Apigenin Loaded Lipoid–PLGA–TPGS Nanoparticles for Colon Cancer Therapy: Characterization, Sustained Release, Cytotoxicity, and Apoptosis Pathways
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Material
2.2. Method of Preparation of LPHyNPs
2.3. Characterization of Prepared LPHyNPs
2.3.1. Determination of Particle Size, PDI and Zeta Potential
2.3.2. Drug Entrapment and Loading Efficiency of Prepared LPHyNP
2.3.3. Differential Scanning Calorimeter (DSC) Analysis
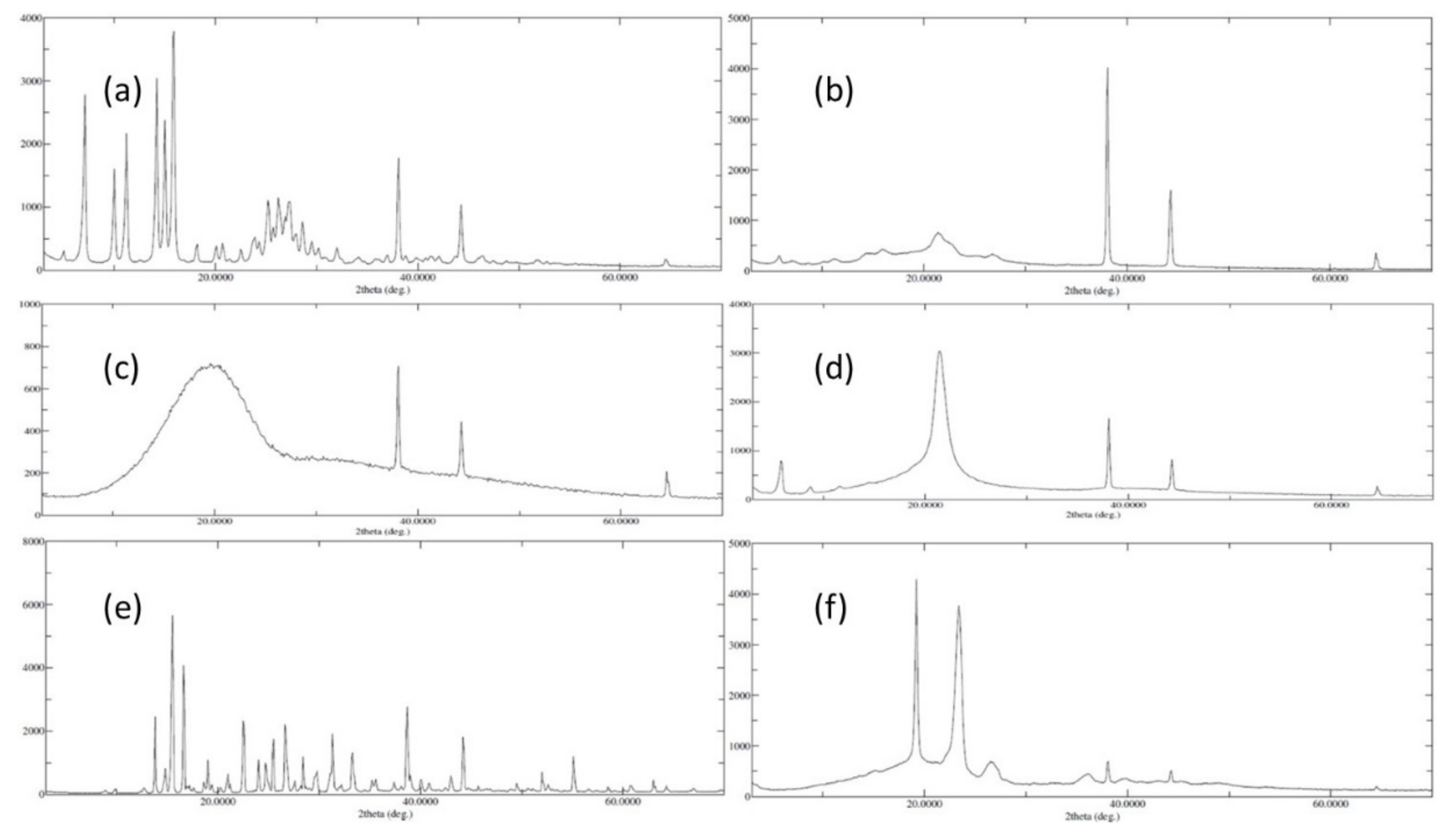
2.3.4. X-ray Diffraction Analysis
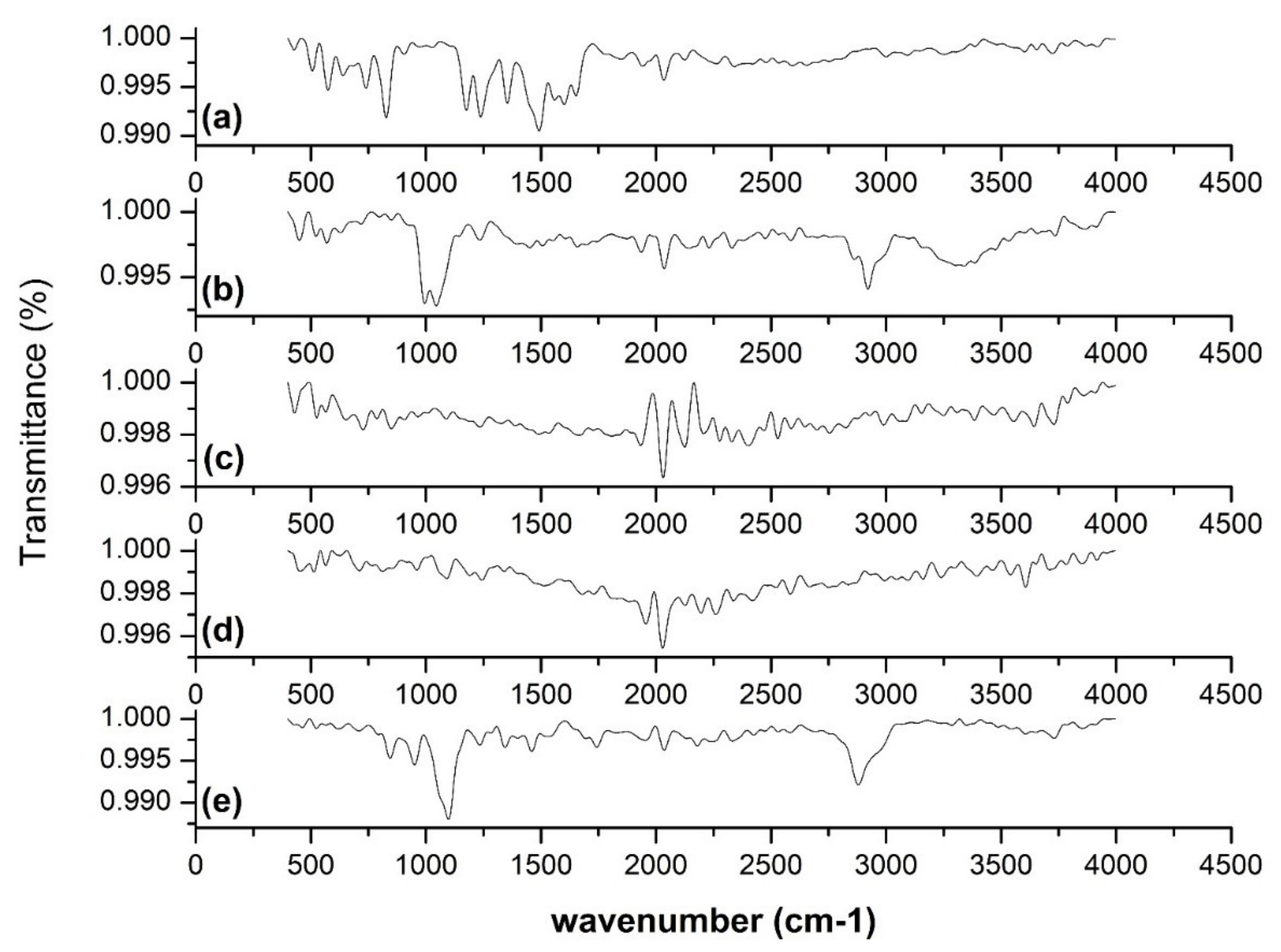
2.3.5. FT-IR Analysis
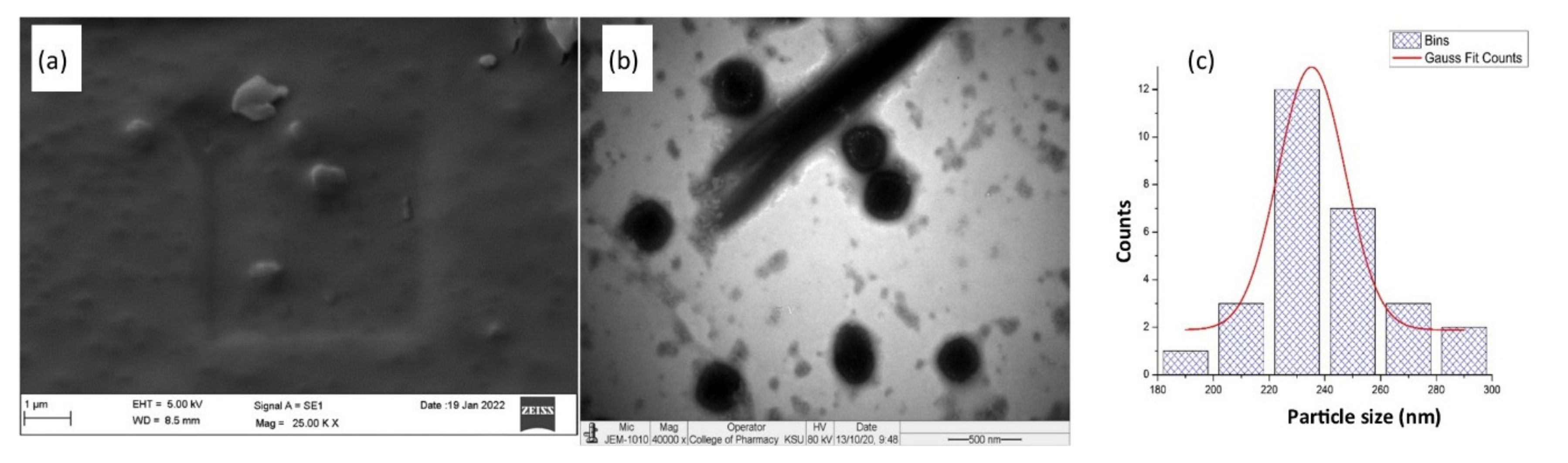
2.3.6. Morphological Analysis
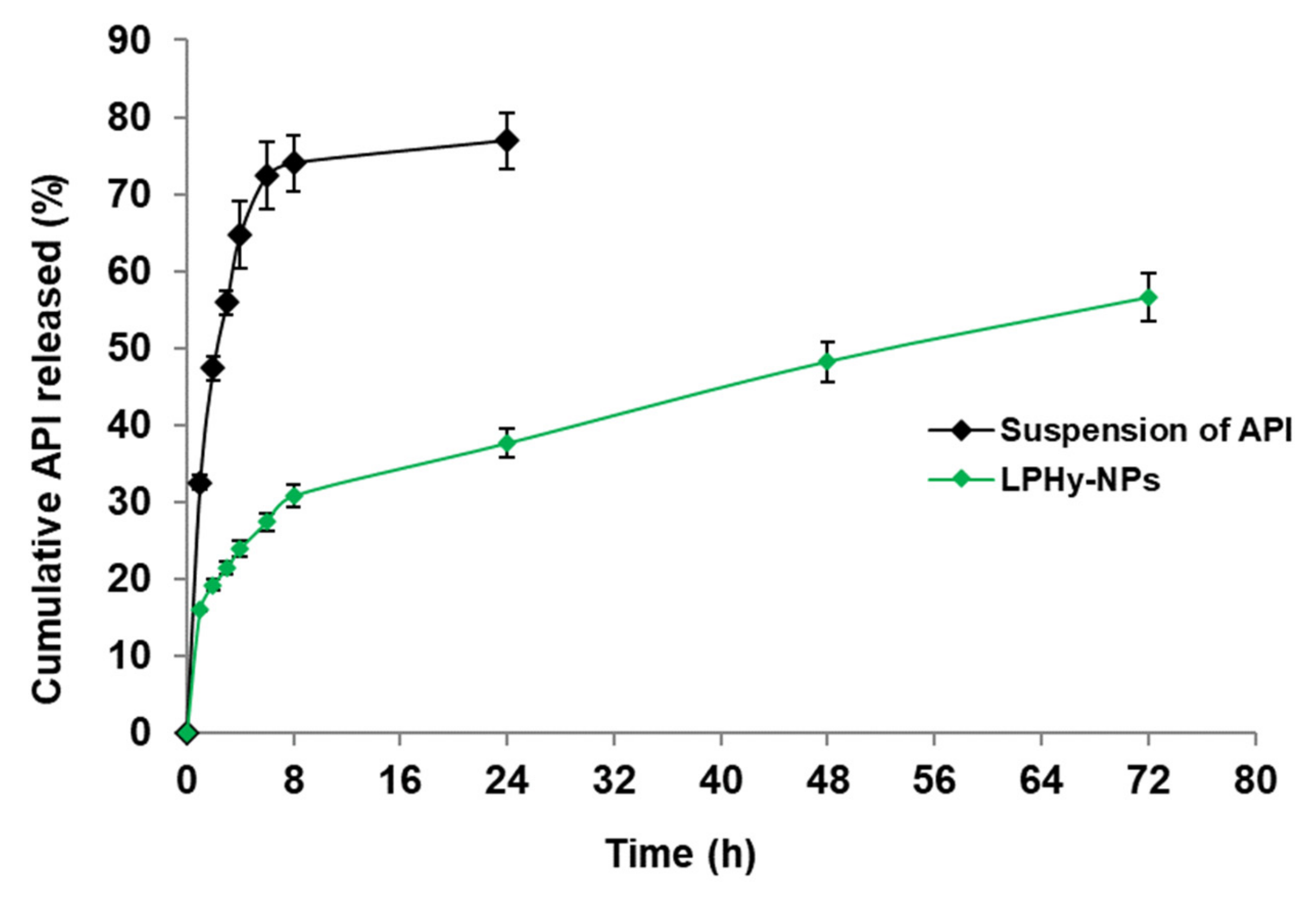
2.4. Study of In Vitro Release Pattern
2.5. Cell Viability Assay
2.6. Cell Cycle and Apoptosis Analysis Using Flow Cytometry
2.7. Gene Expression of the Various Carcinogenic Marker Using RT-PCR
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Particle Size, PDI, and Surface Potential of LPHyNP
3.2. Drug Entrapment and Loading Efficiency of Prepared LPHyNP
3.3. Differential Scanning Calorimeter (DSC) Analysis
3.4. X-ray Diffraction Analysis
3.5. FT-IR Analysis
3.6. Morphological Analysis
3.7. Study of In Vitro Release Pattern
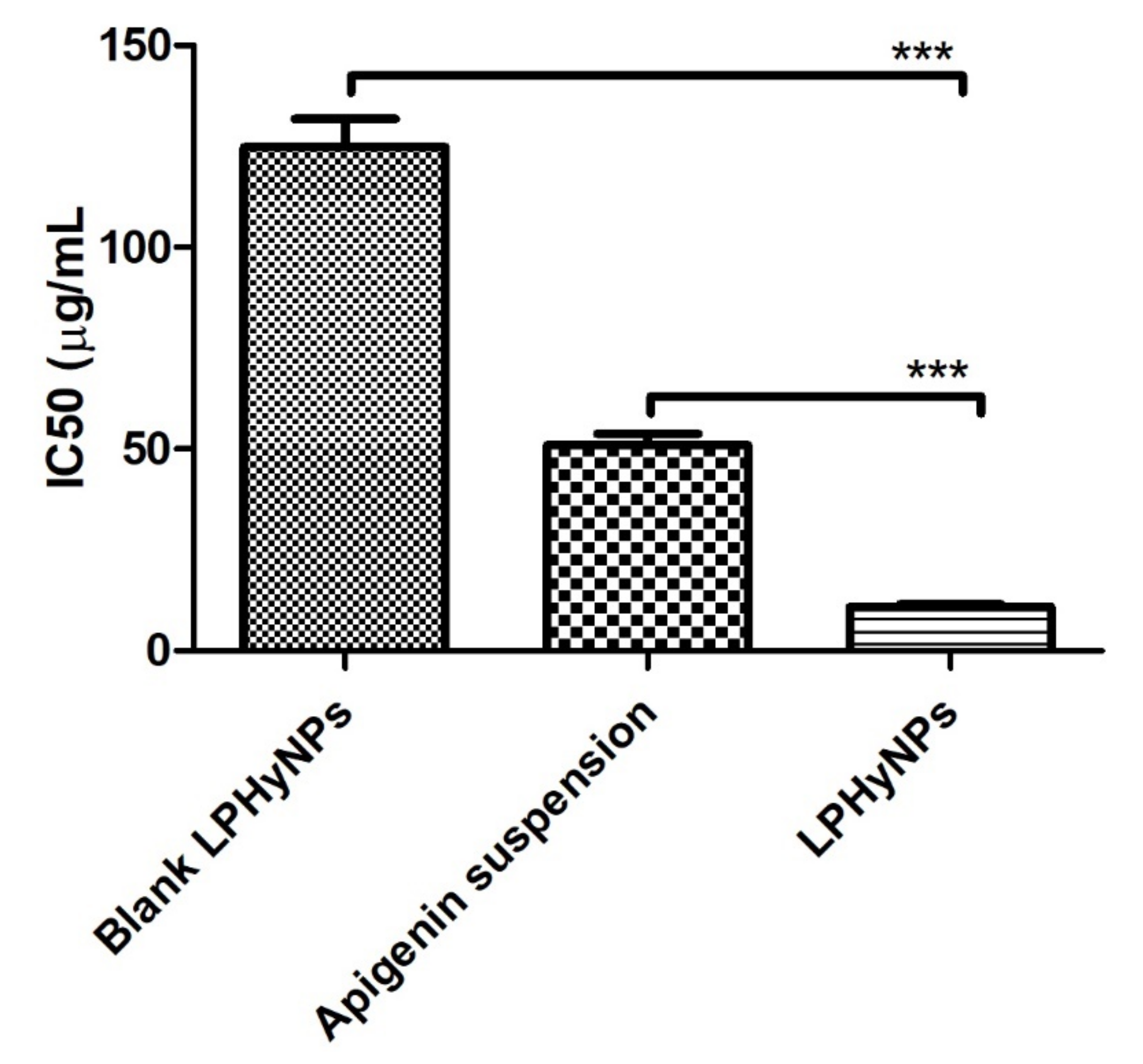
3.8. Cell Viability Analysis (MTT Assay)
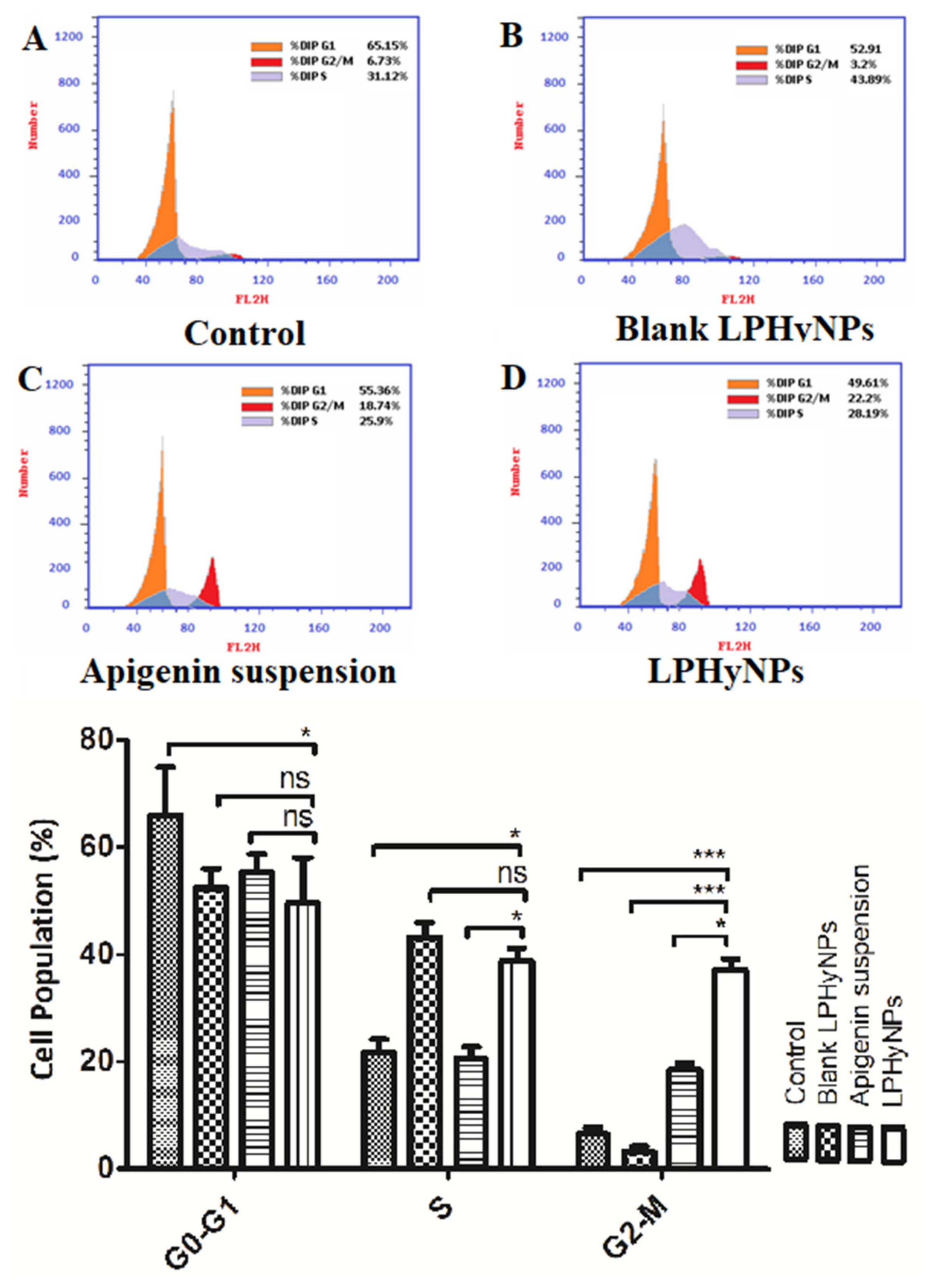
3.9. Effect of LPHyNPs on Cell Cycle
3.10. Apoptosis Assay Using Flow Cytometry
