Synthesis, Characterization and Catalytic Property Studies for Isoprene Polymerization of Iron Complexes Bearing Unionized Pyridine-Oxime Ligands
Abstract
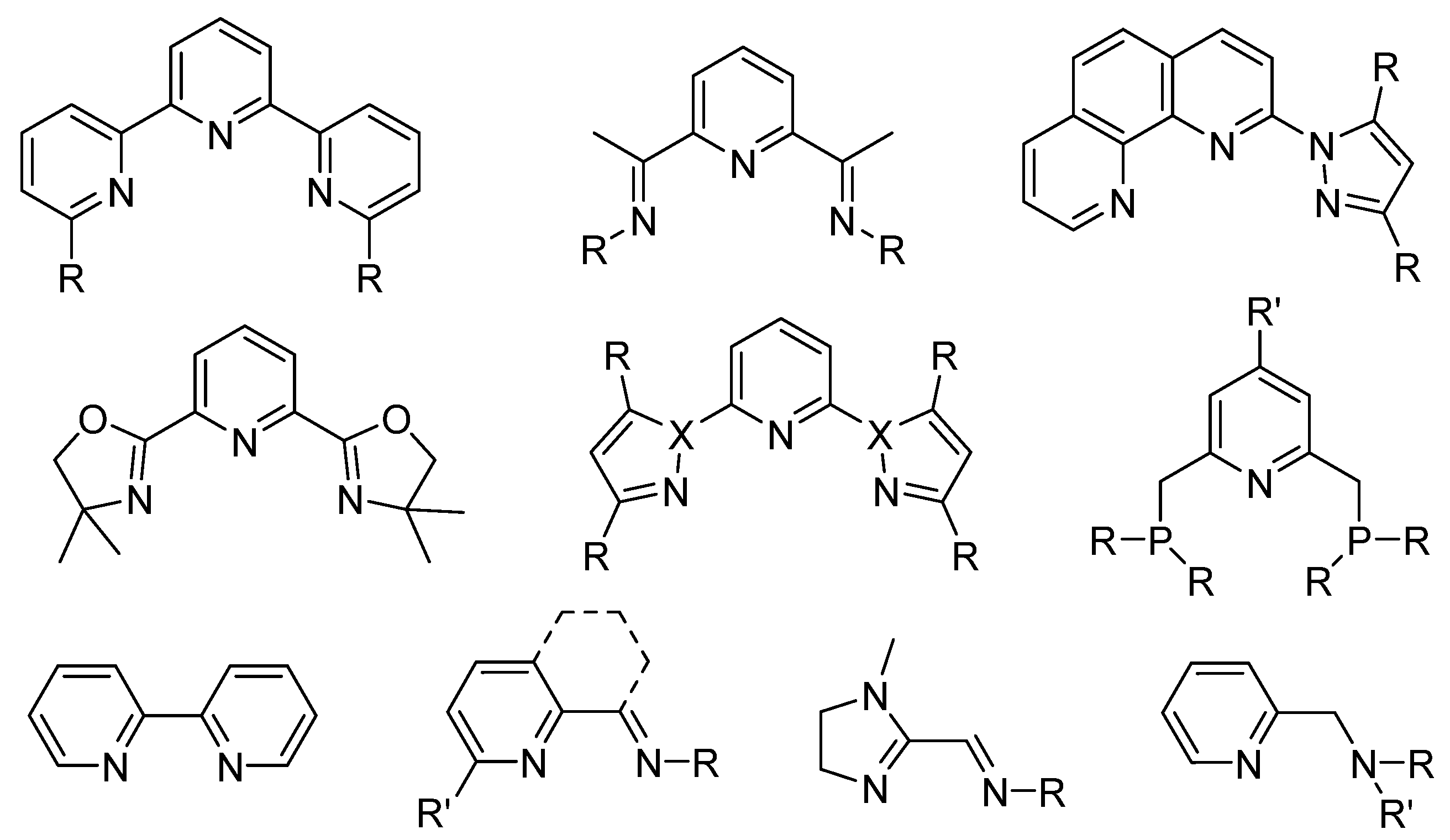
:1. Introduction
2. Materials & Methods
2.1. Materials
2.2. Preparation of Ligands L2 and L4
2.3. Preparation of Complexes Fe1–Fe4
2.4. Polymerization Procedure
3. Results and Discussion
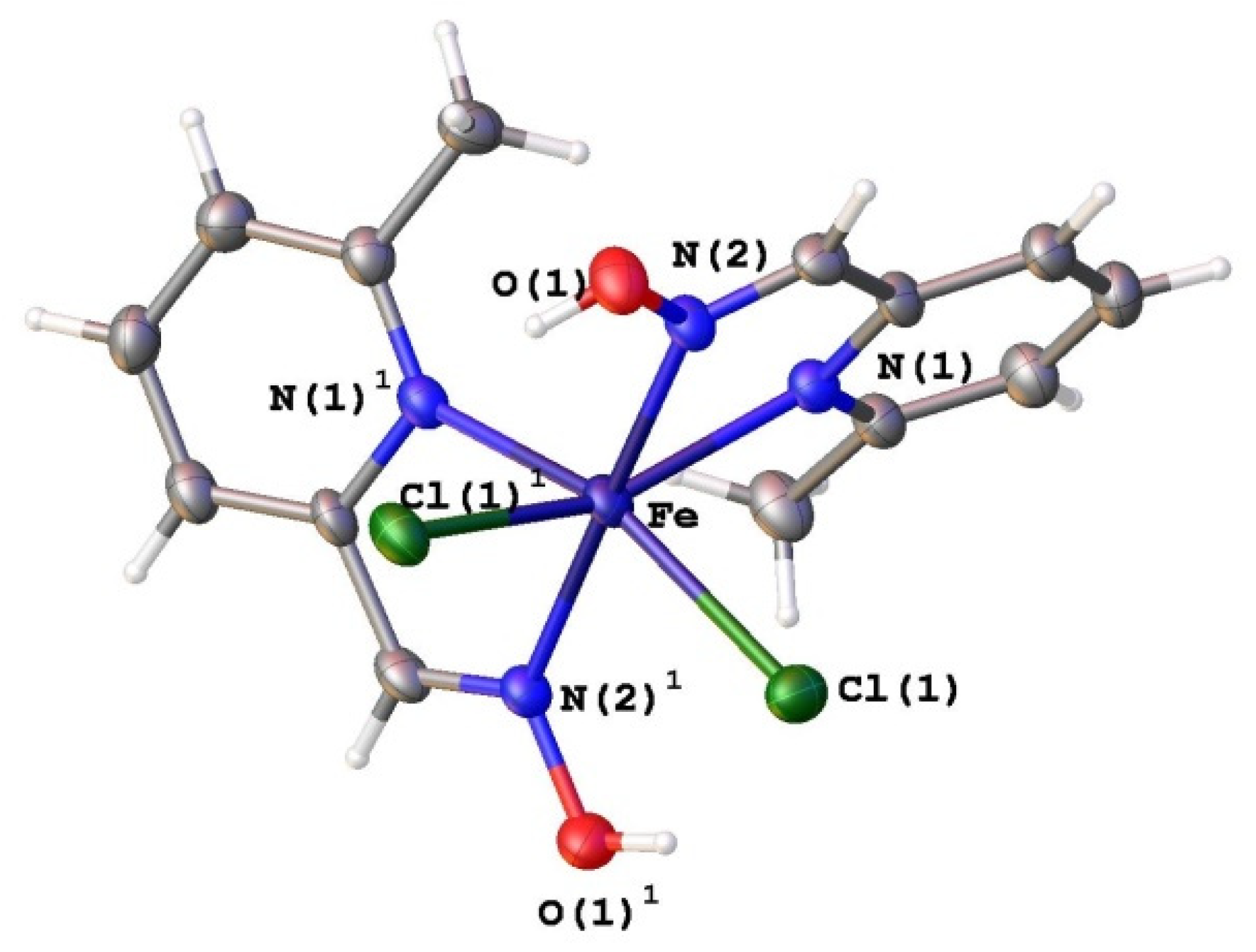
3.1. Synthesis and Characterization of Ligands L2, L4 and Fe(II) Complexes
3.2. Isoprene Polymerization with Binary Catalytic System
3.3. Isoprene Polymerization with Ternary Catalytic System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moad, G. Reversible addition-fragmentation chain transfer (co)polymerization of conjugated diene monomers: Butadiene, isoprene and chloroprene. Polym. Int. 2017, 66, 26–41. [Google Scholar] [CrossRef]
- Ouardad, S.; Bakleh, M.-E.; Kostjuk, S.V.; Ganachaud, F.; Puskas, J.E.; Deffieux, A.; Peruch, F. Bio-inspired cationic polymerization of isoprene and analogues: State-of-the-art. Polym. Int. 2012, 61, 149–156. [Google Scholar] [CrossRef]
- Osakada, K.; Takeuchi, D. Coordination polymerization of dienes, allenes, and methylenecycloalkanes. Polym. Syn. 2004, 171, 137–194. [Google Scholar]
- Zhu, G.Q.; Wang, L.; Kuang, J.; Xu, G.Q.; Zhang, Y.; Wang, Q.G. High double bond content of polyisoprene synthesis via cationic polymerization synergistically catalyzed by Tf2NH-ionic liquids. Macromolecules 2021, 54, 6109–6116. [Google Scholar] [CrossRef]
- Thiele, S.K.H.; Wilson, D.R. Alternate transition metal complex based diene polymerization. J. Macromol. Sci.-Pol. Rev. 2003, C43, 581–628. [Google Scholar] [CrossRef]
- Tinyakova, E.I.; Yakovlev, V.A. New aspects of stereospecific polymerization of butadiene and isoprene with coordination catalysts. Polym. Sci. Ser. B 2003, 45, 219–236. [Google Scholar]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coordin. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Gong, D.R.; Jia, X.Y.; Wang, B.L.; Zhang, X.Q.; Huang, K.-W. Trans-1,4 selective polymerization of 1,3-butadiene with symmetry pincer chromium complexes activated by MMAO. J. Organomet. Chem. 2014, 766, 79–85. [Google Scholar] [CrossRef]
