Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine
Abstract
:1. Introduction
- (1)
- obtaining collagen–chitosan composite biomaterials in the absence or the presence of an antibiotic reagent, with potential use in regenerative medicine;
- (2)
- characterization of the morphology of the composite biomaterials obtained, compared to the biomaterials resulting from raw materials (collagen and chitosan);
- (3)
- highlighting the structural characteristics of the obtained biomaterials (highlighting the functional chemical groups, the interactions between molecules, and the triple helix structure from collagen in simple or composite biomaterials;
- (4)
- evaluation of the antimicrobial properties of the simple or composite biomaterials;
- (5)
- evaluation of the cytotoxicity of the obtained simple biomaterials/composite biomaterials;
- (6)
- selection of composite biomaterial(s) with potential applications in regenerative medicine.
2. Materials and Methods
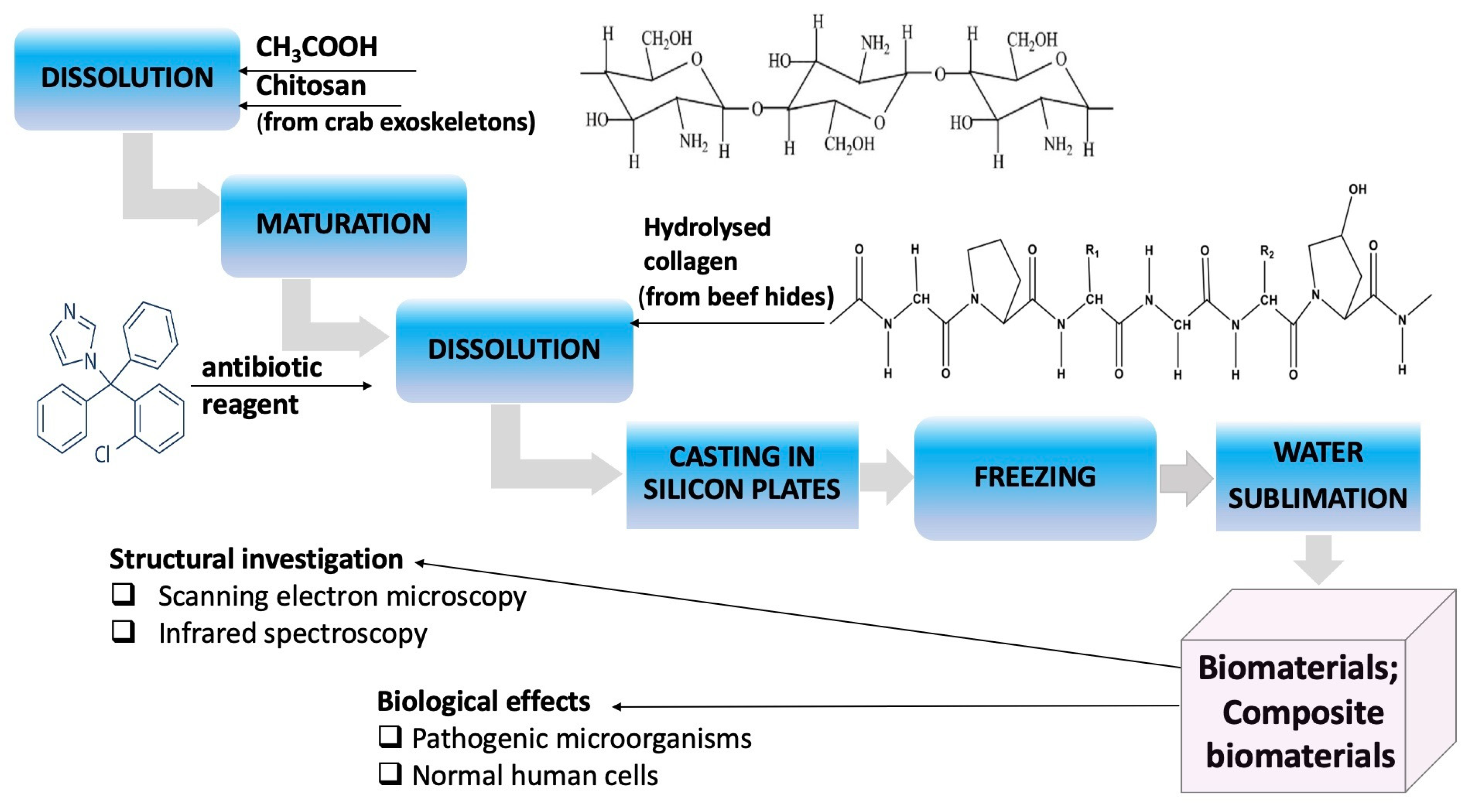
2.1. Obtaining Collagen Composite Materials: Chitosan: Clotrimazole
2.1.1. Source of Chitosan
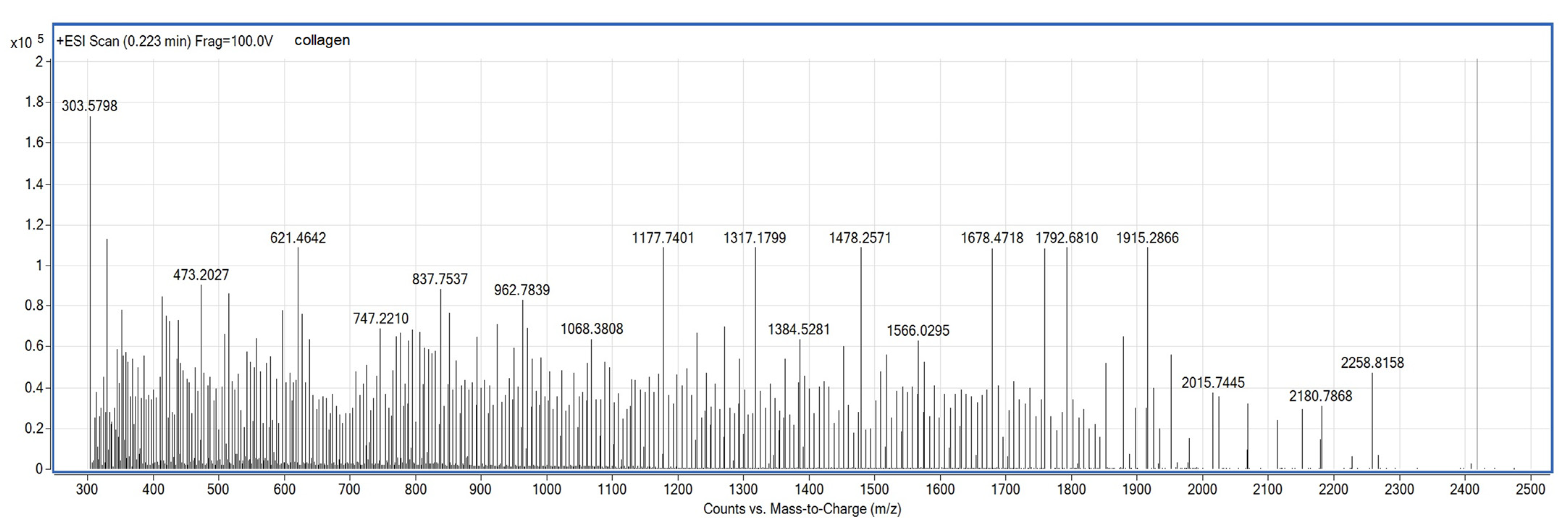
2.1.2. Source of Collagen
2.1.3. Functionalization Reagent
2.1.4. Obtaining Composite Biomaterials with Collagen-Chitosan-Clotrimazole
2.2. Scanning Electron Microscopy Analysis
2.3. Infrared Spectra Analysis
2.4. Antimicrobial Activities
2.5. Cell Proliferation Assay
3. Results
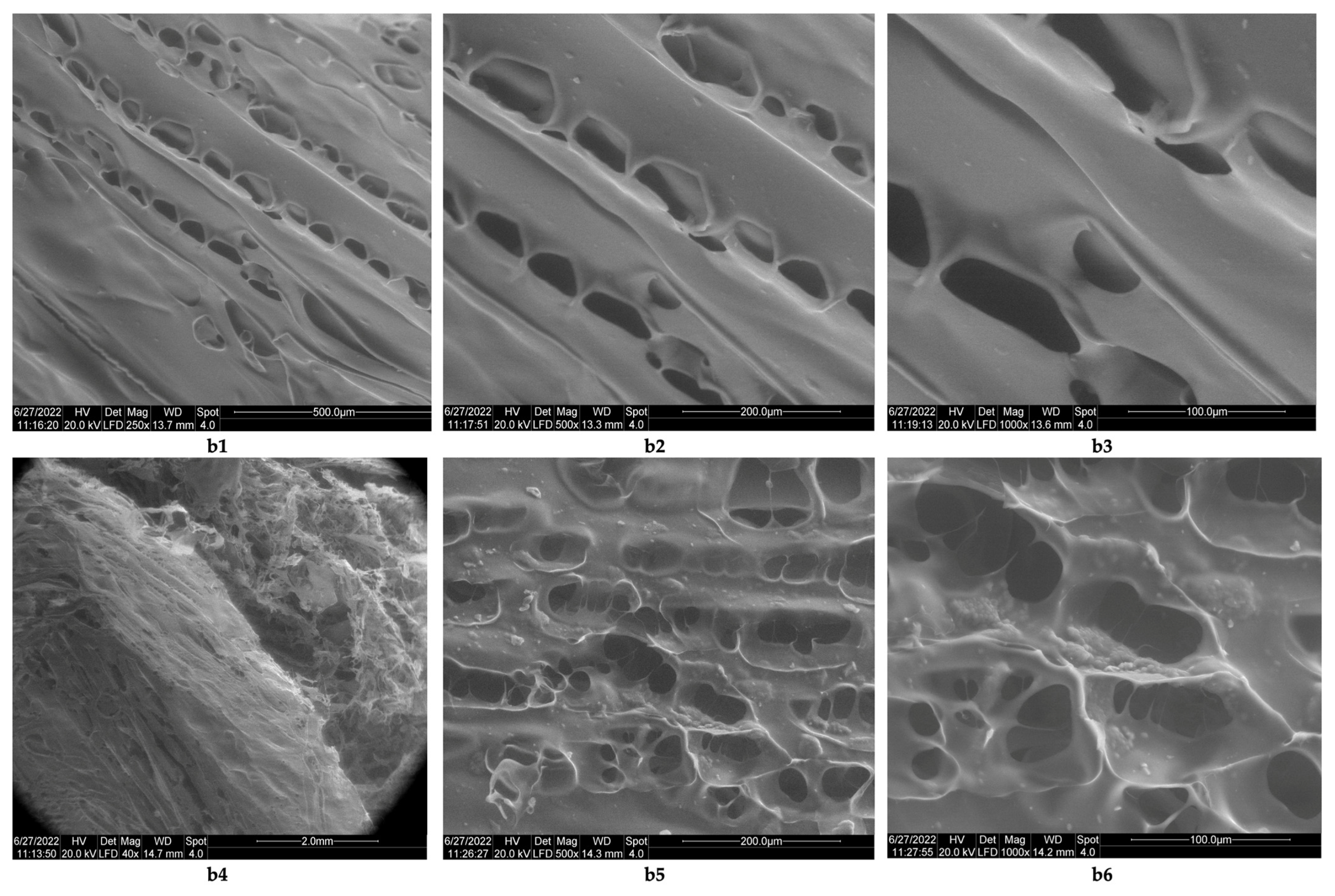
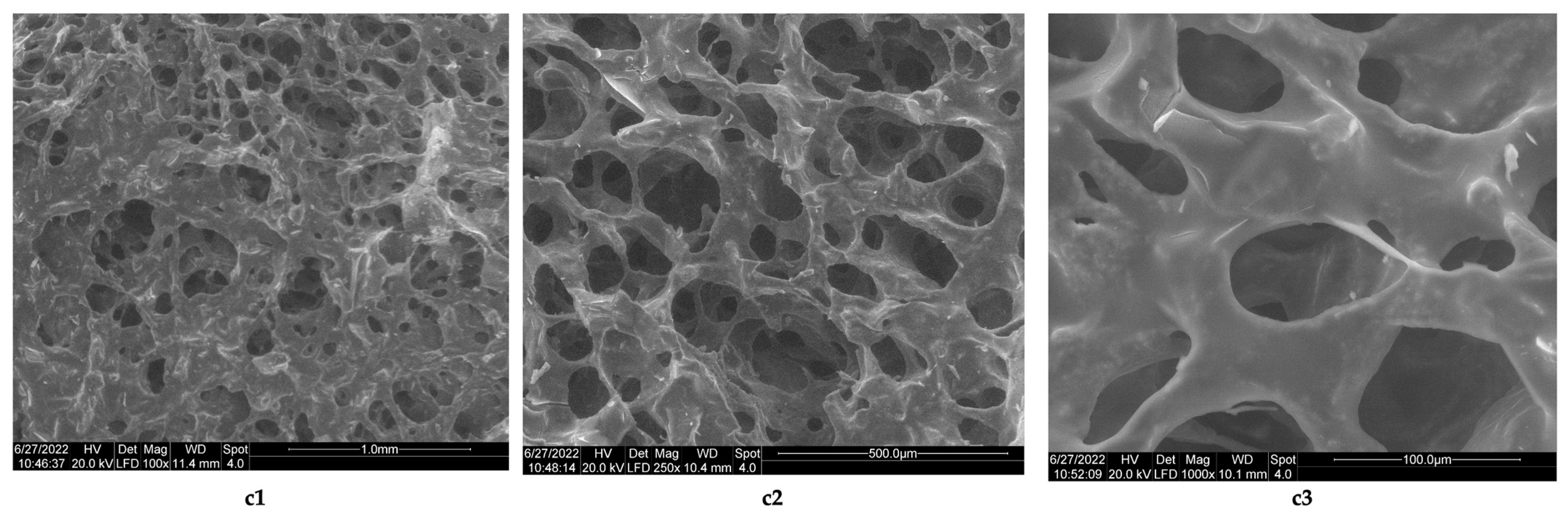
3.1. Morphology Analysis
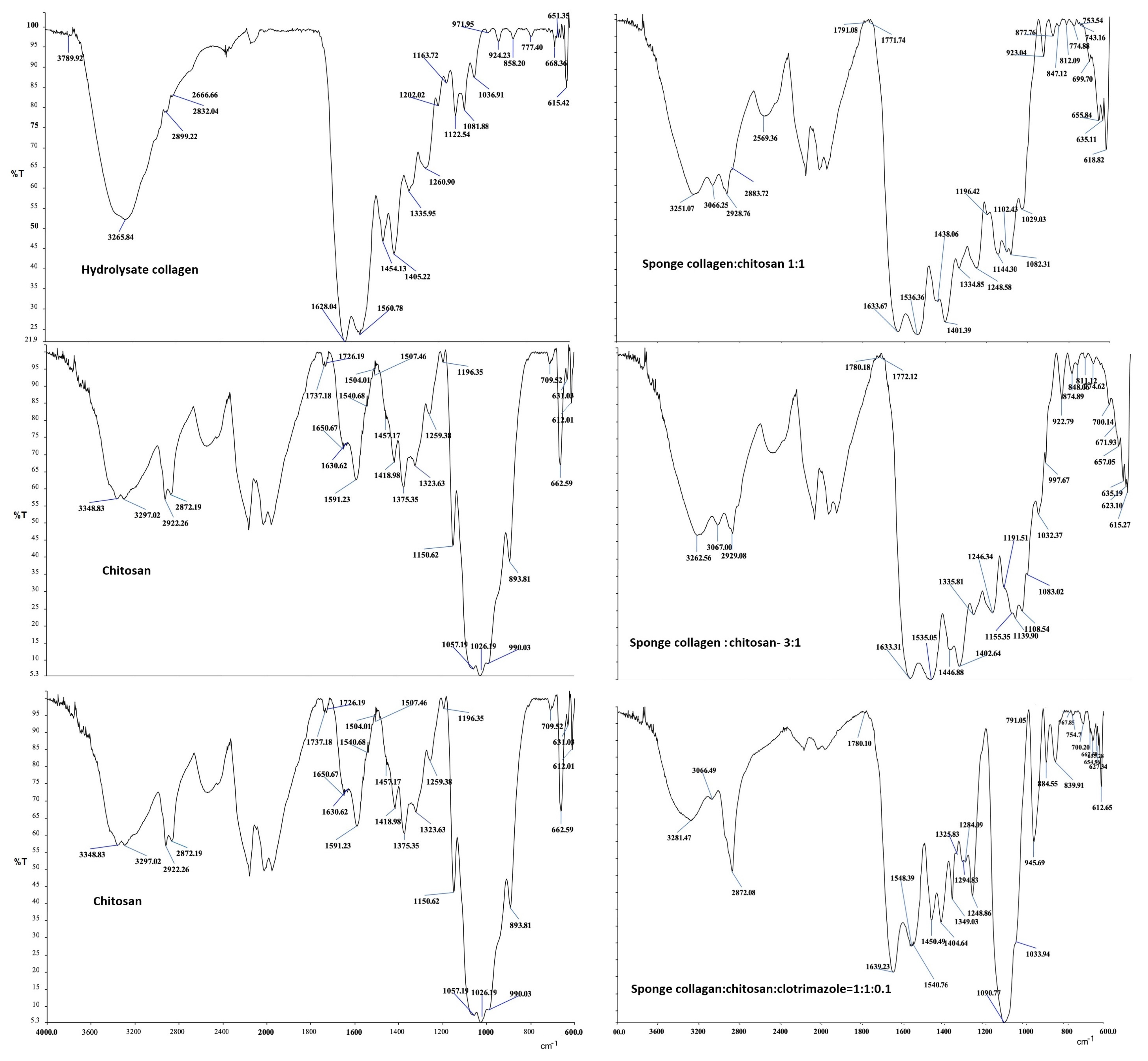
3.2. Infrared Spectra Analysis
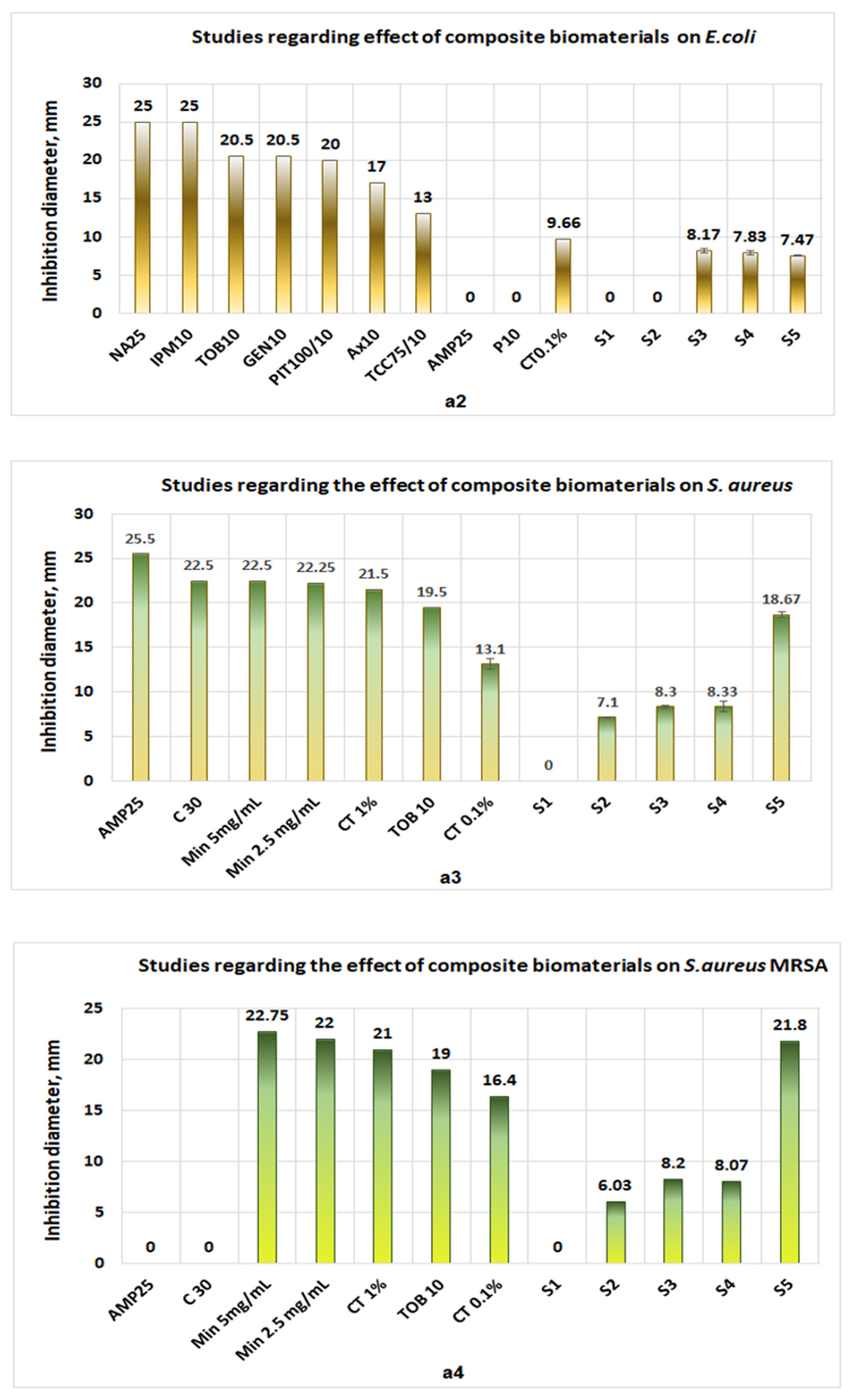
3.3. Antimicrobial Activity
- (1)
- Collagen sponges (S1) do not exhibit antimicrobial activities for either microorganism (Figure 11a1–a4);
- (2)
- The sponges of chitosan (S2) and composite biomaterials which contain only chitosan (S2), only collagen–chitosan 1:1 (S3), collagen–chitosan 3:1 (S4), and collagen–chitosan–clotrimazole 1:1:0.1 (S4) do not exhibit antimicrobial activities against P. aeruginosa;
- (3)
