Silsesquioxane-Doped Electrospun Nanofibrillar Membranes for Separation Systems
Abstract
:1. Introduction
- Cell culture growth matrices [23];
2. Materials and Methods
2.1. Materials
2.2. Organosilicon Precursors (Spherosilicates and Silsesquioxanes)
2.3. Preparation of the PLA Solution for the Electrospinning Process
2.4. Process Conditions
2.5. Analytical Methods
3. Results
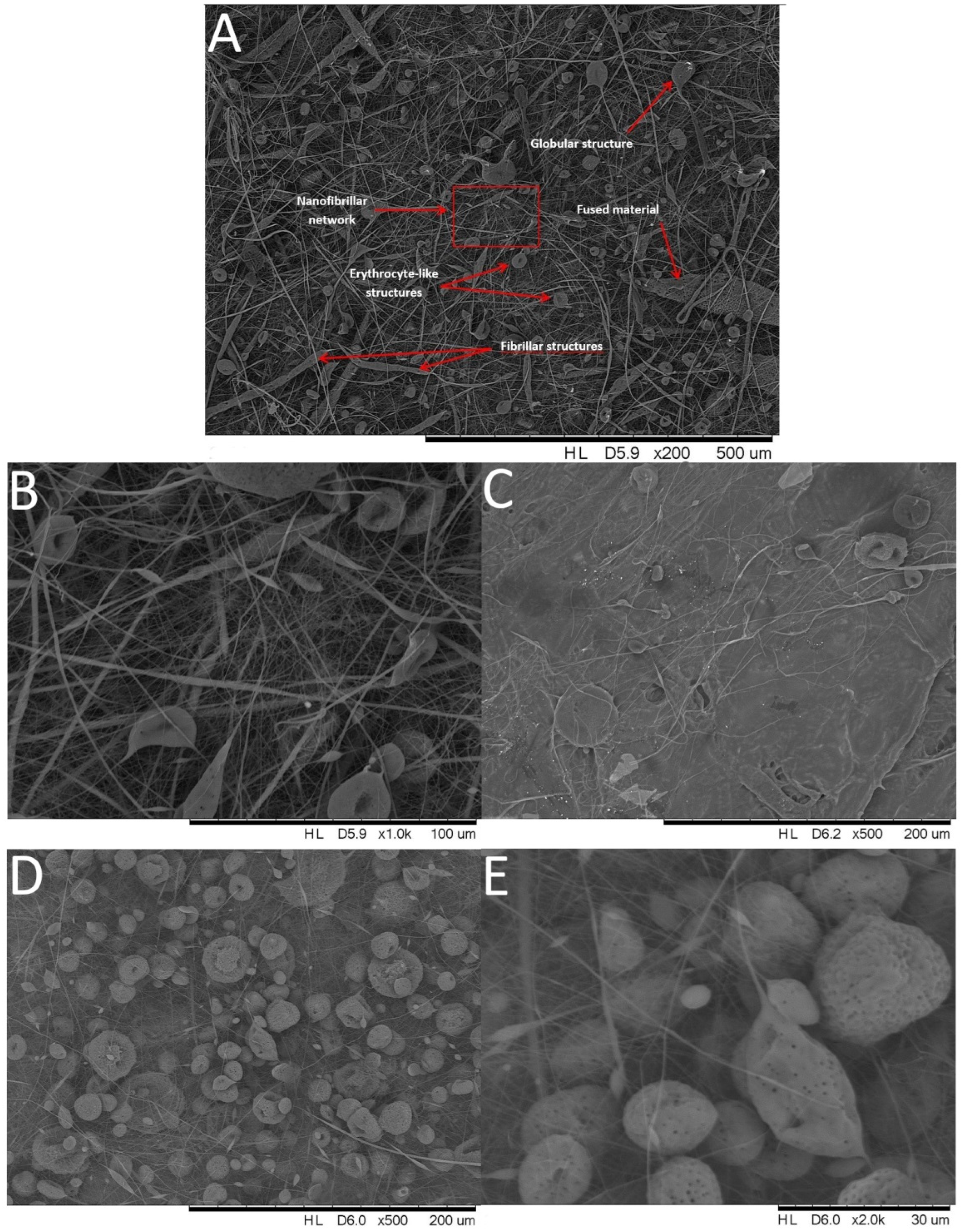
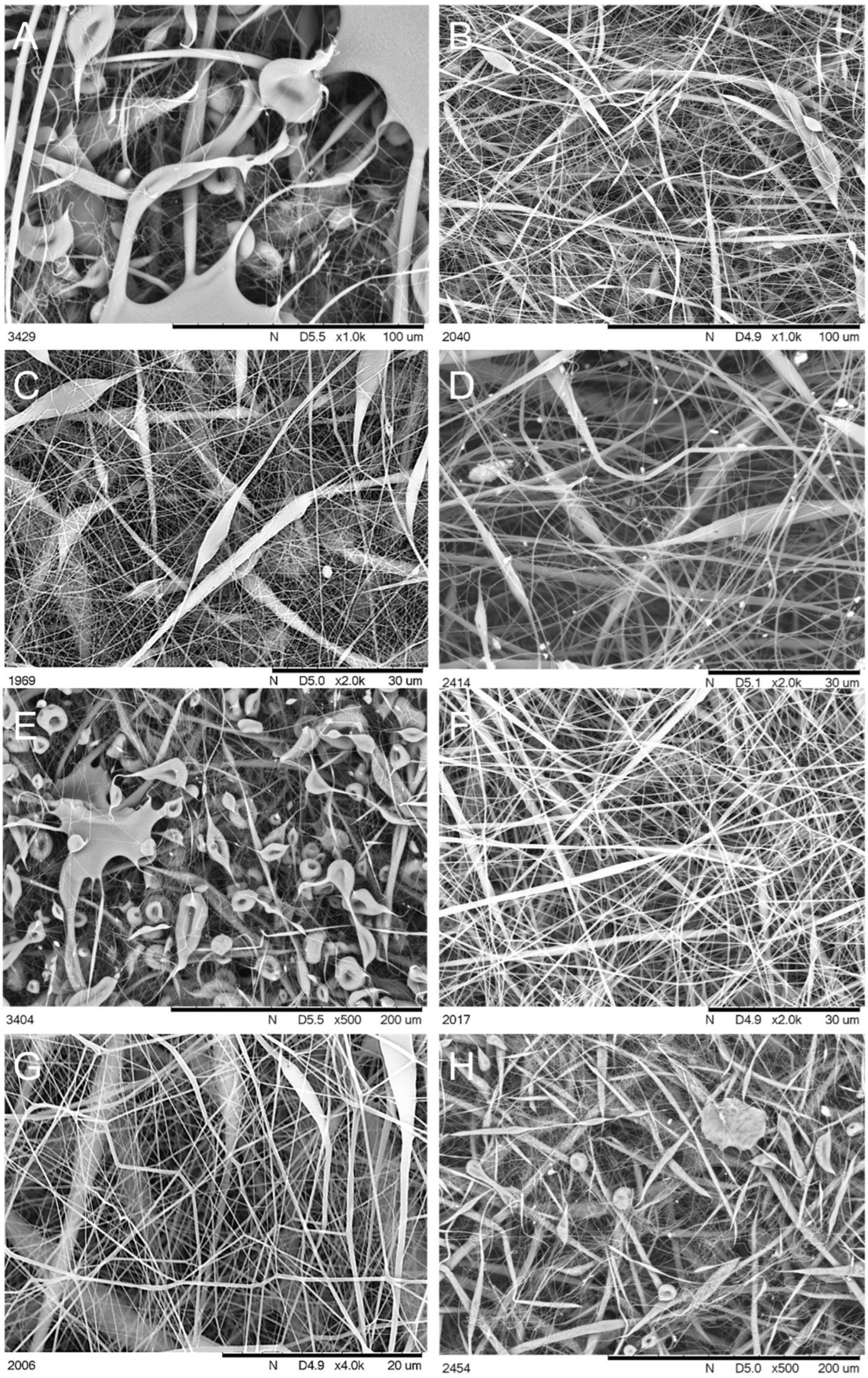
3.1. Scanning Electron Microscopy
3.2. Thermal Analysis
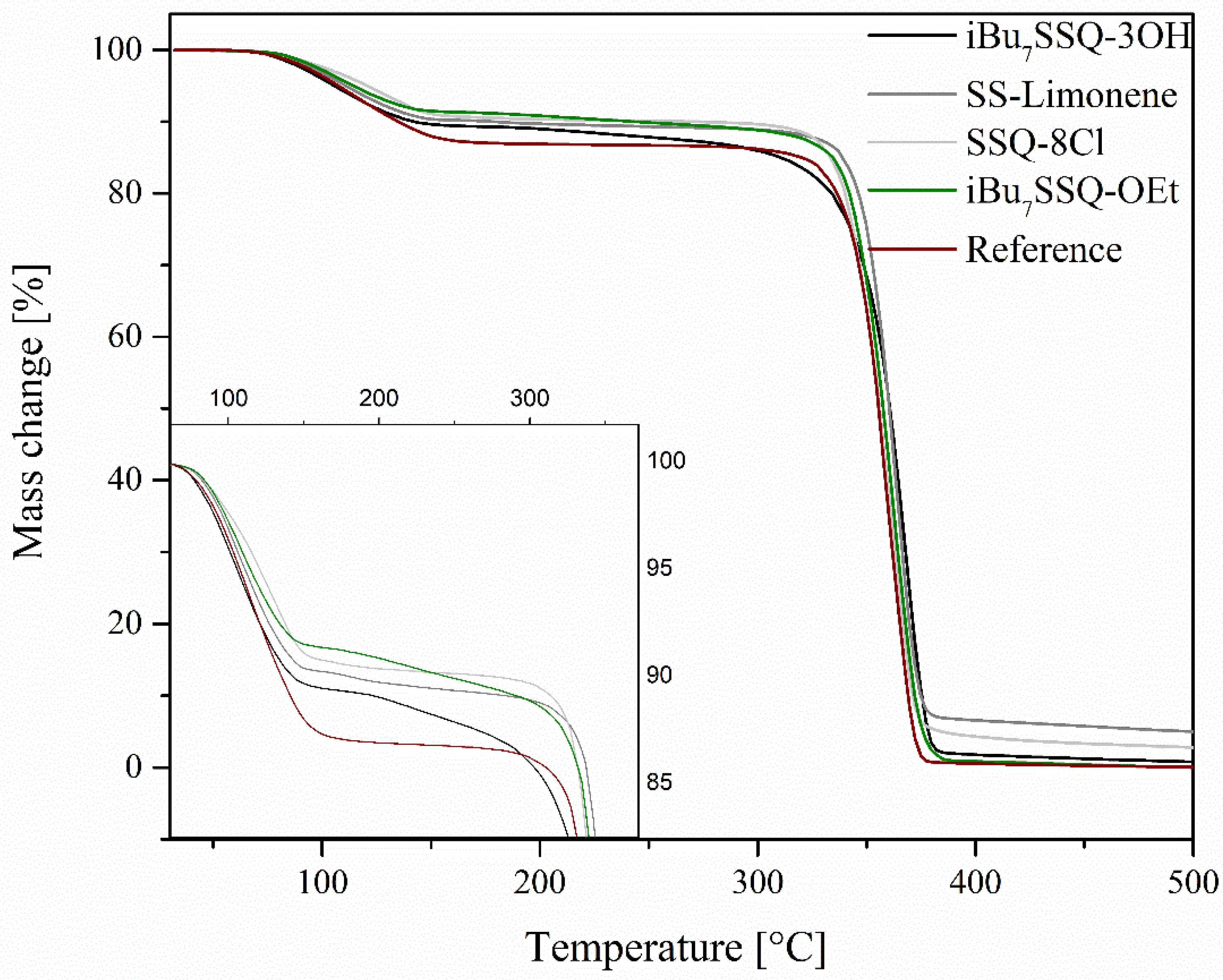
3.2.1. TGA Analysis
3.2.2. DSC Analysis
3.3. Contact Angle Analysis
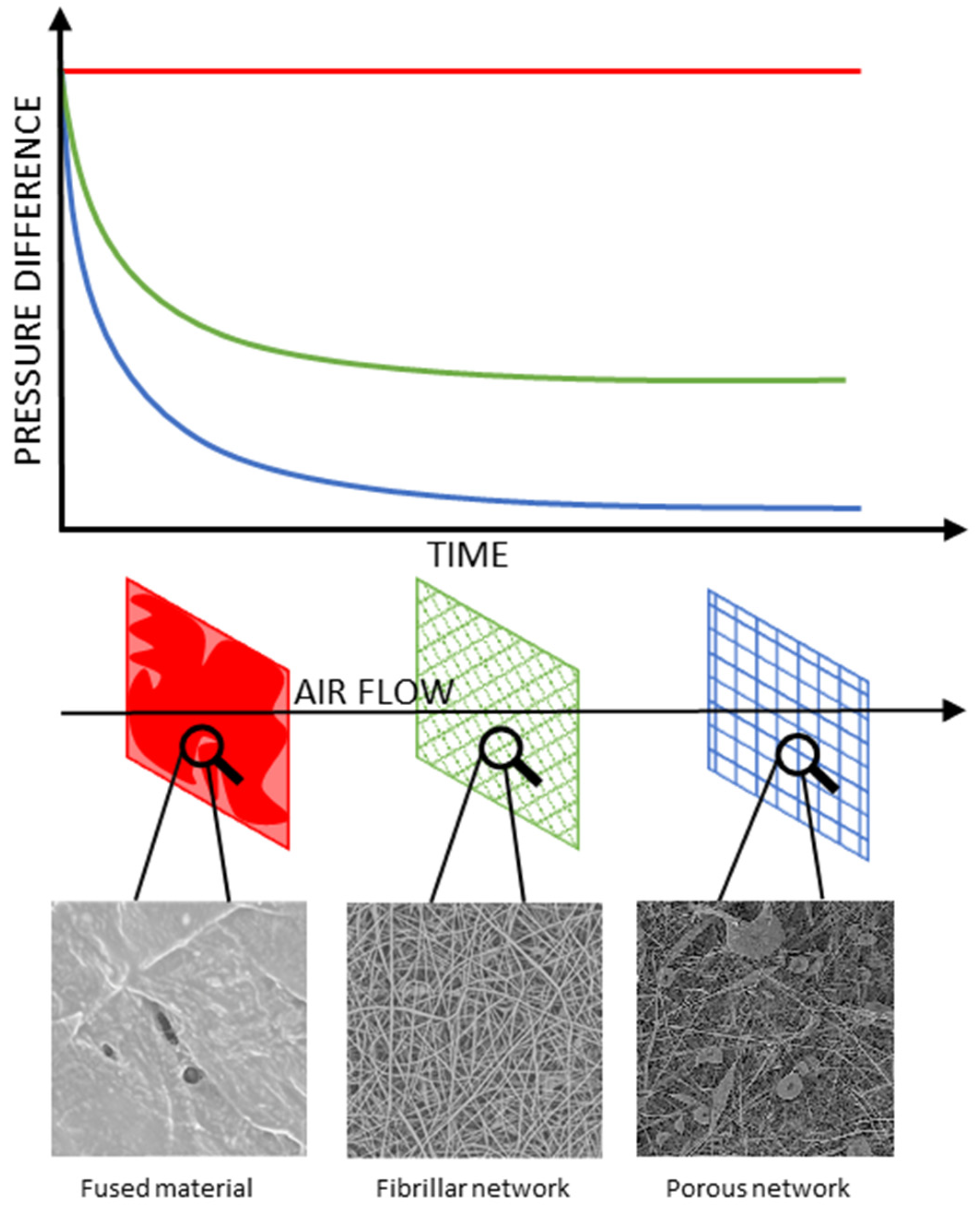
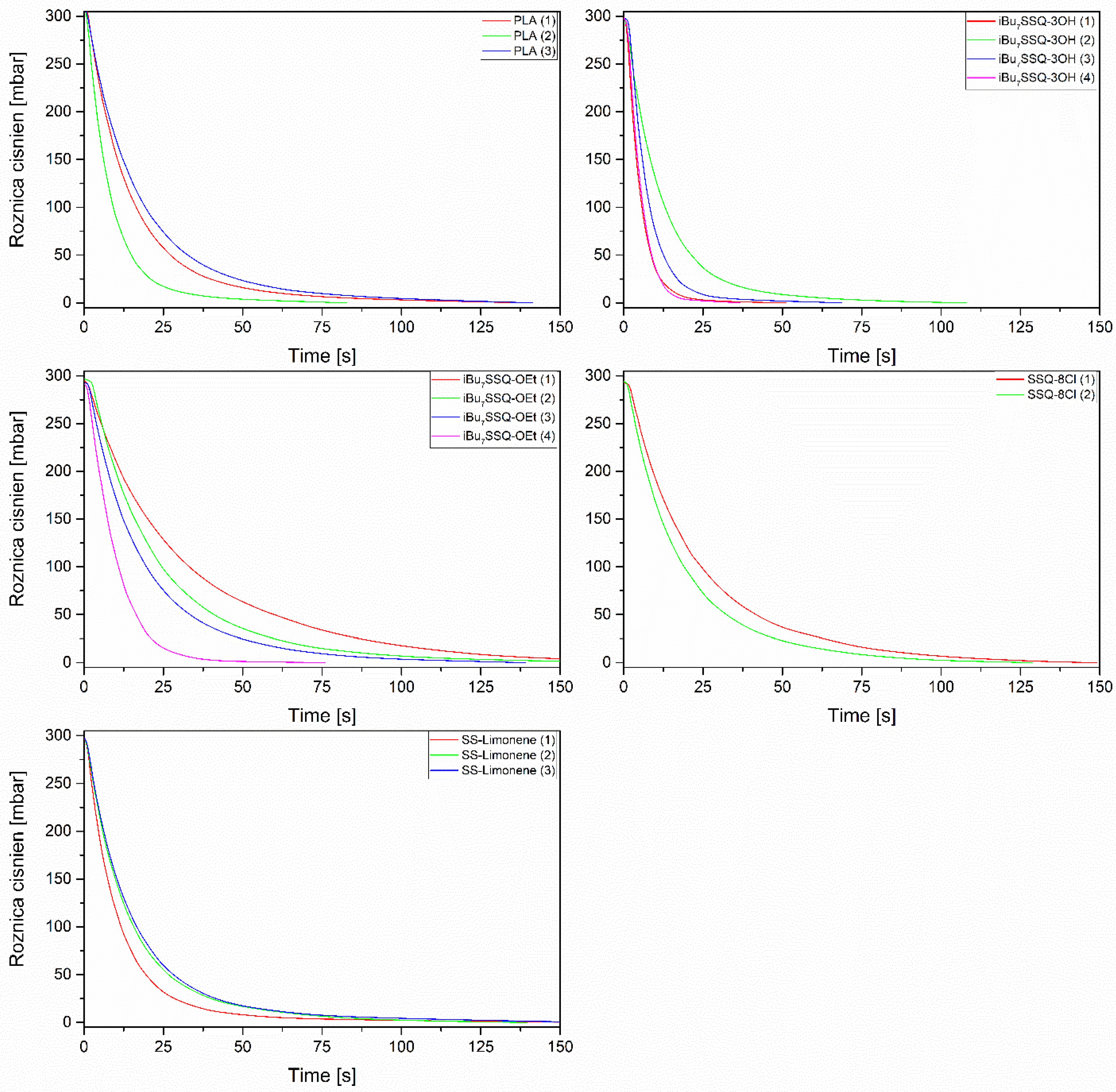
3.4. Membrane Air Flow Analysis
- The main diameter of the fibres, the nanofibers providing higher flow resistance than large fibrils;
- Formation of microglobules instead of fibres, resulting in lower packing density and lower flow resistance;
- Percolation effect between fibrillar and globular structures, increasing packing density and flow resistance;
- Formation of fused material regions, blocking the gas flow in the affected region.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar]
- He, J.-H.; Wan, Y.-Q.; Yu, J.-Y. Scaling Law in Electrospinning: Relationship between Electric Current and Solution Flow Rate. Polymer 2005, 46, 2799–2801. [Google Scholar]
- Jirsak, O.; Sanetrnik, F.; Lukas, D.; Kotek, V.; Martinova, L.; Chaloupek, J. Method of Nanofibres Production from a Polymer Solution Using Electrostatic Spinning and a Device for Carrying out the Method. U.S. Patent 7,585,437, 2004. [Google Scholar]