3.11. Effect of LPHyNPs on the Gene Expression of Bcl-2, BAX, NF-κB, mTOR, JNK, and MDR-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Condello, M.; Meschini, S. Role of Natural Antioxidant Products in Colorectal Cancer Disease: A Focus on a Natural Compound Derived from Prunus Spinosa, Trigno Ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zheng, X.; Li, M.; Zhang, L.; Yu, J. MTOR Inhibitors Induce Apoptosis in Colon Cancer Cells via CHOP-Dependent DR5 Induction on 4E-BP1 Dephosphorylation. Oncogene 2016, 35, 148–157. [Google Scholar] [CrossRef]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.; Samowitz, W.S.; Wolff, R.K.; Stevens, J.R.; Herrick, J.S. The NF-ΚB Signalling Pathway in Colorectal Cancer: Associations between Dysregulated Gene and MiRNA Expression. J. Cancer Res. Clin. Oncol. 2018, 144, 269–283. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Iqubal, A.; Garg, V.K.; Varol, M.; Sharma, U.; Chauhan, A.; Yerer, M.B.; Dhama, K.; Jain, M.; et al. STAT Signaling as a Target for Intervention: From Cancer Inflammation and Angiogenesis to Non-Coding RNAs Modulation. Mol. Biol. Rep. 2022, 1–13. [Google Scholar] [CrossRef]
- Dan, H.C.; Adli, M.; Baldwin, A.S. Regulation of Mammalian Target of Rapamycin Activity in PTEN-Inactive Prostate Cancer Cells by I Kappa B Kinase Alpha. Cancer Res. 2007, 67, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 0731. [Google Scholar] [CrossRef]
- Fatima, M.; Iqubal, M.K.; Iqubal, A.; Kaur, H.; Gilani, S.J.; Rahman, M.H.; Ahmadi, A.; Rizwanullah, M. Current Insight into the Therapeutic Potential of Phytocompounds and Their Nanoparticle-Based Systems for Effective Management of Lung Cancer. Anticancer. Agents Med. Chem. 2021, 21, 668–686. [Google Scholar] [CrossRef]