- Regarding Candida albicans, the best activities were obtained for composite biomaterials obtained in the presence of clotrimazole (S3), giving an inhibition diameter of 23.9 mm (Figure 11a1), whereas the biomaterials S2, S3, and S4 show poor antimicrobial activity (diameter of inhibition under 6.3 mm) on this fungus.

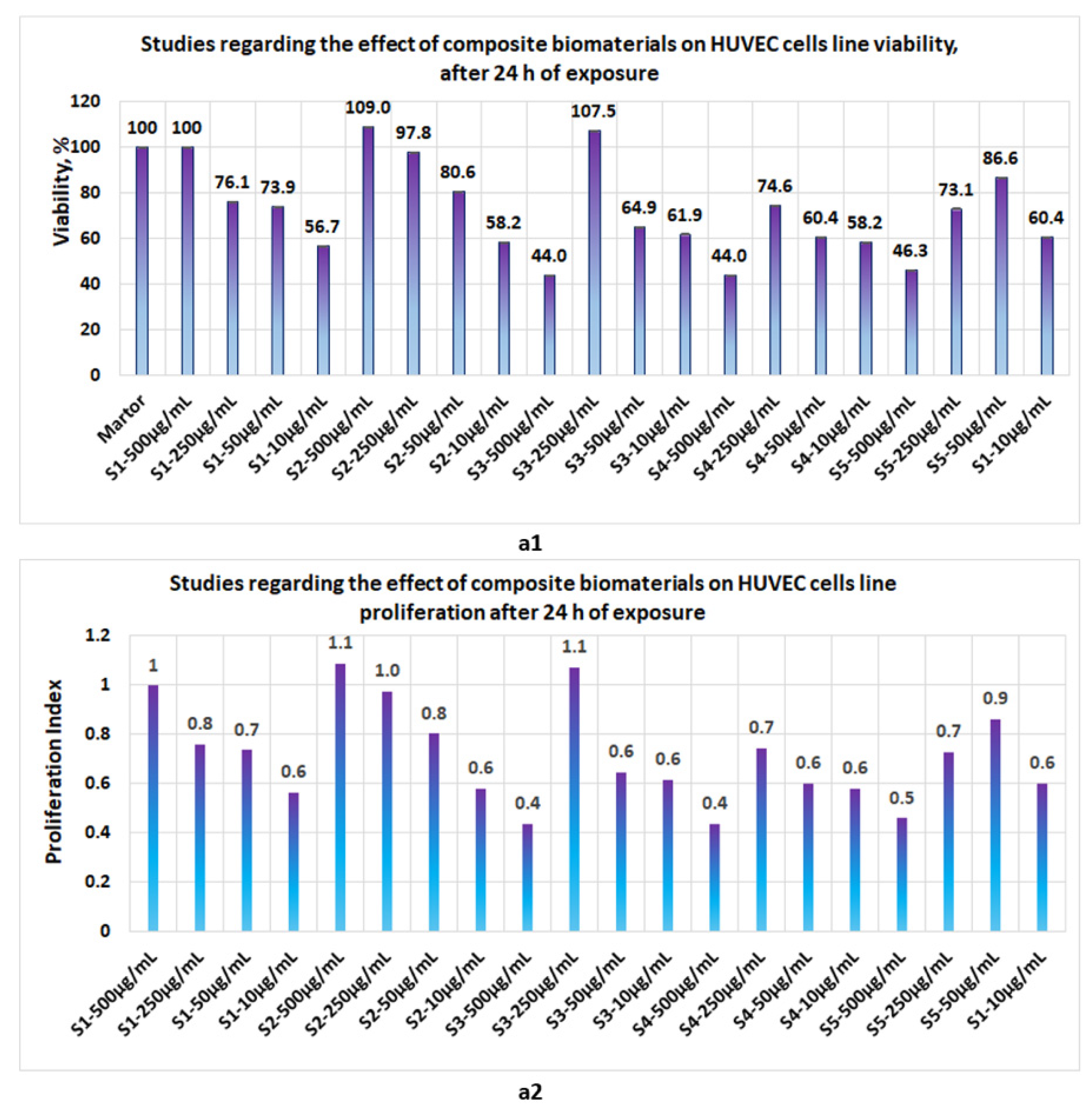
3.4. Proliferation Studies
- -
- At 24 h, the presence of sponges with chitosan or collagen appears to stimulate the cell viability, so that at 500 μg/mL, in the case of S1, the cell viability attains 100% and, in the case of chitosan, it attains 109%. The cell viability increases proportionally with the concentration of S1 and S2 in the culture media. At the low concentrations of S1 and S2, cell viability attains 56.7% and 44%, respectively (Figure 12a1). The proliferation index obtained at the maximum concentration in the case of S1 and S2 attains a value = 1 and, respectively, 1.1, whereas at the low concentrations (10 μg/mL), the PI attains 0.6 (Figure 12a2). In the case of composite biomaterials with collagen and chitosan (biomaterials S3 and S4), cell viability attains the maximum value at 250 μg/mL, with 107.5% and 74.6%, respectively, with a corresponding PI of 1.1 and 0.7, respectively. At concentrations under 250 μg/mL, the values of cell viability are less than 64%. In the case of composite biomaterial S5, the maximum value for cell viability is obtained at 50 μg/mL, and the corresponding PI = 0.9;
- -
- -
- Measurements performed after 72 h of exposure (Figure 14c1,c2) show that the cell viability was greater than 93% for all samples except the S3 at c = 500 μg/mL, for which the viability decreased at 57.8%. This data confirm low cytotoxicity for samples tested and are in agreement with data reported by other scientists [35,44,46,47] for composite biomaterials types, such as collagen–biocellulose, chitosan–biocellulose, and collagen–chitosan–biocellulose, with or without the presence of the antibiotic reagent.


4. Discussion