- Yalcinkaya, F. Preparation of Various Nanofiber Layers Using Wire Electrospinning System. Arab. J. Chem. 2019, 12, 5162–5172. [Google Scholar]
- Rożek, Z.; Kaczorowski, W.; Lukáš, D.; Louda, P.; Mitura, S. Potential applications of nanofiber textile covered by carbon coatings. J. Achiev. Mater. Manuf. Eng. 2008, 27, 35–38. [Google Scholar]
- El-Newehy, M.H.; Al-Deyab, S.S.; Kenawy, E.-R.; Abdel-Megeed, A. Nanospider Technology for the Production of Nylon-6 Nanofibers for Biomedical Applications. J. Nanomater. 2011, 2011, 1–8. [Google Scholar]
- Gopal, R.; Kaur, S.; Ma, Z.; Chan, C.; Ramakrishna, S.; Matsuura, T. Electrospun Nanofibrous Filtration Membrane. J. Membr. Sci. 2006, 281, 581–586. [Google Scholar]
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and Dye Filtration Property of Electrospun Polyhydroxybutyrate–Calcium Alginate/Carbon Nanotubes Composite Nanofibrous Filtration Membrane. Sep. Purif. Technol. 2016, 161, 69–79. [Google Scholar]
- Homaeigohar, S.S.; Buhr, K.; Ebert, K. Polyethersulfone Electrospun Nanofibrous Composite Membrane for Liquid Filtration. J. Membr. Sci. 2010, 365, 68–77. [Google Scholar]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2016, 302, 1600353. [Google Scholar]
- Bhattarai, S.R.; Bhattarai, N.; Yi, H.K.; Hwang, P.H.; Cha, D.I.; Kim, H.Y. Novel Biodegradable Electrospun Membrane: Scaffold for Tissue Engineering. Biomaterials 2004, 25, 2595–2602. [Google Scholar]
- Kim, H.-W.; Lee, H.-H.; Knowles, J.C. Electrospinning Biomedical Nanocomposite Fibers of Hydroxyapatite/Poly(Lactic Acid) for Bone Regeneration. J. Biomed. Mater. Res. Part A 2006, 79A, 643–649. [Google Scholar]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly(Lactide-Co-Glycolide)/Hydroxyapatite