- Faisca Phillips, A.M.; Suo, H.Y.; Guedes da Silva, M.d.F.C.; Pombeiro, A.J.L.; Sun, W.-H. Recent developments in vanadium-catalyzed olefin coordination polymerization. Coordin. Chem. Rev. 2020, 416, 213332–213360. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Jiang, M.-H.; Dong, K.-X.; He, A.-H. Species on the activated TiCl4/MgCl2 pre-catalyst for diene polymerizations. Catal. Lett. 2021, 152, 2543–2551. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar]
- Zhu, H.; Zuo, X.L.; Zhang, S.; Ma, X.L.; Liu, Y.F.; Wu, Y.X. Progress in rare earth catalysts and their use in the synthesis of rubber and elastomer. Polym. Bull. 2014, 5, 65–87. [Google Scholar]
- Pan, Y.; Cai, Y.C.; Song, D.Y.; He, G.H. Development of rare earth metal catalyst systems for cis-1,4-polymerization of 1,3-conjugated dienes. Chin. Rare Earths 2016, 37, 100–107. [Google Scholar]
- Huang, J.M.; Liu, Z.H.; Cui, D.M.; Liu, X.L. Precisely controlled polymerization of styrene and conjugated dienes by group 3 single-site catalysts. Chemcatchem 2018, 10, 42–61. [Google Scholar] [CrossRef]
- Takeuchi, D.; Osakada, K. New polymerization of dienes and related monomers catalyzed by late transition metal complexes. Polymer 2008, 49, 4911–4924. [Google Scholar] [CrossRef] [Green Version]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes polymerization: Where we are and what lies ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Liu, L.J.; Wang, F.; Zhang, C.Y.; Liu, H.; Wu, G.F.; Zhang, X.Q. Thermally robust alpha-diimine nickel and cobalt complexes for cis-1,4 selective 1,3-butadiene polymerizations. Mol. Catal. 2022, 517, 112044. [Google Scholar] [CrossRef]
- Bi, J.F.; Ge, J.N.; Zhang, X.Q. Isoprene polymerization behaviors by using Fe(2-EHA)3/Al(i-Bu)3/AIBN catalyst system. J. Nat. Sci. Heilongjiang Univ. 2011, 28, 690–695. [Google Scholar]
- Hu, Y.M.; Dong, W.M.; Jiang, L.S.; Zhang, X.Q. Polymerization of isoprene using an iron-based catalyst with diethyl phosphate as third component. Chin. Synth. Rubber Ind. 2004, 27, 344–347. [Google Scholar]