- In the collagen hydrolyzate used, collagen had the triple helix structure, the structure that is also preserved in the composite biomaterials obtained by lyophilization, for which the ratio between the two peaks is slightly shifted to the lower wave numbers, (the obtained values for the three composite biomaterials S4, S5 and S6 are as follows: 3, 2.14, and 1.5) [46,47];
- The theoretical degree of deacetylation of pharmaceutical chitosan calculated with Equation (1) is 65% [10]. Most probably, this degree of deacetylation represents the reason for inhomogeneities that appear in the morphology of the chitosan sponge (Figure 6b6). These inhomogeneities also appear in the morphology of the biomaterial composite of collagen–chitosan–clotrimazole, obtained at a mass ratio of 1:1:0.1 (Figure 9e2);
- In all of the three composite biomaterials, the presence of hydrogen bonds between the chitosan and collagen molecules was highlighted.
5. Conclusions
- (1)
- It exhibits significant antimicrobial activity for gram-positive microorganisms, such as Staphylococcus aureus and, respectively, for antibiotic-resistant microorganisms, such as Staphylococcus aureus MRSA;
- (2)
- It does not exhibit cytotoxicity;
- (3)
- It has a potential for application in regenerative medicine, as a result of its own components (collagen, chitosan, and clotrimazole) that support tissue regeneration; it inhibits the development of pathogenic microorganisms, such as Candida albicans, Staphylococcus aureus, or antibiotic-resistant microorganisms, such as Staphylococcus aureus MRSA.
- Testing the obtained composite biomaterials obtained on different tumor cell lines;
- Carrying out the preclinical tests on the lab animals in order to evaluate the antimicrobial activity “in vivo”;
- Carrying out preclinical tests on lab animals to confirm the absence of cytotoxicity “in vivo”.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| NHEK | Primary Normal Human Epidermal Keratinocytes (NHEK) represent standardized cell lines, isolated from the epidermis of adult skin. |
| NHDF | Normal Human Dermal Fibroblasts (NHDF) represent standardized cell lines, isolated from the dermis of adult skin. |