Nanofibrous Scaffolds Fabricated by Electrospinning for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1873–1884. [Google Scholar]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A Biodegradable Nanofiber Scaffold by Electrospinning and Its Potential for Bone Tissue Engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for Tissue Engineering Scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional Electrospun Nanofibrous Scaffolds for Biomedical Applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar]
- Pourhojat, F.; Shariati, S.; Sohrabi, M.; Mahdavi, H.; Asadpour, L. Preparation of antibacterial electrospun Poly lactic-co–glycolic acid nanofibers containing Hypericum Perforatum with bedsore healing property and evaluation of its drug release performance. Int. J. Nano Dimens. 2018, 9, 286–297. [Google Scholar]
- Khil, M.-S.; Cha, D.-I.; Kim, H.-Y.; Kim, I.-S.; Bhattarai, N. Electrospun Nanofibrous Polyurethane Membrane as Wound Dressing. J. Biomed. Mater. Res. 2003, 67B, 675–679. [Google Scholar]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 183–188. [Google Scholar]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A Review on Wound Dressings with an Emphasis on Electrospun Nanofibrous Polymeric Bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable Electrospun Fibers for Drug Delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of Glial Cell Migration and Axonal Growth on Electrospun Nanofibers of Poly-ε-Caprolactone and a Collagen/Poly-ε-Caprolactone Blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar]