- Linn, S.; Giaccone, G. MDR1/P-Glycoprotein Expression in Colorectal Cancer. Eur. J. Cancer 1995, 31, 1291–1294. [Google Scholar] [CrossRef]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-Mutagenesis and Anti-Promotion by Apigenin, Robinetin and Indole-3-Carbinol. Carcinogenesis 1986, 7, 959–963. [Google Scholar] [CrossRef]
- Mabrouk Zayed, M.M.; Sahyon, H.A.; Hanafy, N.A.N.; El-Kemary, M.A. The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics 2022, 14, 1160. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the Therapeutic Potential of Apigenin against Cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics Classification and Intestinal Absorption Study of Apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, Z.; Song, J.; Cheng, X.; Jiang, J.; Jia, X. Enhanced Bioavailability of Apigenin via Preparation of a Carbon Nanopowder Solid Dispersion. Int. J. Nanomed. 2014, 9, 2327–2333. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Silva, L.B.; Castro, K.A.D.F.; Botteon, C.E.A.; Oliveira, C.L.P.; da Silva, R.S.; Marcato, P.D. Hybrid Nanoparticles as an Efficient Porphyrin Delivery System for Cancer Cells to Enhance Photodynamic Therapy. Front. Bioeng. Biotechnol. 2021, 9, 679128. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Quarta, A.; Di Corato, R.; Dini, L.; Nobile, C.; Tasco, V.; Carallo, S.; Cascione, M.; Malfettone, A.; Soukupova, J.; et al. Hybrid Polymeric-Protein Nano-Carriers (HPPNC) for Targeted Delivery of TGFβ Inhibitors to Hepatocellular Carcinoma Cells. J. Mater. Sci. Mater. Med. 2017, 28, 120. [Google Scholar] [CrossRef]