| Vero cell lines | represent standardized cell lines, the original Vero cell line was established from the kidney of an African monkey. |
| HUVEC | primary Human Umbilical Vein Endothelial Cells (HUVEC), represent a standardized cell lines, isolated from the human umbilical vein. |
References
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Masoud, M. Production of hydrolyzed bovine collagen with orange juice concentrate. Egypt. J. Agric. Res. 2017, 95, 1205–1217. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Mitelut, A.C.; Tanase, E.E.; Popa, V.I.; Popa, M.E. Sustainable alternative for food packaging: Chitosan biopolymer—A review. AgroLife Sci. J. 2015, 4, 52–61. [Google Scholar]
- Rufato, K.B.; Galdino, J.P.; Ody, K.S.; Pereira, A.G.; Corradini, E.; Martins, A.F.; Paulino, A.T.; Fajardo, A.R.; Aouada, F.A.; Porta, F.A.L.; et al. Hydrogels Based on Chitosan and Chitosan Derivatives for Biomedical Applications. In Hydrogels—Smart Materials for Biomedical Applications; Popa, L., Ghica, M.V., Dinu-Pîrvu, C., Eds.; IntechOpen: London UK, 2018. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2019; p. 338. [Google Scholar]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Lopes, L.S.; Michelon, M.; Duarte, L.G.R.; Prediger, P.; Cunha, R.L.; Picone, C.S.F. Effect of chitosan structure modification and complexation to whey protein isolate on oil/water interface stabilization. Chem. Eng. Sci. 2021, 230, 116124. [Google Scholar] [CrossRef]
- Lamarra, J.; Damonte, L.; Rivero, S.; Pinotti, A. Structural Insight into Chitosan Supports Functionalized with Nanoparticles. Adv. Mater. Sci. Eng. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 36005. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure 1 to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Reves, B.T.; Jennings, J.A.; Bumgardner, J.D.; Haggard, W.O. Osteoinductivity Assessment of BMP-2 Loaded Composite Chitosan-Nano-Hydroxyapatite Scaffolds in a Rat Muscle Pouch. Materials 2011, 4, 1360–1374. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Śmieszek, A.; Jarmoluk, A.; Nowak, U.; Marycz, K. Potential Biomedical Application of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges—Biocompatible Scaffolds Inducing Chondrogenic Differentiation of Human Adipose Derived Multipotent Stromal Cells. Polymers 2016, 8, 320. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of Porous Materials from Natural/Synthetic Biopolymers and Their Composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef]
- Paun, I.A.; Popescu, R.C.; Calin, B.S.; Mustaciosu, C.C.; Dinescu, M.; Luculescu, C.R. 3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis. Int. J. Mol. Sci. 2018, 19, 495. [Google Scholar] [CrossRef]
- Bealer, E.J.; Kavetsky, K.; Dutko, S.; Lofland, S.; Hu, X. Protein and Polysaccharide-Based Magnetic Composite Materials for Medical Applications. Int. J. Mol. Sci. 2020, 21, 186. [Google Scholar] [CrossRef]
- de Menezes, F.L.; Andrade Neto, D.M.; Rodrigues, M.d.L.L.; Lima, H.L.S.; Paiva, D.V.M.; da Silva, M.A.S.; Fechine, L.M.U.D.; Sombra, A.S.B.; Freire, R.M.; Denardin, J.C.; et al. From Magneto-Dielectric Biocomposite Films to Microstrip Antenna Devices. J. Compos. Sci. 2020, 4, 144. [Google Scholar] [CrossRef]
- Fiejdasz, S.; Gilarska, A.; Strączek, T.; Nowakowska, M.; Kapusta, C. Magnetic Properties of Collagen—Chitosan Hybrid Materials with Immobilized Superparamagnetic Iron Oxide Nanoparticles (SPIONs). Materials 2021, 14, 7652. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Roffi, A.; Kon, E.; Perdisa, F.; Fini, M.; Di Martino, A.; Parrilli, A.; Salamanna, F.; Sandri, M.; Sartori, M.; Sprio, S.; et al. A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration. Int. J. Mol. Sci. 2019, 20, 2227. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Pin, J.M.; Zasada, L.; Sonne, M.M.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Kleszczyński, K. Assessment of Melatonin-Cultured Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials for Wound Healing. Antioxidants 2022, 11, 570. [Google Scholar] [CrossRef]