- Jung, H.-R.; Ju, D.-H.; Lee, W.-J.; Zhang, X.; Kotek, R. Electrospun Hydrophilic Fumed Silica/Polyacrylonitrile Nanofiber-Based Composite Electrolyte Membranes. Electrochim. Acta 2009, 54, 3630–3637. [Google Scholar]
- Kim, J.; Reneker, D.H. Mechanical Properties of Composites Using Ultrafine Electrospun Fibers. Polym. Compos. 1999, 20, 124–131. [Google Scholar]
- Brząkalski, D.; Przekop, R.E.; Dobrosielska, M.; Sztorch, B.; Marciniak, P.; Marciniec, B. Highly Bulky Spherosilicates as Functional Additives for Polyethylene Processing—Influence on Mechanical and Thermal Properties. Polym. Compos. 2020, 41, 3389–3402. [Google Scholar]
- Voronkov, M.G.; Lavrent’yev, V.I. Inorganic ring systems. In Topics in Current Chemistry; Bosche, F.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1982; Volume 102. [Google Scholar]
- Lickiss, P.D.; Cordess, D.B. Fully Condensed Polyhedral Oligosilsesquioxanes (POSS): From synthesis to application. In Advances in Organometallic Chemistry; Hill, A.F., Fink, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 55. [Google Scholar]
- Hartmann-Thompson, C. (Ed.) Applications of polyhedral oligomeric silsesquioxanes. In Advances in Silicon Science; Springer: Berlin/Heidelberg, Germany, 2011; Volume 3. [Google Scholar]
- Scott, D.W. Thermal Rearrangement of Branched-Chain Methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar]
- Du, Y.; Liu, H. Cage-like Silsesquioxanes-Based Hybrid Materials. Dalt. Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced Functional Materials Based on Polyhedral Oligomeric Silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar]
