Development of Clinical Weekly-Dose Teriparatide Acetate Encapsulated Dissolving Microneedle Patch for Efficient Treatment of Osteoporosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TA-Loaded Polymer Solutions
2.2. Evaluation of TA Activity in CL-Mimic Conditions
2.3. Evaluation of the Homogeneity of the TA-Loaded Polymer Solution
2.4. Fabrication of TA-DMNs
2.5. Physical Properties of TA-DMNs
2.6. Insertion of TA-DMNs into Porcine Skin
2.7. Release Profile of TA
2.8. In Vivo Pharmacokinetic Analysis after TA Delivery
2.9. Evaluation of Adverse Effects on Skin
2.10. Statistical Analysis
3. Results
3.1. Optimization of TA Polymer Formulation
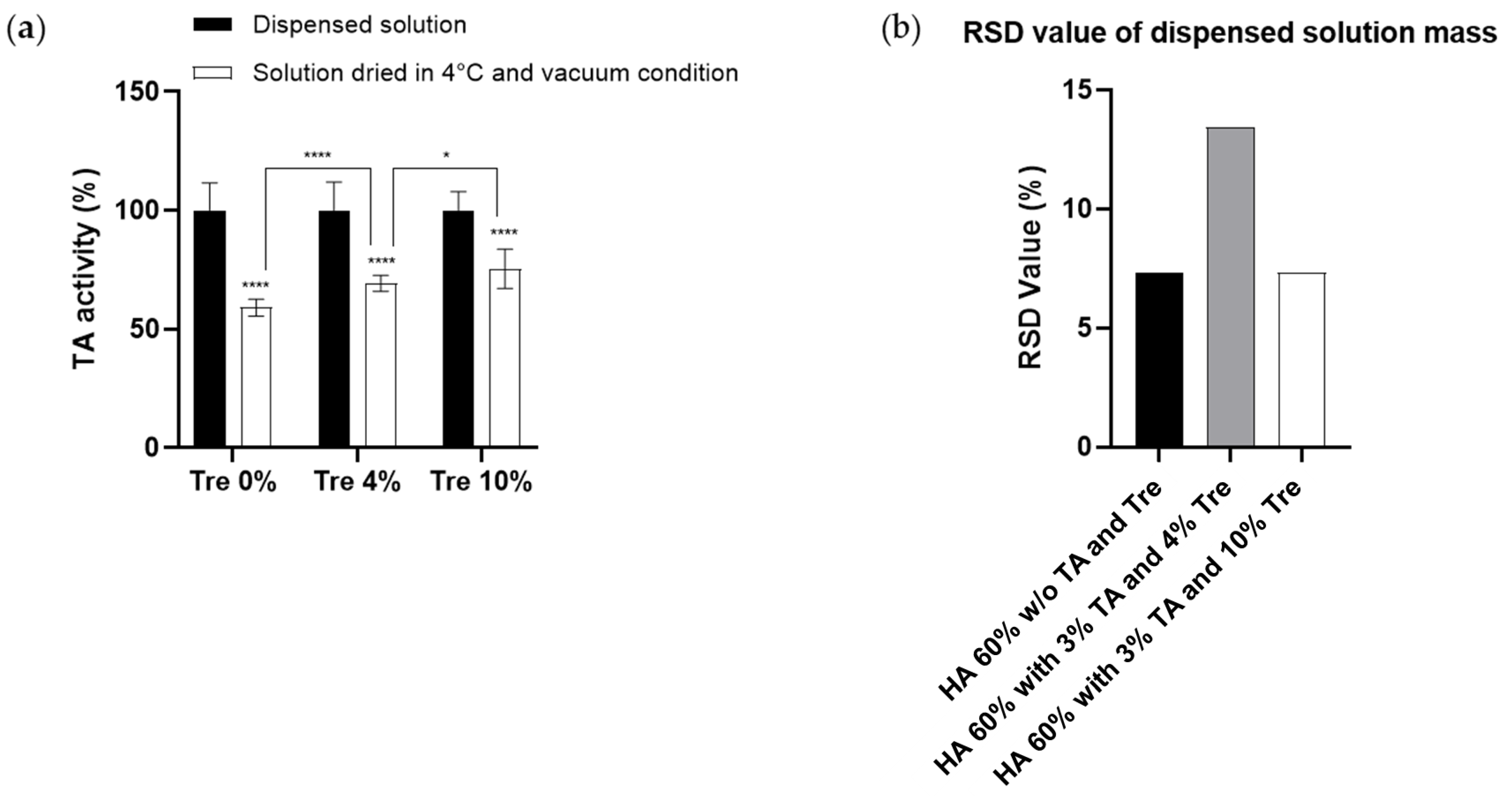
3.1.1. Effect of Trehalose on the Prevention of TA Activity during the Drying Process
3.1.2. Homogeneity of TA Polymer Solution Droplets
3.2. TA-DMN Fabrication by CL
3.3. Evaluation of In Vitro Skin Penetration of TA-DMNs
3.4. In Vitro TA Release Profiles of TA-DMNs
3.5. Pharmacokinetic Profiles of TA-DMN Patch Applications
3.6. Skin Adverse Reaction after TA-DMN Patch Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Lewiecki, E.M.; Miller, P.D.; Baim, S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): What they mean to the bone densitometrist and bone technologist. J. Clin. Densitom. 2008, 11, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Asafo-Adjei, T.A.; Chen, A.J.; Najarzadeh, A.; Puleo, D.A. Advances in Controlled Drug Delivery for Treatment of Osteoporosis. Curr. Osteoporos. Rep. 2016, 14, 226–238. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- Lupsa, B.C.; Insogna, K. Bone Health and Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2015, 44, 517–530. [Google Scholar] [CrossRef]
- Rivadeneira, F.; Mäkitie, O. Osteoporosis and Bone Mass Disorders: From Gene Pathways to Treatments. Trends Endocrinol. Metab. 2016, 27, 262–281. [Google Scholar] [CrossRef]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am. J. Med. 2005, 118 (Suppl. S12B), 64–73. [Google Scholar] [CrossRef]
- Gennari, L.; Merlotti, D.; Nuti, R. Selective estrogen receptor modulator (SERM) for the treatment of osteoporosis in postmenopausal women: Focus on lasofoxifene. Clin. Interv. Aging 2010, 5, 19–29. [Google Scholar] [CrossRef]
- Chung, P.-L.; Zhou, S.; Eslami, B.; Shen, L.; LeBoff, M.S.; Glowacki, J. Effect of Age on Regulation of Human Osteoclast Differentiation. J. Cell. Biochem. 2014, 115, 1412–1419. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Jansen, J.P.; Bergman, G.J.; Huels, J.; Olson, M. The Efficacy of Bisphosphonates in the Prevention of Vertebral, Hip, and Nonvertebral-Nonhip Fractures in Osteoporosis: A Network Meta-Analysis. Semin. Arthritis Rheum. 2011, 40, 275–284.e2. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate Therapy for Osteoporosis: Benefits, Risks, and Drug Holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; San Martin, J.; Miller, P.D.; Civitelli, R.; Bandeira, F.; Omizo, M.; Donley, D.W.; Dalsky, G.P.; Eriksen, E.F. Opposite Bone Remodeling Effects of Teriparatide and Alendronate in Increasing Bone Mass. Arch. Intern. Med. 2005, 165, 1762–1768. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Woo, S.-B.; Hellstein, J.W.; Kalmar, J.R. Systematic Review: Bisphosphonates and Osteonecrosis of the Jaws. Ann. Intern. Med. 2006, 144, 753–761. [Google Scholar] [CrossRef]