- Râpă, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Valencia Zapata, M.E.; Restrepo, Y.J.; Mina Hernandez, J.H.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/Polyvinyl Alcohol/Tea Tree Essential Oil Composite Films for Biomedical Applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Xu, Y.; Xia, J.; Xu, Z.; Zhu, S.; Jin, M. Preparation of Chitosan/Recombinant Human Collagen-Based Photo-Responsive Bioinks for 3D Bioprinting. Gels 2022, 8, 314. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Eufrásio Cruz, M.A.; Aguilar, G.J.; Tapia-Blácido, D.R.; da Silva Ferreira, M.E.; Maniglia, B.C.; Bottini, M.; Ciancaglini, P.; Ramos, A.P. Synthesis of Antibacterial Hybrid Hydroxyapatite/Collagen/Polysaccharide Bioactive Membranes and Their Effect on Osteoblast Culture. Int. J. Mol. Sci. 2022, 23, 7277. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Yao, Z.; Feng, L.; Liu, T.; Wei, S.; Xu, P.; Guo, R.; Cheng, B.; Wang, X. Antibiotic-loaded chitosan-gelatin scaffolds for infected seawater immersion wound healing. Int. J. Biol. Macromol. 2020, 159, 1140–1155. [Google Scholar] [CrossRef]
- Sionkowska, A.; Walczak, M.; Michalska-Sionkowska, M. Preparation and characterization of collagen/chitosan composites with silver nanoparticles. Polym. Compos. 2020, 41, 951–957. [Google Scholar] [CrossRef]
- Tallapaneni, V.; Pamu, D.; Mude, L. Dual-Drug Loaded Biomimetic Chitosan-Collagen Hybrid Nanocomposite Scaffolds for Ameliorating Potential Tissue Regeneration in Diabetic Wounds. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tripathi, S.; Singh, B.N.; Singh, D.; Kumar, G.; Srivastava, P. Optimization and evaluation of ciprofloxacin-loaded collagen/chitosan scaffolds for skin tissue engineering. 3 Biotech 2020, 11, 160. [Google Scholar] [CrossRef]
- Ioan, D.-C.; Rău, I.; Albu Kaya, M.G.; Radu, N.; Bostan, M.; Zgârian, R.G.; Tihan, G.T.; Dinu-Pîrvu, C.-E.; Lupuliasa, A.; Ghica, M.V. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers 2020, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yao, Q.; Liang, Y.; Dan, Y.; Wang, Y.; Wen, H.; Yang, Y.; Dan, W. Chitosan based antibacterial composite materials for leather industry: A review. J. Leather Sci Eng. 2021, 3, 12. [Google Scholar] [CrossRef]