- Marciniec, B. (Ed.) Hydrosilylation; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Cicala, G.; Blanco, I.; Latteri, A.; Ognibene, G.; Agatino Bottino, F.; Fragalà, M. PES/POSS Soluble Veils as Advanced Modifiers for Multifunctional Fiber Reinforced Composites. Polymers 2017, 9, 281. [Google Scholar]
- Deng, N.; Wang, L.; Liu, Y.; Zhong, C.; Kang, W.; Cheng, B. Functionalized Polar Octa(γ-Chloropropyl) Polyhedral Oligomeric Silsesquioxane Assisted Polyimide Nanofiber Composite Membrane with Excellent Ionic Conductivity and Wetting Mechanical Strength towards Enhanced Lithium-Ion Battery. Compos. Sci. Technol. 2020, 192, 108080. [Google Scholar]
- Zhang, Q.; Liu, Y.; Ma, J.; Zhang, M.; Ma, X.; Chen, F. Preparation and Characterization of Polypropylene Supported Electrospun POSS-(C3H6Cl)8/PVDF Gel Polymer Electrolytes for Lithium-Ion Batteries. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123750. [Google Scholar]
- Zhang, M.; Ma, X.; Liu, Y.; Ma, J.; Chen, F.; Zhang, Q. High-Performance Electrospun POSS-(PMMA46)8/PVDF Hybrid Gel Polymer Electrolytes with PP Support for Li-Ion Batteries. Ionics 2018, 25, 2595–2605. [Google Scholar]
- Zhao, H.; Deng, N.; Yan, J.; Kang, W.; Ju, J.; Wang, L.; Li, Z.; Cheng, B. Effect of OctaphenylPolyhedral Oligomeric Silsesquioxane on the Electrospun Poly-m-Phenylene Isophthalamid Separators for Lithium-Ion Batteries with High Safety and Excellent Electrochemical Performance. Chem. Eng. J. 2019, 356, 11–21. [Google Scholar]
- Jeong, H.-G.; Han, Y.-S.; Jung, K.-H.; Kim, Y.-J. Poly(Vinylidene Fluoride) Composite Nanofibers Containing Polyhedral Oligomeric Silsesquioxane–Epigallocatechin Gallate Conjugate for Bone Tissue Regeneration. Nanomaterials 2019, 9, 184. [Google Scholar]
