Effect of Increased Powder–Binder Adhesion by Backbone Grafting on the Properties of Feedstocks for Ceramic Injection Molding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Compounds and Feedstocks
2.2.2. Preparation of Compression Molded Plates
2.2.3. Attenuated Total Reflection Spectroscopy
2.2.4. Morphology Analysis
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. Rheological Characteristics of Compounds
3. Results and Discussion
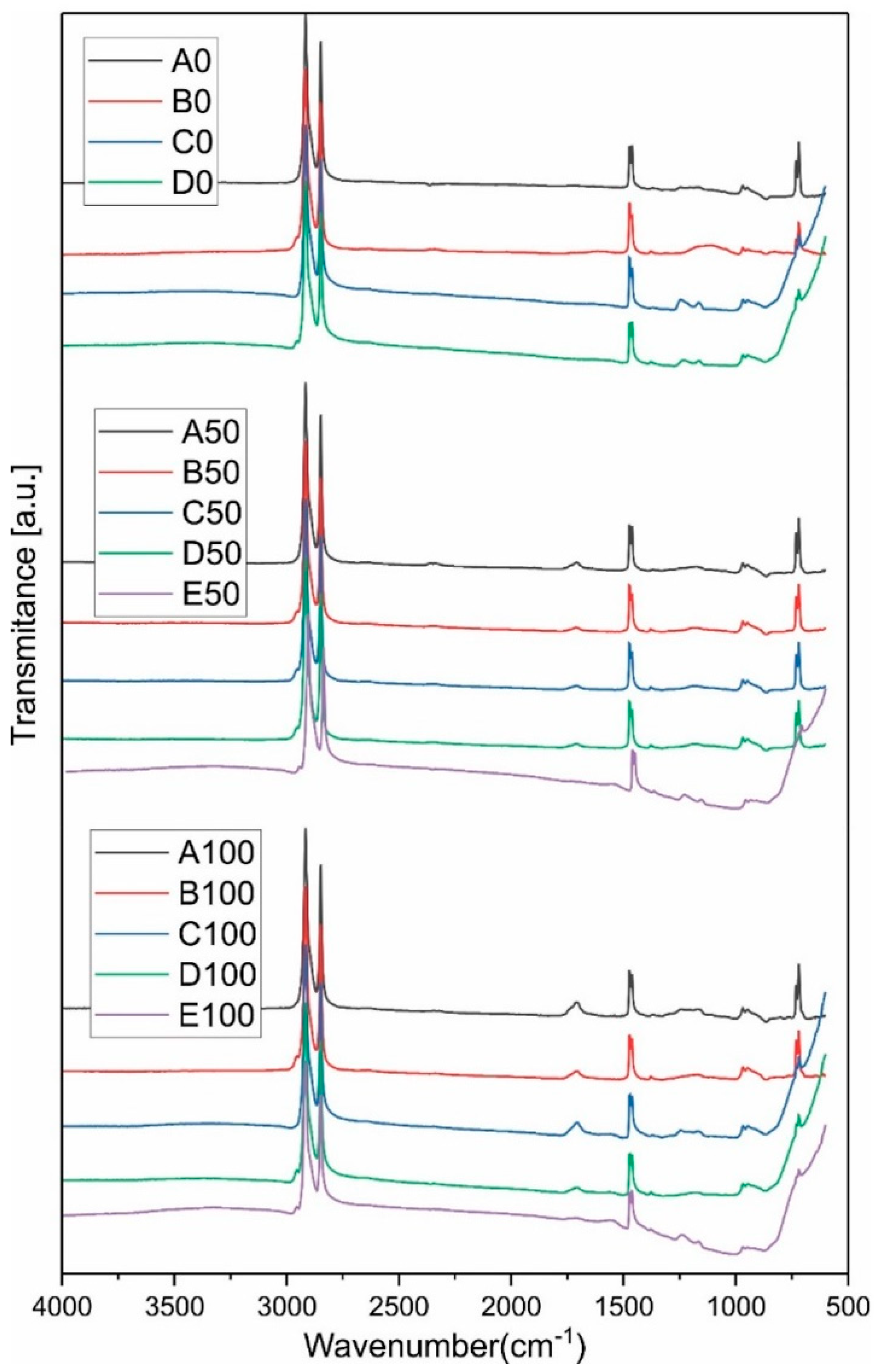
3.1. ATR
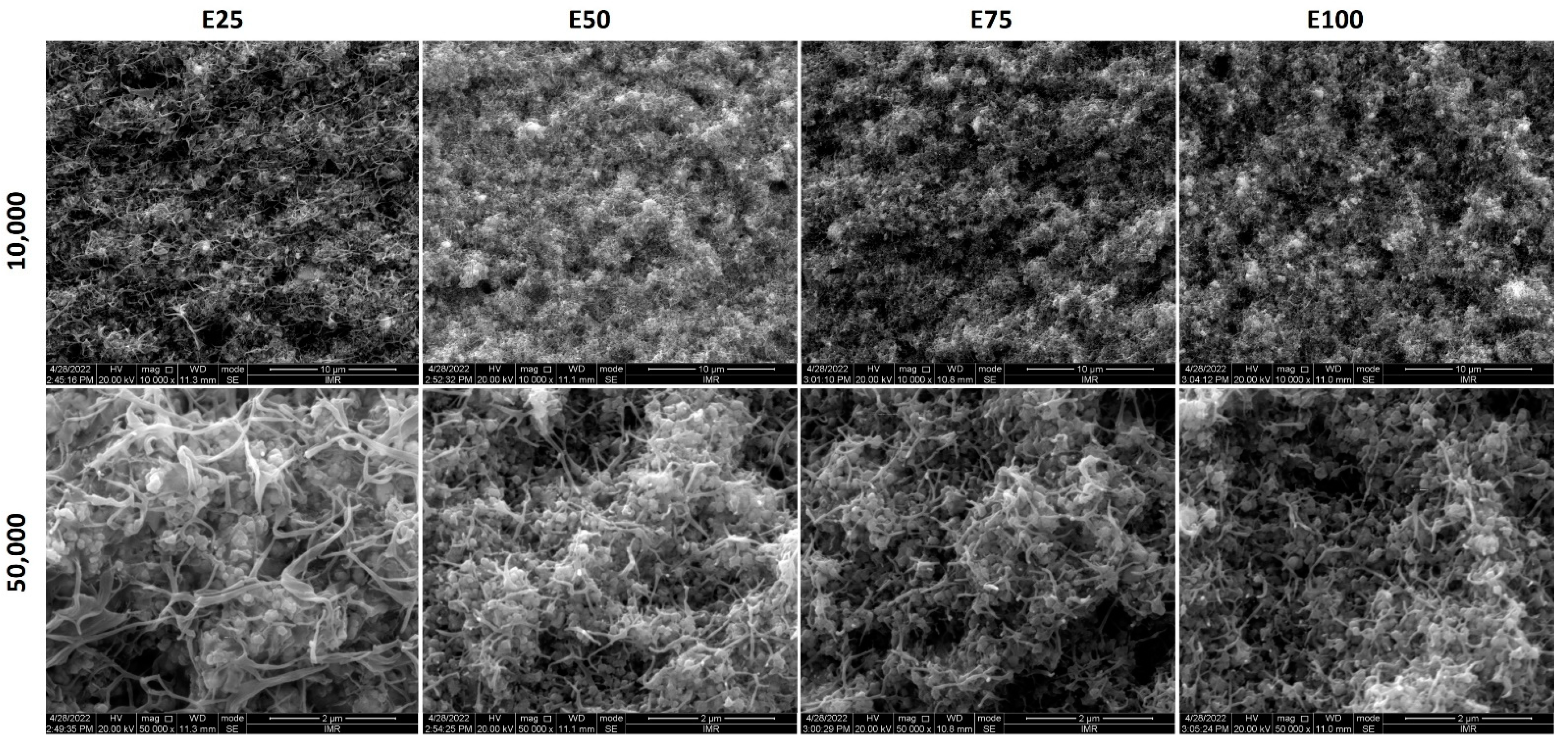
3.2. Morphology
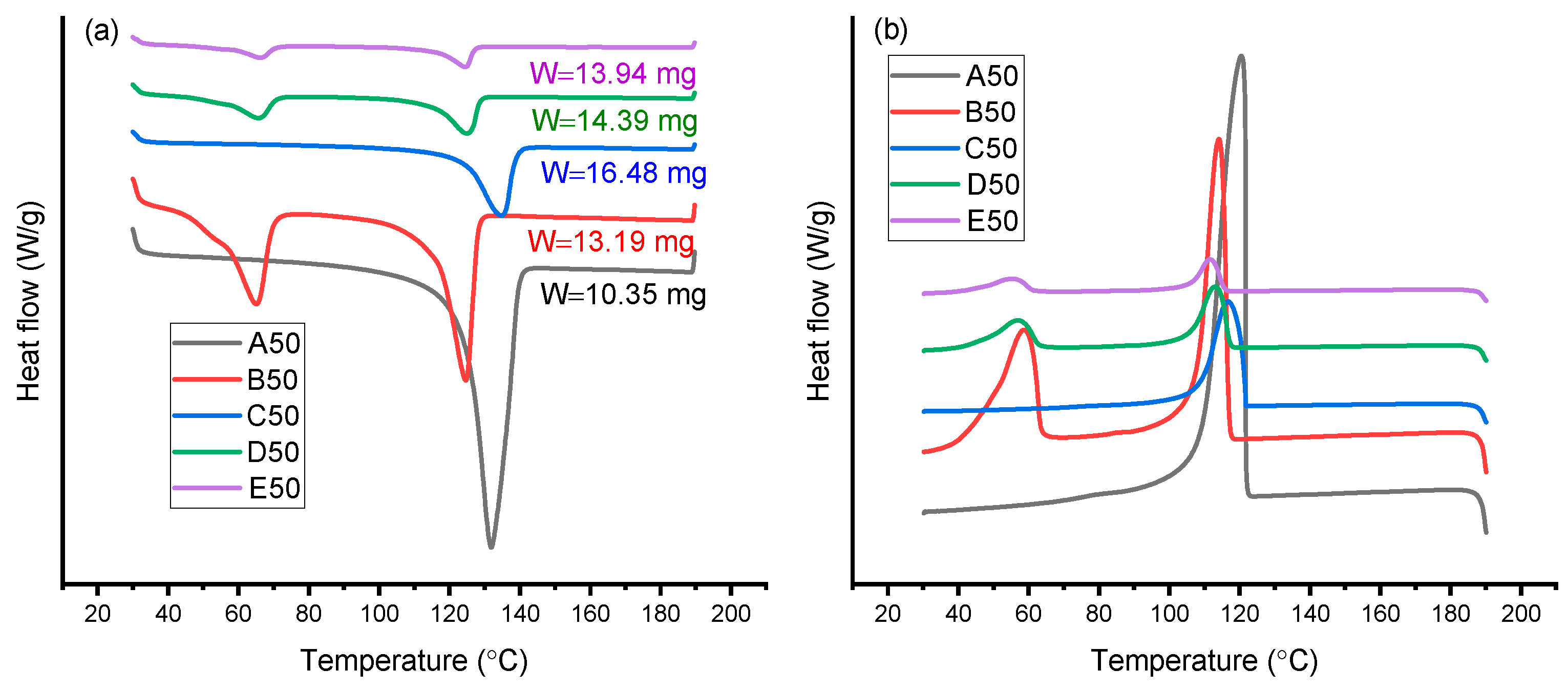
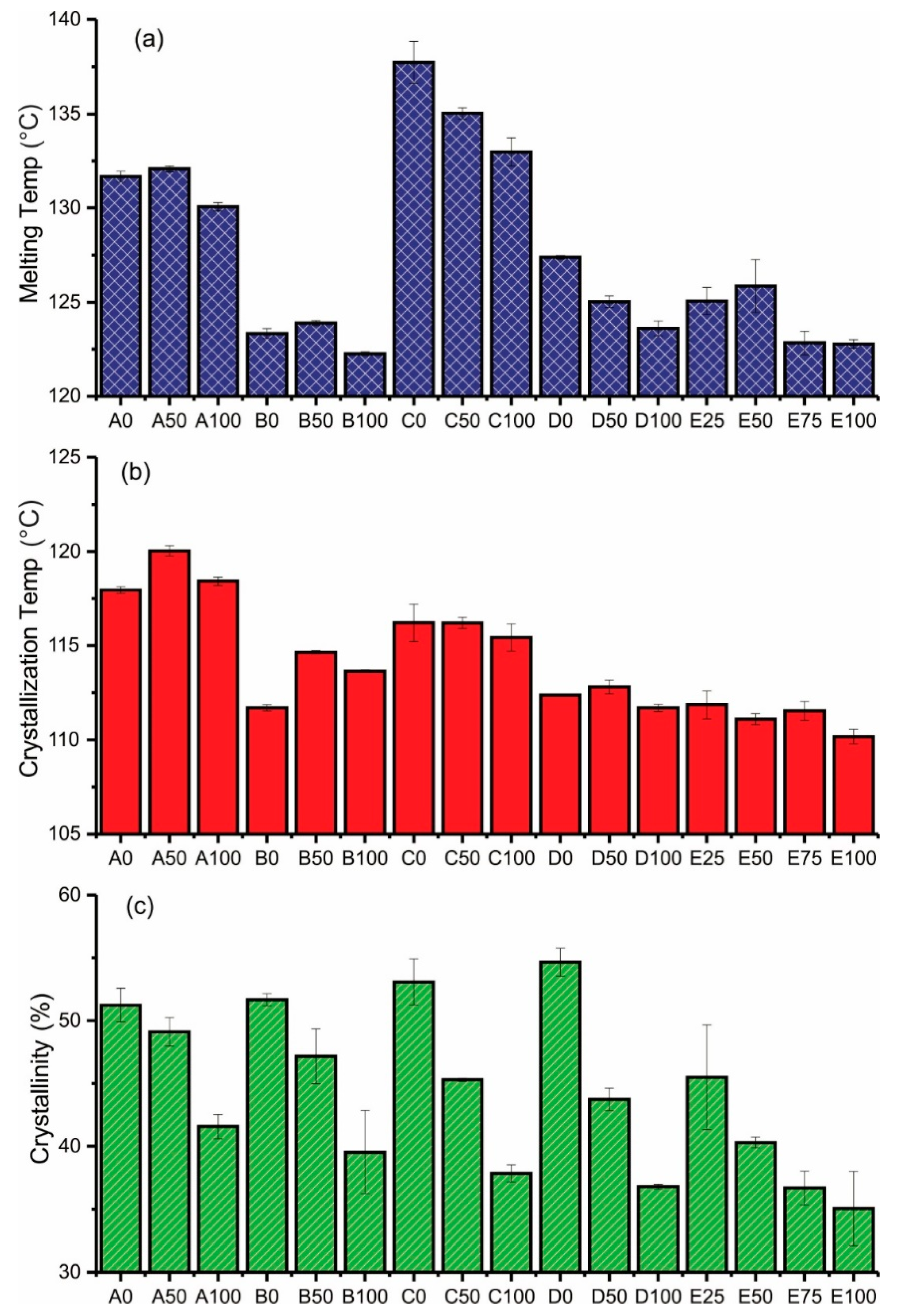
3.3. DSC Analysis
3.4. Rheological Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tülümen, H.M.; Hanemann, T.; Piotter, V.; Stenzel, D. Investigation of Feedstock Preparation for Injection Molding of Oxide–Oxide Ceramic Composites. J. Manuf. Mater. Process. 2019, 3, 9. [Google Scholar] [CrossRef]
- Huang, R.; El Rassi, J.; Kim, M.; Jo, K.-H.; Lee, S.-K.; Morscher, G.N.; Choi, J.-W. Material extrusion and sintering of bind-er-coated zirconia: Comprehensive characterizations. Addit. Manuf. 2021, 45, 102073. [Google Scholar]
- Delaroa, C.; Fulchiron, R.; Lintingre, E.; Buniazet, Z.; Cassagnau, P. Impact of Polymer Binders on the Structure of Highly Filled Zirconia Feedstocks. Polymers 2020, 12, 2247. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.; Bo, T.; Yang, X. Injection molding of surface modified powders with high solid loadings: A case for fabrica-tion of translucent alumina ceramics. J. Eur. Ceram. Soc. 2011, 31, 1611–1617. [Google Scholar] [CrossRef]