- Salzano, G.; Marra, M.; Porru, M.; Zappavigna, S.; Abbruzzese, A.; La Rotonda, M.I.; Leonetti, C.; Caraglia, M.; De Rosa, G. Self-Assembly Nanoparticles for the Delivery of Bisphosphonates into Tumors. Int. J. Pharm. 2011, 403, 292–297. [Google Scholar] [CrossRef]
- Hanafy, N.A.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials 2018, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Wan, F.; Bohr, S.S.-R.; Kłodzińska, S.N.; Jumaa, H.; Huang, Z.; Nylander, T.; Thygesen, M.B.; Sørensen, K.K.; Jensen, K.J.; Sternberg, C.; et al. Ultrasmall TPGS-PLGA Hybrid Nanoparticles for Site-Specific Delivery of Antibiotics into Pseudomonas Aeruginosa Biofilms in Lungs. ACS Appl. Mater. Interfaces 2020, 12, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Ishak, R.A.H.; Mostafa, N.M.; Kamel, A.O. Stealth Lipid Polymer Hybrid Nanoparticles Loaded with Rutin for Effective Brain Delivery—Comparative Study with the Gold Standard (Tween 80): Optimization, Characterization and Biodistribution. Drug Deliv. 2017, 24, 1874–1890. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial Lipid-Nanosystem for Dermal Delivery of 5-Fluorouracil and Resveratrol against Skin Cancer: Delineation of Improved Dermatokinetics and Epidermal Drug Deposition Enhancement Analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Ghorbani, M.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Targeted Hyaluronic Acid-Based Lipid Nanoparticle for Apigenin Delivery to Induce Nrf2-Dependent Apoptosis in Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2019, 49, 268–276. [Google Scholar] [CrossRef]