- Liu, B.; Yao, T.; Ren, L.; Zhao, Y.; Yuan, X. Antibacterial PCL Electrospun Membranes Containing Synthetic Polypeptides for Biomedical Purposes. Colloids Surf. B Biointerfaces 2018, 172, 330–337. [Google Scholar]
- Brząkalski, D.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, R.E. Limonene Derivative of Spherosilicate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar]
- Ye, M.; Wu, Y.; Zhang, W.; Yang, R. Synthesis of incompletely caged silsesquioxane (T7-POSS) compounds via a versatile three-step approach. Res. Chem. Intermed. 2018, 44, 4277–4294. [Google Scholar] [CrossRef]
- Vieira, E.G.; Dal-Bó, A.G.; Frizon, T.E.A.; Dias Filho, N.L. Synthesis of two new Mo(II) organometallic catalysts immobilized on POSS for application in olefin oxidation reactions. J. Organomet. Chem. 2017, 834, 73–82. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Frydrych, M.; Pakuła, D.; Dobrosielska, M.; Sztorch, B.; Marciniec, B. Where ppm Quantities of Silsesquioxanes Make a Difference—Silanes and Cage Siloxanes as TiO2 Dispersants and Stabilizers for Pigmented Epoxy Resins. Materials 2022, 15, 494. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of Injection Moulded, Natural Fibre-Reinforced Composites with PP and PLA as Matrices. J. Thermoplast. Compos. Mater. 2011, 25, 927–948. [Google Scholar]
- Naeem, S.; Gilani, S.Q.Z.; Baheti, V.; Wiener, J.; Militky, J.; Javed, S.; Ali, A.; Javed, Z.; ul Hassan, S.Z. Electrical Conductivity of PLA Films Reinforced with Carbon Nano Particles from Waste Acrylic Fibers. In Advances in Natural Fibre Composites; Springer: Cham, Switzerland, 2017; pp. 205–222. [Google Scholar]
- Brown, J.F.; Vogt, L.H. The Polycondensation of Cyclohexylsilanetriol. J. Am. Chem. Soc. 1965, 87, 4313–4317. [Google Scholar] [CrossRef]