- Tseng, W.J.; Liu, D.-M.; Hsu, C.-K. Influence of stearic acid on suspension structure and green microstructure of injec-tion-molded zirconia ceramics. Ceram. Int. 1999, 25, 191–195. [Google Scholar] [CrossRef]
- Wagner, M.A.; Hadian, A.; Sebastian, T.; Clemens, F.; Schweizer, T.; Rodriguez-Arbaizar, M.; Carreño-Morelli, E.; Spolenak, R. Fused filament fabrication of stainless steel structures—From binder development to sintered properties. Addit. Manuf. 2022, 49, 102472. [Google Scholar] [CrossRef]
- Enneti, R.K.; Onbattuvelli, V.P.; Atre, S.V. Powder binder formulation and compound manufacture in metal injection molding (MIM). In Handbook of Metal Injection Molding; Heaney, D.F., Ed.; Woodhead Publishing in Materials; Woodhead Publishing: Cambridge, UK; Philadelphia, PA, USA, 2012; Volume 70. [Google Scholar]
- Li, H.W.; Zhao, Y.P.; Chen, G.Q.; Li, M.H.; Fu, X.S.; Zhou, W.L. Synergy of low-and high-density polyethylene in a binder system for powder injection molding of SiC ceramics. Ceram. Int. 2022, 48, 25513–25520. [Google Scholar] [CrossRef]
- Cano, S.; Gonzalez-Gutierrez, J.; Sapkota, J.; Spoerk, M.; Arbeiter, F.; Schuschnigg, S.; Holzer, C.; Kukla, C. Additive manufac-turing of zirconia parts by fused filament fabrication and solvent debinding: Selection of binder formulation. Addit. Manuf. 2019, 26, 117–128. [Google Scholar]