- Shane, E. Evolving Data about Subtrochanteric Fractures and Bisphosphonates. N. Engl. J. Med. 2010, 362, 1825–1827. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, H.; Pan, B.; Lu, L.; Liu, J.; Kang, Y.; Yao, X.; Feng, S. Effectiveness of Teriparatide on Fracture Healing: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0168691. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.-Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of Parathyroid Hormone (1–34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Rowshan, H.H.; Parham, M.A.; Baur, D.A.; McEntee, R.D.; Cauley, E.; Carriere, D.T.; Wood, J.C.; Demsar, W.J.; Pizarro, J.M. Effect of Intermittent Systemic Administration of Recombinant Parathyroid Hormone (1–34) on Mandibular Fracture Healing in Rats. J. Oral Maxillofac. Surg. 2010, 68, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Alkhiary, Y.M.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of Experimental Fracture-Healing by Systemic Administration of Recombinant Human Parathyroid Hormone (PTH 1–34). J. Bone Jt. Surg. 2005, 87, 731–741. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, J.H.; Jung, H.-D.; Jung, Y.-S. Treatment of Medication-Related Osteonecrosis of the Jaw Around the Dental Implant with a Once-Weekly Teriparatide: A Case Report and Literature Review. J. Oral Implant. 2019, 45, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Santabalbina, F.J.; Aguilella-Fernández, L. Bisphosphonate long-term treatment related bilateral subtrochanteric femoral fracture. Can teriparatide be useful? Aging Clin. Exp. Res. 2013, 25, 605–609. [Google Scholar] [CrossRef]
- Yuan, F.; Peng, W.; Yang, C.; Zheng, J. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: A meta-analysis. Int. J. Surg. 2019, 66, 1–11. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Scheele, W.H.; Paul, S.; Adami, S.; Syversen, U.; Diez-Perez, A.; Kaufman, J.-M.; Clancy, A.D.; Gaich, G.A. The Effect of Teriparatide [Human Parathyroid Hormone (1–34)] Therapy on Bone Density in Men With Osteoporosis. J. Bone Miner. Res. 2003, 18, 9–17. [Google Scholar] [CrossRef]
- Cosman, F.; Lane, N.E.; Bolognese, M.A.; Zanchetta, J.R.; García-Hernandez, P.A.; Sees, K.; Matriano, J.A.; Gaumer, K.; Daddona, P.E. Effect of Transdermal Teriparatide Administration on Bone Mineral Density in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2010, 95, 151–158. [Google Scholar] [CrossRef]
- Honeywell, M.; Phillips, S.; Branch, E., III; Vo, K.A.; Marks, E.I.; Thompson, M. Teriparatide for osteoporosis: A clinical review. Drug Forecast. 2003, 28, 713–716. [Google Scholar]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving Microneedle Patch for Transdermal Delivery of Human Growth Hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef]