- Spirk, S.; Nieger, M.; Belaj, F.; Pietschnig, R. Formation and hydrogen bonding of a novel POSS-trisilanol. Dalton Trans. 2009, 2009, 163–167. [Google Scholar] [CrossRef]
- Ozdemir, E.; Lekesiz, T.O.; Hacaloglu, J. Polylactide/Organically Modified Montmorillonite Composites; Effects of Organic Modifier on Thermal Characteristics. Polym. Degrad. Stab. 2016, 134, 87–96. [Google Scholar]
- Mayes, A. Softer at the boundary. Nature Mater. 2005, 4, 651–652. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Sztorch, B.; Jakubowska, P.; Jałbrzykowski, M.; Marciniec, B. Silsesquioxane Derivatives as Functional Additives for Preparation of Polyethylene-Based Composites: A Case of Trisilanol Melt-Condensation. Polymers 2020, 12, 2269. [Google Scholar] [CrossRef]



| Code | Structure |
|---|---|
| iBu7SSQ-3OH | 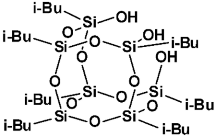 |
| SS-Limonene | 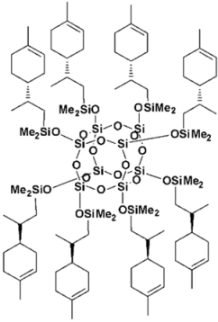 |
| SSQ-8Cl | 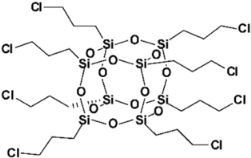 |
| iBu7SSQ-OEt |  |
| Conditions Number | Voltage [kV] | Electrospinning Lead Rotation [rpm] | Time [min] |
|---|---|---|---|
| 1 | 80 | 24 | 10 |
| 2 | 80 | 12 | 20 |
| 3 | 54 | 12 | 20 |
| 4 | 54 | 24 | 10 |
| T5% [°C] | Tonset [°C] [a] | 1st Stage | 2nd Stage | Total Mass Loss [%] | ||
|---|---|---|---|---|---|---|
| Tmax1 [°C] | Mass Loss [%] | Tmax2 [°C] | ||||
| iBu7SSQ-3OH | 104.7 | 350.7 | 105.8 | 10.75 | 372.5 | 100 |
| iBu7SSQ-OEt | 114.6 | 348.8 | 109.3 | 9.75 | 364.2 | 96.63 |
| SSQ-8Cl | 119.7 | 344.5 | 109.4 | 8.64 | 359.5 | 100 |
| SS-Limonene | 109.6 | 340.9 | 120.5 | 9.23 | 362.1 | 98.53 |
| Reference | 107.3 | 352.5 | 114.9 | 12.90 | 359.4 | 100 |