- Villanueva-Ornelas, G.E.; Nunez-Anita, R.E.; Arenas-Arrocena, M.C.; Luevano-Colmenero, G.H.; Acosta-Torres, L.S. Biocidal and bioresorbable chitosan/triclosan/collagen matrixes. Innovation 2019, 2, e1. [Google Scholar] [CrossRef]
- Krishnan, R.; Pandiaraj, S.; Muthusamy, S.; Panchal, H.; Alsoufi, M.H.; Ibrahim, A.M.M.; Elsheikh, A. Biodegradable magnesium metal matrix composites for biomedical implants: Synthesis, mechanical performance, and corrosion behavior—A review. J. Mater. Res. Technol. 2022, 20, 650–670. [Google Scholar] [CrossRef]
- Gopal, N.; Palaniyandi, P.; Ramasamy, P.; Panchal, H.; Ibrahim, A.; Alsoufi, M.S.; Elsheikh, A.H. In Vitro Degradability, Microstructural Evaluation, and Biocompatibility of Zn-Ti-Cu-Ca-P Alloy. Nanomaterials 2022, 12, 1357. [Google Scholar] [CrossRef]
- Ferdes, M.; Ungureanu, C.; Radu, N.; Chirvase, A. Antimicrobial effect of Monascus sp. red rice against some bacterial and fungal strains. Chem. Eng. Trans. 2009, 17, 1089–1094. [Google Scholar]
- Zaharie, M.-G.O.; Radu, N.; Pirvu, L.; Bostan, M.; Voicescu, M.; Begea, M.; Constantin, M.; Voaides, C.; Babeanu, N.; Roman, V. Studies Regarding the Pharmaceutical Potential of Derivative Products from Plantain. Plants 2022, 11, 1827. [Google Scholar] [CrossRef]
- Radu, N.; Salageanu, A.; Ferdes, M.; Rau, I. Cytotoxicity Study Regarding Some Products Derived from Monascus sp. Mol. Cryst. Liq. Cryst. 2012, 555, 189–194. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. BioMed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.M.; Amaro Martins, V.C.; de Guzzi Plepis, A.M. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Stalinska, J.; Osyczka, A.M. Biological Properties of Chitosan/Collagen Composites. Key Eng. Mater. 2013, 587, 205–210. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Liu, L.; Zhang, D. The mechanism of a chitosan-collagen composite film used as biomaterial support for MC3T3-E1 cell differentiation. Sci. Rep. 2016, 6, 39322. [Google Scholar] [CrossRef]