- Kim, S.; Park, M.; Yang, H.; Dangol, M.; Lahiji, S.F.; Huh, I.; Kim, M.; Lee, J.; Son, J.; Jung, H. Development of a quantitative method for active epidermal growth factor extracted from dissolving microneedle by solid phase extraction and liquid chromatography electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2016, 131, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagase, Y.; Iga, K.; Kawase, M.; Oka, M.; Yanai, S.; Matsumoto, Y.; Nakagawa, S.; Fukuda, T.; Adachi, H.; et al. Prevention of bone loss in ovariectomized rats by pulsatile transdermal iontophoretic administration of human PTH(1–34). J. Pharm. Sci. 2002, 91, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Zorec, B.; Becker, S.; Reberšek, M.; Miklavčič, D.; Pavšelj, N. Skin electroporation for transdermal drug delivery: The influence of the order of different square wave electric pulses. Int. J. Pharm. 2013, 457, 214–223. [Google Scholar] [CrossRef]
- Herwadkar, A.; Sachdeva, V.; Taylor, L.F.; Silver, H.; Banga, A.K. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int. J. Pharm. 2012, 423, 289–296. [Google Scholar] [CrossRef]
- Walther, W.; Siegel, R.; Kobelt, D.; Knösel, T.; Dietel, M.; Bembenek, A.; Aumann, J.; Schleef, M.; Baier, R.; Stein, U.; et al. Novel Jet-Injection Technology for Nonviral Intratumoral Gene Transfer in Patients with Melanoma and Breast Cancer. Clin. Cancer Res. 2008, 14, 7545–7553. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Cahill, E.M.; O’Cearbhaill, E.D. Toward Biofunctional Microneedles for Stimulus Responsive Drug Delivery. Bioconjugate Chem. 2015, 26, 1289–1296. [Google Scholar] [CrossRef]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Microneedles for transdermal drug delivery using clay-based composites. Expert Opin. Drug Deliv. 2022. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Kang, N.-W.; Jeong, H.-R.; Sohn, S.-Y.; Jeon, Y.-E.; Yu, N.-Y.; Hwang, Y.; Kim, S.; Kim, D.-D.; Park, J.-H. The Relationship between the Drug Delivery Properties of a Formulation of Teriparatide Microneedles and the Pharmacokinetic Evaluation of Teriparatide Administration in Rats. Pharm. Res. 2022, 39, 989–999. [Google Scholar] [CrossRef]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid Hormone (1–34)-Coated Microneedle Patch System: Clinical Pharmacokinetics and Pharmacodynamics for Treatment of Osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Naito, C.; Katsumi, H.; Suzuki, T.; Quan, Y.-S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1–34). Pharmaceutics 2018, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.H.; Lee, B.Y.; Kim, S.J.; Sung, C.Y.; Jang, N.K.; Kim, J.D.; Jeong, D.H.; Ryu, H.Y.; Lee, S. A comparative study of dissolving hyaluronic acid microneedles with trehalose and poly(vinyl pyrrolidone) for efficient peptide drug delivery. Biomater. Sci. 2018, 6, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Genheden, S.; Garcia Sakai, V.; Swenson, J. Mechanism of Trehalose-Induced Protein Stabilization from Neutron Scattering and Modeling. J. Phys. Chem. B 2019, 123, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