- Standring, T.; Blackburn, S.; Wilson, P. Investigation into Paraffin Wax and Ethylene Vinyl Acetate Blends for Use as a Carrier Vehicle in Ceramic Injection Molding. Polym.-Plast. Technol. Eng. 2016, 55, 802–817. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Influence of injection process parameters on dimensional stability of wax patterns made by the lost wax process using Taguchi approach. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2013, 227, 52–60. [Google Scholar] [CrossRef]
- Gorjan, L.; Galusca, C.S.; Sami, M.; Sebastian, T.; Clemens, F. Effect of stearic acid on rheological properties and printability of ethylene vinyl acetate based feedstocks for fused filament fabrication of alumina. Addit. Manuf. 2020, 36, 101391. [Google Scholar] [CrossRef]
- Cano, S.; Gooneie, A.; Kukla, C.; Rieß, G.; Holzer, C.; Gonzalez-Gutierrez, J. Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing. Appl. Sci. 2020, 10, 1471. [Google Scholar] [CrossRef] [Green Version]
- Nabiyev, A.A.; Islamov, A.K.; Maharramov, A.M.; Nuriyev, M.A.; Ismayilova, R.S.; Doroshkevic, A.S.; Pawlukojc, A.; Turchenko, V.A.; Olejniczak, A.; Rulev, M.İ.; et al. Structural Studies of dielectric HDPE+ZrO2 polymer nano-composites: Filler concentration dependences. J. Phys. Conf. Ser. 2018, 994, 12011. [Google Scholar] [CrossRef]
- Gorjan, L.; Reiff, L.; Liersch, A.; Clemens, F. Ethylene vinyl acetate as a binder for additive manufacturing of tricalcium phosphate bio-ceramics. Ceram. Int. 2018, 44, 15817–15823. [Google Scholar] [CrossRef]