| System | Contact Angle Value |
|---|---|
| iBu7SSQ-3OH (1) | 138.0 |
| iBu7SSQ-3OH (2) | 140.1 |
| iBu7SSQ-3OH (3) | 144.0 |
| iBu7SSQ-3OH (4) | 137.0 |
| SSQ-8Cl (1) | 132.9 |
| SSQ-8Cl (2) | 136.8 |
| SSQ-8Cl (3) | 136.0 |
| SSQ-8Cl (4) | 129.4 |
| iBu7SSQ-OEt (1) | 138.4 |
| iBu7SSQ-OEt (2) | 131.5 |
| iBu7SSQ-OEt (3) | 140.2 |
| iBu7SSQ-OEt (4) | 139.8 |
| SS-Limonene (1) | 137.3 |
| SS-Limonene (2) | 140.2 |
| SS-Limonene (3) | 129.3 |
| SS-Limonene (4) | 124.7 |
| Reference (1) | 129.9 |
| Reference (2) | 130.2 |
| Reference (3) | 129.9 |
| Reference (4) | 130.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frydrych, M.; Sztorch, B.; Brząkalski, D.; Kozera, R.; Konieczna, R.; Osiecki, T.; Przekop, R.E. Silsesquioxane-Doped Electrospun Nanofibrillar Membranes for Separation Systems. Polymers 2022, 14, 3569. https://doi.org/10.3390/polym14173569
Frydrych M, Sztorch B, Brząkalski D, Kozera R, Konieczna R, Osiecki T, Przekop RE. Silsesquioxane-Doped Electrospun Nanofibrillar Membranes for Separation Systems. Polymers. 2022; 14(17):3569. https://doi.org/10.3390/polym14173569
Chicago/Turabian StyleFrydrych, Miłosz, Bogna Sztorch, Dariusz Brząkalski, Rafał Kozera, Roksana Konieczna, Tomasz Osiecki, and Robert E. Przekop. 2022. "Silsesquioxane-Doped Electrospun Nanofibrillar Membranes for Separation Systems" Polymers 14, no. 17: 3569. https://doi.org/10.3390/polym14173569
APA StyleFrydrych, M., Sztorch, B., Brząkalski, D., Kozera, R., Konieczna, R., Osiecki, T., & Przekop, R. E. (2022). Silsesquioxane-Doped Electrospun Nanofibrillar Membranes for Separation Systems. Polymers, 14(17), 3569. https://doi.org/10.3390/polym14173569