- Merutka, G.; Murphy, B.M.; Payne, R.W.; Wilson, G.A.; Matsuura, J.E.; Henry, C.S.; Manning, M.C. Stability of lyophilized teriparatide, PTH(1–34), after reconstitution. Eur. J. Pharm. Biopharm. 2016, 99, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.; Kang, G.; Lahiji, S.F.; Jang, M.; Kim, Y.M.; Kim, J.-M.; Cho, S.-N.; Jung, H. Centrifugal Lithography: Self-Shaping of Polymer Microstructures Encapsulating Biopharmaceutics by Centrifuging Polymer Drops. Adv. Health Mater. 2017, 6, 1700326. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nakahigashi, T.; Yoshimoto, N.; Ueda, Y.; Hamasaki, N.; Takada, K. Transdermal Insulin Application System with Dissolving Microneedles. Diabetes Technol. Ther. 2012, 14, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, E.; Jarrahian, C.; Hull, H.F.; Zehrung, D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine 2015, 33, 4699–4704. [Google Scholar] [CrossRef]
- Kim, J.-E.; Sykes, J.M. Hyaluronic Acid Fillers: History and Overview. Facial Plast. Surg. 2011, 27, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Hatamipour, M.; Tabatabaei, S.A. Trehalose administration attenuates atherosclerosis in rabbits fed a high-fat diet. J. Cell. Biochem. 2019, 120, 9455–9459. [Google Scholar] [CrossRef] [PubMed]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.-W.; Prausnitz, M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J. Control Release 2016, 239, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fakhraei Lahiji, S.; Kim, Y.; Kang, G.; Kim, S.; Lee, S.; Jung, H. Tissue Interlocking Dissolving Microneedles for Accurate and Efficient Transdermal Delivery of Biomolecules. Sci. Rep. 2019, 9, 7886. [Google Scholar] [CrossRef] [PubMed]
- Ohn, J.; Jang, M.; Kang, B.M.; Yang, H.; Hong, J.T.; Kim, K.H.; Kwon, O.; Jung, H. Dissolving Candlelit Microneedle for Chronic Inflammatory Skin Diseases. Adv. Sci. 2021, 8, 2004873. [Google Scholar] [CrossRef] [PubMed]





| Group | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Administered dose [μg] | 60.6 | 61.4 ± 1.3 | 122.8 ± 2.6 |
| Cmax [pg] | 293.4 ± 17.6 | 149.9 ± 45.9 | 338.8 ± 18.6 |
| Tmax (h) | 0.3 | 0.7 | 0.5 |
| t1/2 (h) | 0.8 | 0.9 | 0.7 |
| AUC (min × pg × mL−1) | 255.9 ± 26.8 | 149.9 ± 45.9 | 338.8 ± 18.6 |
| BA (%) | 100 ± 10.5 | 59.2 ± 18.1 | 66.9 ± 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.; Kang, G.; Yang, H.; Jang, M.; Kim, Y.; Ahn, H.; Kim, M.; Jung, H. Development of Clinical Weekly-Dose Teriparatide Acetate Encapsulated Dissolving Microneedle Patch for Efficient Treatment of Osteoporosis. Polymers 2022, 14, 4027. https://doi.org/10.3390/polym14194027
Sim J, Kang G, Yang H, Jang M, Kim Y, Ahn H, Kim M, Jung H. Development of Clinical Weekly-Dose Teriparatide Acetate Encapsulated Dissolving Microneedle Patch for Efficient Treatment of Osteoporosis. Polymers. 2022; 14(19):4027. https://doi.org/10.3390/polym14194027
Chicago/Turabian StyleSim, Jeeho, Geonwoo Kang, Huisuk Yang, Mingyu Jang, Youseong Kim, Hyeri Ahn, Minkyung Kim, and Hyungil Jung. 2022. "Development of Clinical Weekly-Dose Teriparatide Acetate Encapsulated Dissolving Microneedle Patch for Efficient Treatment of Osteoporosis" Polymers 14, no. 19: 4027. https://doi.org/10.3390/polym14194027
APA StyleSim, J., Kang, G., Yang, H., Jang, M., Kim, Y., Ahn, H., Kim, M., & Jung, H. (2022). Development of Clinical Weekly-Dose Teriparatide Acetate Encapsulated Dissolving Microneedle Patch for Efficient Treatment of Osteoporosis. Polymers, 14(19), 4027. https://doi.org/10.3390/polym14194027