- Durmus, A.; Kasgoz, A.; Macosko, C.W. Linear low density polyethylene (LLDPE)/clay nanocomposites. Part I: Structural characterization and quantifying clay dispersion by melt rheology. Polymer 2007, 48, 4492–4502. [Google Scholar]
- Chen, G.; Ma, H.; Zhou, Z.; Ren, F.; Xu, W. Effect of interaction from the reaction of carboxyl/epoxy hyperbranched polyesters on properties of feedstocks for metal injection molding. Mater. Res. Express 2022, 9, 16506. [Google Scholar] [CrossRef]
- Wongpanit, P.; Khanthsri, S.; Puengboonsri, S.; Manonukul, A. Effects of acrylic acid-grafted HDPE in HDPE-based binder on properties after injection and debinding in metal injection molding. Mater. Chem. Phys. 2014, 147, 238–246. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. (Eds.) Polymer Handbook, 3rd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application. Materials 2018, 11, 1144. [Google Scholar] [CrossRef]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Mannschatz, A.; Höhn, S.; Moritz, T. Powder-binder separation in injection moulded green parts. J. Eur. Ceram. Soc. 2010, 30, 2827–2832. [Google Scholar] [CrossRef]
- Matula, G.; Tomiczek, B.; Król, M.; Szatkowska, A.; Sotomayor, M.E. Application of thermal analysis in the selection of poly-mer components used as a binder for metal injection moulding of Co–Cr–Mo alloy powder. J. Therm. Anal. Calorim. 2018, 134, 391–399. [Google Scholar] [CrossRef]