- Udkhiyati, M.; Rosiati, N.M.; Silvianti, F. The Influence of Chitosan Towards Antibacterial Properties in Natural Leather. Leather Footwear J./Rev. De Piel. Incalt. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.; Aly, M.M.; Sheshtawi, R.M. Antimicrobial Activities of Different Novel Chitosan-Collagen Nanocomposite Films Against Some Bacterial Pathogens. Int. J. Pharm. Phytopharm. Res. 2020, 10, 114–121. [Google Scholar]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Frosini, S.M.; Bond, R. Activity In Vitro of Clotrimazole against Canine Methicillin-Resistant and Susceptible Staphylococcus pseudintermedius. Antibiotics 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimling, B.; Karolewicz, B.; Nawrot, U.; Włodarczyk, K.; Górniak, A. Physicochemical and Antifungal Properties of Clotrimazole in Combination with High-Molecular Weight Chitosan as a Multifunctional Excipient. Mat. Drugs 2020, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics 2021, 10, 757. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babeanu, N.; Radu, N.; Enascuta, C.-E.; Alexandrescu, E.; Ganciarov, M.; Mohammed, M.S.O.; Suica-Bunghez, I.R.; Senin, R.; Ursu, M.; Bostan, M. Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine. Polymers 2022, 14, 3544. https://doi.org/10.3390/polym14173544
Babeanu N, Radu N, Enascuta C-E, Alexandrescu E, Ganciarov M, Mohammed MSO, Suica-Bunghez IR, Senin R, Ursu M, Bostan M. Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine. Polymers. 2022; 14(17):3544. https://doi.org/10.3390/polym14173544
Chicago/Turabian StyleBabeanu, Narcisa, Nicoleta Radu, Cristina-Emanuela Enascuta, Elvira Alexandrescu, Mihaela Ganciarov, Mohammed Shaymaa Omar Mohammed, Ioana Raluca Suica-Bunghez, Raluca Senin, Magdalina Ursu, and Marinela Bostan. 2022. "Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine" Polymers 14, no. 17: 3544. https://doi.org/10.3390/polym14173544
APA StyleBabeanu, N., Radu, N., Enascuta, C.-E., Alexandrescu, E., Ganciarov, M., Mohammed, M. S. O., Suica-Bunghez, I. R., Senin, R., Ursu, M., & Bostan, M. (2022). Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine. Polymers, 14(17), 3544. https://doi.org/10.3390/polym14173544