- Shen, Q.; Guo, X.G.; Xu, X.L.; Liu, G.Z. A novel high active novel iron catalyst Fe(naph)2-Al(i-Bu)3-halides for polymerization of butadiene. Chin. Sci. Bull. 1988, 12, 988–990. [Google Scholar]
- Wang, F.J.; Liu, G.Z.; Qian, B.G. Studies on the polymerization of butadiene in the presence of iron catalyst VI. Effects of nitrogen-containing ligands on the polymerization and the microstructure of polymer. Chin. J. Appl. Chem. 1988, 5, 15–20. (In Chinese) [Google Scholar]
- Bazzini, C.; Giarrusso, A.; Porri, L. Diethyl bis(2,2′-bipyridine) iron/MAO. A very active and stereospecific catalyst for 1, 3-diene polymerization. Macromol. Rapid Commun. 2002, 23, 922–927. [Google Scholar] [CrossRef]
- Ricci, G.; Morganti, D.; Sommazzi, A.; Santi, R.; Masi, F. Polymerization of 1,3-dienes with iron complexes based catalysts. Influence of the ligand on catalyst activity and stereospecificity. J. Mol. Catal. A Chem. 2003, 204, 287–293. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L.; Pirozzi, B.; Napolitano, R. Synthesis and characterization of syndiotactic 3, 4-polyisoprene prepared with diethylbis (2,2′-bipyridine) iron–MAO. Polymer 2004, 45, 2871–2875. [Google Scholar] [CrossRef]
- Pirozzi, B.; Napolitano, R.; Petraccone, V.; Esposito, S. Determination of the crystal structure of syndiotactic 3,4-poly (2-methyl-1,3-butadiene) by molecular mechanics and X-Ray diffraction. Macromol. Chem. Phys. 2004, 205, 1343–1350. [Google Scholar] [CrossRef]
- Nakayama, Y.; Baba, Y.; Yasuda, H.; Kawakita, K.; Ueyama, N. Stereospecific polymerizations of conjugated dienes by single site iron complexes having chelating N,N,N-donor ligands. Macromolecules 2003, 36, 7953–7958. [Google Scholar] [CrossRef]
- Gong, D.R.; Wang, B.L.; Bai, C.X.; Bi, J.F.; Wang, F.; Dong, W.M.; Zhang, X.Q.; Jiang, L.S. Metal dependent control of cis-1,4/trans-1,4 regioselectivity in 1,3-butadiene polymerization catalyzed by transition metal complexes supported by 2,6-bis[1-(iminophenyl)ethyl]pyridine. Polymer 2009, 50, 6259–6264. [Google Scholar] [CrossRef]