- Kukla, C.; Duretek, I.; Gonzalez-Gutierrez, J.; Holzer, C. Rheology of PIM feedstocks. Met. Powder Rep. 2017, 72, 39–44. [Google Scholar] [CrossRef]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Malmberg, A.; Gabriel, C.; Steffl, T.; Münstedt, H.; Löfgren, B. Long-Chain Branching in Metallocene-Catalyzed Polyethylenes Investigated by Low Oscillatory Shear and Uniaxial Extensional Rheometry. Macromolecules 2002, 35, 1038–1048. [Google Scholar] [CrossRef]
- Liang, X.-K.; Luo, Z.; Yang, L.; Wei, J.-T.; Yuan, X.; Zheng, Q. Rheological properties and crystallization behaviors of long chain branched polyethylene prepared by melt branching reaction. J. Polym. Eng. 2018, 38, 7–17. [Google Scholar] [CrossRef]
- Ghosh, P.; Dev, D. Reactive processing of polyethylene: Effect of peroxide-induced graft copolymerization of some acrylic monomers on polymer structure melt rheology and relaxation behavior. Eur. Polym. J. 1998, 34, 1539–1547. [Google Scholar] [CrossRef]
- Bek, M.; Gonzalez-Gutierrez, J.; Kukla, C.; Črešnar, K.P.; Maroh, B.; Perše, L.S. Rheological Behaviour of Highly Filled Materials for Injection Moulding and Additive Manufacturing: Effect of Particle Material and Loading. Appl. Sci. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Heaney, D.F. (Ed.) Handbook of Metal Injection Molding, 2nd ed.; Woodhead Publishing: Cambridge, UK; Philadelphia, PA, USA, 2019. [Google Scholar]
