sodium Waterglass from Waste Glass and Rice Husk Ash as Alternative Activators: A Comparative Study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic Polymers from Alkali Activation of Metakaolin: Effect of Setting and Curing on Structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of Synthesis Parameters on the Mechanical Properties of Fly Ash-Based Geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Eckhard, D.; Zhang, Z.; Provis, J.L.; Kirchheim, A.P. Metakaolin-Based Geopolymers: Efflorescence and Its Effect on Microstructure and Mechanical Properties. Ceram. Int. 2022, 48, 2212–2229. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Identification of Silicooxygen Rings in SiO2 Based on IR Spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 1819–1823. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W.; Galuskin, E.; Galuskina, I. The Non-Ring Cations Influence on Silicooxygen Ring Vibrations. J. Mol. Struct. 2000, 555, 357–362. [Google Scholar] [CrossRef]
- Dal Poggetto, G.; Catauro, M.; Crescente, G.; Leonelli, C. Efficient Addition of Waste Glass in MK-Based Geopolymers: Microstructure, Antibacterial and Cytotoxicity Investigation. Polymers 2021, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Abdollahnejad, Z.; Dalvand, A.; Mastali, M.; Luukkonen, T.; Illikainen, M. Effects of Waste Ground Glass and Lime on the Crystallinity and Strength of Geopolymers. Mag. Concr. Res. 2019, 71, 1218–1231. [Google Scholar] [CrossRef]
- Gougazeh, M. Geopolymers from Jordanian Metakaolin: Influence of Chemical and Mineralogical Compositions on the Development of Mechanical Properties. Jordan J. Civ. Eng. 2013, 7, 236–257. [Google Scholar]






| SiO2 | Al2O3 | TiO2 | Fe2O3 | K2O | V2O5 | SO3 | CaO | ZrO2 | Cr2O3 | ZnO | CuO | PbO | MnO | NiO | MoO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52–54% | 37–44% | 2.5–7.6% | <0.5% | <0.5% | <0.3% | <0.2% | <0.2% | <0.1% | <0.1% | <0.1% | <0.05% | <0.05% | <0.05% | <0.05% | 0.03% |
| A | B | ||
|---|---|---|---|
| Sample | ∆E | Sample | ∆E |
| GP1 | 0 | GP2 | 0 |
| MOGP1 | 44.55 | MOGP2 | 41.31 |
| PPTGP1 | 47.46 | PPTGP2 | 42.37 |
| CRGP1 | 49.32 | CRGP2 | 64.71 |
| BBGP1 | 57.89 | BBGP2 | 56.44 |
| A | B | ||
|---|---|---|---|
| Sample | ∆E | Sample | ∆E |
| GP1 | 0 | GP2 | 0 |
| MOGP1 | 48.14 | MOGP2 | 48.27 |
| PPTGP1 | 70.09 | PPTGP2 | 52.54 |
| CRGP1 | 67.37 | CRGP2 | 59.30 |
| BBGP1 | 61.44 | BBGP2 | 57.47 |
| Samples | Test Parameters | 7 Days | 14 Days | 28 Days | 56 Days |
|---|---|---|---|---|---|
| GP1 | pH | 10.2 * | 9.00 * | 10.5 * | 9.8 * |
| Resistance | No | Yes | Yes | Yes | |
| Water | Clear | Clear | Clear | Clear | |
| GP2 | pH | 10.4 * | 9.3 * | 10 * | 10.1 * |
| Resistance | Yes | Yes | Yes | Yes | |
| Water | Clear | Clear | Clear | Clear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, A.; Dal Poggetto, G.; Piccolella, S.; Leonelli, C.; Catauro, M. Characterisation of White Metakaolin-Based Geopolymers Doped with Synthetic Organic Dyes. Polymers 2022, 14, 3380. https://doi.org/10.3390/polym14163380
D’Angelo A, Dal Poggetto G, Piccolella S, Leonelli C, Catauro M. Characterisation of White Metakaolin-Based Geopolymers Doped with Synthetic Organic Dyes. Polymers. 2022; 14(16):3380. https://doi.org/10.3390/polym14163380
Chicago/Turabian StyleD’Angelo, Antonio, Giovanni Dal Poggetto, Simona Piccolella, Cristina Leonelli, and Michelina Catauro. 2022. "Characterisation of White Metakaolin-Based Geopolymers Doped with Synthetic Organic Dyes" Polymers 14, no. 16: 3380. https://doi.org/10.3390/polym14163380