- Wang, B.L.; Bi, J.F.; Zhang, C.Y.; Dai, Q.; Bai, C.X.; Zhang, X.Q.; Hu, Y.M.; Jiang, L.S. Highly active and trans-1,4 specific polymerization of 1,3-butadiene catalyzed by 2-pyrazolyl substituted 1,10-phenanthroline ligated iron (II) complexes. Polymer 2013, 54, 5174–5181. [Google Scholar] [CrossRef]
- Zhang, J.S.; Gao, W.; Lang, X.M.; Wu, Q.L.; Zhang, L.; Mu, Y. Ni(II) and Fe(II) complexes based on bis(imino)aryl pincer ligands: Synthesis, structural characterization and catalytic activities. Dalton Trans. 2012, 41, 9639–9645. [Google Scholar] [CrossRef]
- Gong, D.R.; Jia, X.Y.; Wang, B.L.; Zhang, X.Q.; Jiang, L.S. Synthesis, characterization, and butadiene polymerization of iron(III), iron(II) and cobalt(II) chlorides bearing 2,6-bis(benzimidazolyl)pyridyl or 2,6-bis(pyrazol)pyridine ligand. J. Organomet. Chem. 2012, 702, 10–18. [Google Scholar] [CrossRef]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-catalyzed polymerization of isoprene and other 1,3-dienes. Angew. Chem. Int. Ed. 2012, 51, 11805–11808. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Jing, X.Y.; Xiong, S.Y.; Liu, W.J.; Liu, Y.L.; Liu, Z.; Chen, C.L. Influences of alkyl and aryl substituents on iminopyridine Fe(II)- and Co(II)-catalyzed isoprene polymerization. Polymers 2016, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, O.H.; Champouret, Y.; Visseaux, M. Highly active iminopyridyl iron-based catalysts for the polymerization of isoprene. Molecules 2019, 24, 3024. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Zhang, X.H.; Zhao, M.M.; Wang, L.; Jing, C.Y.; Wang, P.; Wang, X.W.; Wang, Q.G. Influences of fluorine substituents on iminopyridine Fe(II)- and Co(II)-catalyzed isoprene polymerization. Polymers 2018, 10, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhu, G.Q.; Mahmood, Q.; Zhao, M.M.; Wang, L.; Jing, C.Y.; Wang, X.W.; Wang, Q.G. Iminoimidazole-based Co(II) and Fe(II) complexes: Syntheses, characterization, and catalytic behaviors for isoprene polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 767–775. [Google Scholar] [CrossRef]
- Zhao, M.M.; Wang, L.; Mahmood, Q.; Jing, C.Y.; Zhu, G.Q.; Zhang, X.H.; Wang, X.W.; Wang, Q.G. Controlled isoprene polymerization mediated by iminopyridine-iron(II) acetylacetonate pre-catalysts. Appl. Organometal. Chem. 2019, 33, e4836. [Google Scholar] [CrossRef]