- Md, S.; Abdullah, S.; Alhakamy, N.A.; Alharbi, W.S.; Ahmad, J.; Shaik, R.A.; Ansari, M.J.; Ibrahim, I.M.; Ali, J. Development, Optimization, and In Vitro Evaluation of Novel Oral Long-Acting Resveratrol Nanocomposite In-Situ Gelling Film in the Treatment of Colorectal Cancer. Gels 2021, 7, 276. [Google Scholar] [CrossRef]
- Rimkiene, L.; Baranauskaite, J.; Marksa, M.; Jarukas, L.; Ivanauskas, L. Development and Evaluation of Ginkgo Biloba L. Extract Loaded into Carboxymethyl Cellulose Sublingual Films. Appl. Sci. 2021, 11, 270. [Google Scholar] [CrossRef]
- Senthamarai Kannan, M.; Hari Haran, P.S.; Sundar, K.; Kunjiappan, S.; Balakrishnan, V. Fabrication of Anti-Bacterial Cotton Bandage Using Biologically Synthesized Nanoparticles for Medical Applications. Prog. Biomater. 2022, 11, 229–241. [Google Scholar] [CrossRef]
- Almehmady, A.M.; Elsisi, A.M. Development, Optimization, and Evaluation of Tamsulosin Nanotransfersomes to Enhance Its Permeation and Bioavailability. J. Drug Deliv. Sci. Technol. 2020, 57, 101667. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; Md, S. Development and in Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer. Polymers 2021, 13, 884. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and in Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; Shadab, M. Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer. Pharmaceuticals 2021, 14, 729. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Elshal, M.F.; Kumosani, T.A.; Aldahlawi, A.M.; Basbrain, T.A.; Alshehri, F.A.; Choudhry, H. L-Asparaginase Isolated from Phaseolus Vulgaris Seeds Exhibited Potent Anti-Acute Lymphoblastic Leukemia Effects In-Vitro and Low Immunogenic Properties In-Vivo. Int. J. Environ. Res. Public Health 2016, 13, 1008. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Dina, F.; Soliman, G.M.; Fouad, E.A. Development and in Vitro/in Vivo Evaluation of Liposomal Gels for the Sustained Ocular Delivery of Latanoprost. J. Clin. Exp. Ophthalmol. 2015, 6, 16–19. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlȩga, B.; Misiak, L.E.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. FTIR, 1H NMR and EPR Spectroscopy Studies on the Interaction of Flavone Apigenin with Dipalmitoylphosphatidylcholine Liposomes. Biochim. Biophys. Acta—Biomembr. 2013, 1828, 518–527. [Google Scholar] [CrossRef]
- Liu, G.; Li, K.; Wang, H. Polymeric Micelles Based on PEGylated Chitosan-g-Lipoic Acid as Carrier for Efficient Intracellular Drug Delivery. J. Biomater. Appl. 2017, 31, 1039–1048. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in Vivo Virtue of Dermal Targeted Combinatorial Lipid Nanocolloidal Based Formulation of 5-Fluorouracil and Resveratrol against Skin Cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.I.; Sambamoorthy, U.; Kumar, S.A. D-ɑ-Tocopheryl Polyethylene Glycol Succinate: A Review of Multifarious Applications in Nanomedicines. OpenNano 2022, 6, 100036. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-Cancer Potential of Polysaccharide Extracted from Hawthorn (Crataegus.) on Human Colon Cancer Cell Line HCT116 via Cell Cycle Arrest and Apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Li, X.-Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Lin, A.W. Apoptosis in Cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.M. Apoptosis and Colorectal Cancer. Gut 2004, 53, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. Onco. Targets. Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Hasan, S.; Taha, R.; Omri, H. El Current Opinions on Chemoresistance: An Overview. Bioinformation 2018, 14, 80–85. [Google Scholar] [CrossRef]
- Tournier, C. The 2 Faces of JNK Signaling in Cancer. Genes Cancer 2013, 4, 397–400. [Google Scholar] [CrossRef]
- Elia, S.G.; Al-Karmalawy, A.A.; Nasr, M.Y.; Elshal, M.F. Loperamide Potentiates Doxorubicin Sensitivity in Triple-negative Breast Cancer Cells by Targeting MDR1 and JNK and Suppressing MTOR and Bcl-2: In Vitro and Molecular Docking Study. J. Biochem. Mol. Toxicol. 2022, 36, e22938. [Google Scholar] [CrossRef]




| Target | Primer Used |
|---|---|
| BAX | F: 5′-CTGCAGAGGATGATTGCCG-3′ R: 5′-TGCCACTCGGAAAAAGACCT-3′ |
| Bcl-2 | F: 5′-GACTTCGCCGAGATGTCCAG-3′ R: 5′-GAACTCAAAGAAGGCCACAATC-3′ |
| mTOR | F: 5′-GCTTGATTTGGTTCCCAGGACAGT3 R: 5′-GTGCTGAGTTTGCTGTACCCATGT3′ |
| JNK | F: 5′ -GTGT-GGAATCAAGCACCTTC-3′ R: 5′ -AGGCGTCATCATAAAACTCGTTC-3 |
| NF-κB | F: 5’- CGCATCCAGACCAACAACA-3’ R: 5’- TGCCAGAGTTTCGGTTCAC-3’ |
| MDR1 | F: 5′-CCC ATC ATT GCA ATA GCA GG-3′ R: 5′-TGT TCA AAC TTC TGC TCC TGA-3′ |
| β-actin | F: 5′-AGAGCTACGAGCTGCCTGAC-3′ R: 5′-AGCACTGTGTTGGCGTACAG-3′ |
| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|
| Blank LPHyNPs | 200.26 ± 9.19 | 0.34 ± 0.10 | −4.14 ± 0.81 | - | - |
| LPHyNPs | 234.80 ± 12.28 | 0.11 ± 0.04 | −5.15 ± 0.70 | 55.18 ± 3.61 | 11.04 ± 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaleh, M.A.; Hashem, A.M.; Abujamel, T.S.; Alhakamy, N.A.; Kalam, M.A.; Riadi, Y.; Md, S. Apigenin Loaded Lipoid–PLGA–TPGS Nanoparticles for Colon Cancer Therapy: Characterization, Sustained Release, Cytotoxicity, and Apoptosis Pathways. Polymers 2022, 14, 3577. https://doi.org/10.3390/polym14173577
Alfaleh MA, Hashem AM, Abujamel TS, Alhakamy NA, Kalam MA, Riadi Y, Md S. Apigenin Loaded Lipoid–PLGA–TPGS Nanoparticles for Colon Cancer Therapy: Characterization, Sustained Release, Cytotoxicity, and Apoptosis Pathways. Polymers. 2022; 14(17):3577. https://doi.org/10.3390/polym14173577
Chicago/Turabian StyleAlfaleh, Mohamed A., Anwar M. Hashem, Turki S. Abujamel, Nabil A. Alhakamy, Mohd Abul Kalam, Yassine Riadi, and Shadab Md. 2022. "Apigenin Loaded Lipoid–PLGA–TPGS Nanoparticles for Colon Cancer Therapy: Characterization, Sustained Release, Cytotoxicity, and Apoptosis Pathways" Polymers 14, no. 17: 3577. https://doi.org/10.3390/polym14173577
APA StyleAlfaleh, M. A., Hashem, A. M., Abujamel, T. S., Alhakamy, N. A., Kalam, M. A., Riadi, Y., & Md, S. (2022). Apigenin Loaded Lipoid–PLGA–TPGS Nanoparticles for Colon Cancer Therapy: Characterization, Sustained Release, Cytotoxicity, and Apoptosis Pathways. Polymers, 14(17), 3577. https://doi.org/10.3390/polym14173577