- Sahu, S.K.; Badgayan, N.D.; Sreekanth, P.S.R. Rheological Properties of HDPE based Thermoplastic Polymeric Nanocomposite Reinforced with Multidi-mensional Carbon-based Nanofillers, Biointerface Res. Appl. Chem. 2021, 12, 5709–5715. [Google Scholar]
- Gonzalez-Gutierrez, J.; Duretek, I.; Kukla, C.; Poljšak, A.; Bek, M.; Emri, I.; Holzer, C. Models to Predict the Viscosity of Metal Injection Molding Feedstock Materials as Function of Their Formulation. Metals 2016, 6, 129. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.-J.; Sun, Z. Effects of polyethylene-grafted maleic anhydride (PE-g-MA) on thermal properties, morphology, and tensile properties of low-density polyethylene (LDPE) and corn starch blends. J. Appl. Polym. Sci. 2003, 88, 2904–2911. [Google Scholar] [CrossRef]
- Fowlks, A.C.; Narayan, R. The effect of maleated polylactic acid (PLA) as an interfacial modifier in PLA-talc composites. J. Appl. Polym. Sci. 2010, 118, 2810–2820. [Google Scholar] [CrossRef]



| Binder Composition (vol%) | Powder Content (vol%) | |||
|---|---|---|---|---|
| HDPE | AAHDPE | PW | ||
| A0 | 100 | |||
| A50 | 50 | 50 | ||
| A100 | 100 | |||
| Ax | 100 | |||
| B0 | 50 | 50 | ||
| B50 | 25 | 25 | 50 | |
| B100 | 50 | 50 | ||
| C0 | 100 | 30 | ||
| C50 | 50 | 50 | 30 | |
| C100 | 100 | 30 | ||
| Cx | 100 | 30 | ||
| D0 | 50 | 50 | 30 | |
| D50 | 25 | 25 | 50 | 30 |
| D100 | 50 | 50 | 30 | |
| E0 | 50 | 50 | 50 | |
| E25 | 37.5 | 12.5 | 50 | 50 |
| E50 | 25 | 25 | 50 | 50 |