- Jing, C.Y.; Wang, L.; Mahmood, Q.; Zhao, M.M.; Zhu, G.Q.; Zhang, X.H.; Wang, X.W.; Wang, Q.G. Synthesis and characterization of aminopyridine iron(ii) chloride catalysts for isoprene polymerization: Sterically controlled monomer enchainment. Dalton Trans. 2019, 48, 7862–7874. [Google Scholar] [CrossRef]
- Jing, C.Y.; Wang, L.; Zhu, G.Q.; Hou, H.B.; Zhou, L.; Wang, Q.G. Enhancing thermal stability in aminopyridine iron(II)-catalyzed polymerization of conjugated dienes. Organometallics 2020, 39, 4019–4026. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.W.; Hou, H.B.; Zhu, G.Q.; Han, Z.Y.; Yang, W.Y.; Chen, X.Y.; Wang, Q.G. An unsymmetrical binuclear iminopyridine-iron complex and its catalytic isoprene polymerization. Chem. Commun. 2020, 56, 8846–8849. [Google Scholar] [CrossRef]
- Weyhermüller, T.; Wagner, R.; Khanra, S.; Chaudhuri, P. A magneto structural study of linear NiII MnIII NiII, NiIICrIIINiII and triangular NiII3 species containing (pyridine-2-aldoximato)nickel(II) unit as a building block. Dalton Trans. 2005, 15, 2539–2546. [Google Scholar] [CrossRef]
- Erdogan, D.A.; Özalp-Yaman, Ş. Novel Pt(II) complexes containing pyrrole oxime; synthesis, characterization and DNA binding studies. J. Mol. Struct. 2014, 1064, 50–57. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patel, B.A.; Bhaduri, S. Selective ethylene oligomerization with nickel oxime complexes. Organometallics 2009, 28, 3074–3078. [Google Scholar] [CrossRef]
- Tayade, K.N.; Mane, M.V.; Sen, S.; Murthy, C.N.; Tembe, G.L.; Pillai, S.M.; Vanka, K.; Mukherjee, S. A catalytic and DFT study of selective ethylene oligomerization by nickel(II) oxime-based complexes. J. Mol. Catal. A-Chem. 2013, 366, 238–246. [Google Scholar] [CrossRef]
- Tyagi, D.; Rai, R.K.; Mobin, S.M.; Singh, S.K. N-Substituted iminopyridine arene–Ruthenium complexes for the regioselective catalytic hydration of terminal alkynes. Asian J. Org. Chem. 2017, 6, 1647–1658. [Google Scholar] [CrossRef]
- Mohan, M.; Mittal, S.G.; Khera, H.C.; Srivastava, A.K. Transition metal chemistry of oxime containing ligands: Part III. Complexes of iron(II), cobalt(II), and nickel(II) with pyridine-2-aldoxime and 6-methylpyridine-2-aldoxime. Indian J. Chem. A 1977, 15, 696–699. [Google Scholar] [CrossRef]
- Mohan, M.; Paramhans, B.D. Transition metal chemistry of oxime-containing ligands, part XIV. Iron (II) complexes of syn-phenyl-2-pyridylketoxime and syn-methyl-2-pyridylketoxime. Croat. Chem. Acta 1981, 54, 173–182. [Google Scholar]
- Krause, R.A.; Colthup, N.B.; Busch, N.B. Infrared spectra of complexes of 2-pyridinaldoxime. J. Phys. Chem. 1961, 65, 2216–2219. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Weyhermüller, T.; Wagner, R.; Khanra, S.; Biswas, B.; Bothe, E.; Bill, E. Tridentate facial ligation of tris(pyridine-2-aldoximato)nickel(II) and tris(imidazole-2-aldoximato)nickel(II) to generate NiIIFeIIINiII, MnIIINiII, NiIINiII, and ZnIINiII and the electrooxidized MnIVNiII, NiIINiIII, and ZnIINiIII species: A magnetostructural, electrochemical, and EPR spectroscopic study. Inorg. Chem. 2007, 46, 9003–9016. [Google Scholar]


| Entry | Cat. | Time (min) | Yield b (%) | Act. c (×10−5) | Mnd (×10−4) | Mw/Mnd | Microstructure (%) e | ||
|---|---|---|---|---|---|---|---|---|---|
| trans-1,4 | cis-1,4 | 3,4 | |||||||
| 1 | Fe1 | 10 | >99 | 8.2 | 47.5 | 1.8 | 0 | 48 | 52 |
| 2 | Fe2 | 10 | 49 | 4.0 | 11.5 | 1.8 | 11 | 43 | 46 |
| 3 | Fe2 | 120 | 86 | 0.6 | 7.8 | 2.3 | 18 | 39 | 43 |
| 4 | Fe2 | 300 | 98 | 0.3 | 5.9 | 3.5 | 20 | 37 | 43 |
| 5 | Fe3 | 10 | >99 | 8.2 | 32.3 | 2.1 | 0 | 46 | 54 |
| 6 | Fe4 | 10 | >99 | 8.2 | 57.2 | 2.3 | 0 | 42 | 58 |
| Entry | Cat. | [Fe]/[MAO] | Time (min) | Yield b (%) | Act. c (×10−5) | Mnd (×10−5) | Mw/Mnd | Microstructure (%) e | |
|---|---|---|---|---|---|---|---|---|---|
| cis-1,4 | 3,4 | ||||||||
| 1 | Fe1 | 1/100 | 10 | >99 | 8.2 | 3.5 | 1.8 | 47 | 53 |
| 2 | Fe1 | 1/100 | 1 | 56 | 45.7 | 4.9 | 1.9 | 45 | 55 |
| 3 | Fe1 | 1/50 | 10 | 91 | 8.1 | 2.6 | 2.0 | 47 | 53 |
| 4 | Fe1 | 1/10 | 10 | 54 | 6.9 | 2.1 | 1.9 | 48 | 52 |
| 5 | Fe1 | 1/10 | 60 | 98 | 1.3 | 2.3 | 1.7 | 48 | 52 |
| 6 | Fe1 | 1/5 | 30 | 3 | 0.2 | 8.7 | 3.1 | 56 | 44 |
| 7 | Fe1 | 1/5 | 120 | 92 | 0.6 | 4.7 | 1.8 | 49 | 51 |
| 8 | Fe4 | 1/100 | 10 | >99 | 8.2 | 5.8 | 2.3 | 42 | 58 |