| E75 | 12.5 | 37.5 | 50 | 50 |
| E100 | 50 | 50 | 50 | |
| Stage (No) | Temperature (°C) | Pressure (bar) | Time (min) |
|---|---|---|---|
| 1 | 160 | 0 | 10 |
| 2 | 160 | 50 | 5 |
| 3 | 30 | 50 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi-Mobarakeh, L.; Cano, S.; Momeni, V.; Liu, D.; Duretek, I.; Riess, G.; Kukla, C.; Holzer, C. Effect of Increased Powder–Binder Adhesion by Backbone Grafting on the Properties of Feedstocks for Ceramic Injection Molding. Polymers 2022, 14, 3653. https://doi.org/10.3390/polym14173653
Ghasemi-Mobarakeh L, Cano S, Momeni V, Liu D, Duretek I, Riess G, Kukla C, Holzer C. Effect of Increased Powder–Binder Adhesion by Backbone Grafting on the Properties of Feedstocks for Ceramic Injection Molding. Polymers. 2022; 14(17):3653. https://doi.org/10.3390/polym14173653
Chicago/Turabian StyleGhasemi-Mobarakeh, Laleh, Santiago Cano, Vahid Momeni, Dongyan Liu, Ivica Duretek, Gisbert Riess, Christian Kukla, and Clemens Holzer. 2022. "Effect of Increased Powder–Binder Adhesion by Backbone Grafting on the Properties of Feedstocks for Ceramic Injection Molding" Polymers 14, no. 17: 3653. https://doi.org/10.3390/polym14173653
APA StyleGhasemi-Mobarakeh, L., Cano, S., Momeni, V., Liu, D., Duretek, I., Riess, G., Kukla, C., & Holzer, C. (2022). Effect of Increased Powder–Binder Adhesion by Backbone Grafting on the Properties of Feedstocks for Ceramic Injection Molding. Polymers, 14(17), 3653. https://doi.org/10.3390/polym14173653