| 9 | Fe4 | 1/100 | 1 | 80 | 65.3 | 6.5 | 2.0 | 42 | 58 |
| 10 | Fe4 | 1/50 | 10 | 93 | 7.6 | 3.4 | 2.9 | 42 | 58 |
| 11 | Fe4 | 1/10 | 10 | 71 | 5.8 | 4.5 | 3.4 | 46 | 54 |
| 12 | Fe4 | 1/5 | 30 | 19 | 0.5 | 3.5 | 1.8 | 47 | 53 |
| 13 f | Fe1 | 1/100 | 10 | 99 | 8.1 | 4.6 | 2.1 | 62 | 38 |
| 14 g | Fe1 | 1/100 | 10 | 97 | 7.9 | 4.7 | 2.2 | 43 | 57 |
| 15 f | Fe4 | 1/100 | 10 | 97 | 7.9 | 5.1 | 2.2 | 49 | 51 |
| 16 g | Fe4 | 1/100 | 10 | 74 | 6.0 | 4.4 | 2.6 | 42 | 58 |
| 17 h | Fe1 | 1/500 | 120 | 85 | 5.8 | 3.8 | 2.0 | 42 | 58 |
| Entry | Cat. | AlR3 | Time (min) | Yield b (%) | Act. c (×10−5) | Mnd (×10−5) | Mw/Mnd | Microstructure (%) e | ||
|---|---|---|---|---|---|---|---|---|---|---|
| trans-1,4 | cis-1,4 | 3,4 | ||||||||
| 1 | Fe1 | AlEt3 | 2 | 96 | 39.2 | 1.4 | 2.6 | 0 | 44 | 56 |
| 2 | Fe1 | Al(i-Bu)3 | 2 | 64 | 26.1 | 2.8 | 2.2 | 0 | 44 | 56 |
| 3 | Fe2 | AlEt3 | 120 | 0 | - | - | - | - | - | - |
| 4 | Fe2 | Al(i-Bu)3 | 120 | 11 | 0.1 | 4.2 | 3.4 | 41 | 20 | 39 |
| 5 | Fe3 | AlEt3 | 2 | 98 | 40.0 | 3.7 | 3.3 | 3 | 44 | 53 |
| 6 | Fe3 | Al(i-Bu)3 | 2 | 93 | 37.9 | 3.6 | 2.2 | 0 | 45 | 55 |
| 7 | Fe4 | AlEt3 | 2 | 10 | 4.1 | 7.6 | 4.0 | 0 | 43 | 57 |
| 8 | Fe4 | Al(i-Bu)3 | 2 | 51 | 20.8 | 3.1 | 2.2 | 0 | 47 | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Ma, Y.; Zhang, X.; Wang, L.; Zhu, G.; Wang, Q. Synthesis, Characterization and Catalytic Property Studies for Isoprene Polymerization of Iron Complexes Bearing Unionized Pyridine-Oxime Ligands. Polymers 2022, 14, 3612. https://doi.org/10.3390/polym14173612
Zhao M, Ma Y, Zhang X, Wang L, Zhu G, Wang Q. Synthesis, Characterization and Catalytic Property Studies for Isoprene Polymerization of Iron Complexes Bearing Unionized Pyridine-Oxime Ligands. Polymers. 2022; 14(17):3612. https://doi.org/10.3390/polym14173612
Chicago/Turabian StyleZhao, Mengmeng, Ying Ma, Xianhui Zhang, Liang Wang, Guangqian Zhu, and Qinggang Wang. 2022. "Synthesis, Characterization and Catalytic Property Studies for Isoprene Polymerization of Iron Complexes Bearing Unionized Pyridine-Oxime Ligands" Polymers 14, no. 17: 3612. https://doi.org/10.3390/polym14173612





