Slurry Homopolymerization of Ethylene Using Thermostable α-Diimine Nickel Catalysts Covalently Linked to Silica Supports via Substituents on Acenaphthequinone-Backbone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Supported α-Diimine Nickel(II) Catalysts
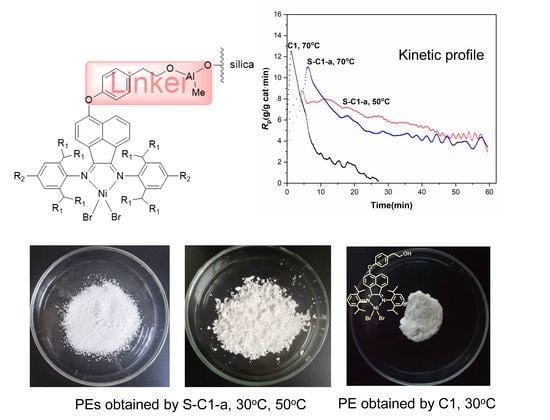
2.4. Polymerization
3. Results and Discussion
3.1. Synthesis and Characterization of Supported Catalysts
3.2. Ethylene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(Ⅱ)- and Ni(Ⅱ)-based catalysts for polymerization of ethylene and a-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- AlObaidi, F.; Ye, Z.; Zhu, S. Ethylene polymerization with homogeneous nickel–diimine catalysts: Effects of catalyst structure and polymerization conditions on catalyst activity and polymer properties. Polymer 2004, 45, 6823–6829. [Google Scholar] [CrossRef]
- Song, C.-L.; Tang, L.-M.; Li, Y.-G.; Li, X.-F.; Chen, J.; Li, Y.-S. Preparation of linear α-olefins to high-molecular weight polyethylenes using cationic α-diimine nickel(II) complexes containing chloro-substituted ligands. J. Polym. Sci. Polym. Chem. 2006, 44, 1964–1974. [Google Scholar] [CrossRef]
- Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti, A.O.; Rieger, B. New Nickel(II) Diimine Complexes and the Control of Polyethylene Microstructure by Catalyst Design. J. Am. Chem. Soc. 2007, 129, 9182–9191. [Google Scholar] [CrossRef]
- Bahuleyan, B.K.; Son, G.W.; Park, D.-W.; Ha, C.-S.; Kim, I. Ethylene polymerization by sterically and electronically modulated Ni(II) α-diimine complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1066–1082. [Google Scholar] [CrossRef]
- Liu, F.-S.; Hu, H.-B.; Xu, Y.; Guo, L.-H.; Zai, S.-B.; Song, K.-M.; Gao, H.-Y.; Zhang, L.; Zhu, F.-M.; Wu, Q. Thermostable α-Diimine Nickel(II) Catalyst for Ethylene Polymerization: Effects of the Substituted Backbone Structure on Catalytic Properties and Branching Structure of Polyethylene. Macromolecules 2009, 42, 7789–7796. [Google Scholar] [CrossRef]
- Popeney, C.S.; Guan, Z. Effect of Ligand Electronics on the Stability and Chain Transfer Rates of Substituted Pd(II) α-Diimine Catalysts. Macromolecules 2010, 43, 4091–4097. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Mitchell, N.E.; Long, B.K. Enhancing α-Diimine Catalysts for High-Temperature Ethylene Polymerization. ACS Catal. 2014, 4, 2501–2504. [Google Scholar] [CrossRef]
- Du, S.; Kong, S.; Shi, Q.; Mao, J.; Guo, C.-Y.; Yi, J.; Liang, T.; Sun, W.-H. Enhancing the Activity and Thermal Stability of Nickel Complex Precatalysts Using 1-[2,6-Bis(bis(4-fluorophenyl)methyl)-4-methyl phenylimino]-2-aryliminoacenaphthylene Derivatives. Organometallics 2015, 34, 582–590. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Chen, C. Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization. Polymers 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Solanad, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, C.; Du, H.; Pang, W.; Chen, C. Influence of Ligand Backbone Structure and Connectivity on the Properties of Phosphine-Sulfonate Pd(II)/Ni(II) Catalysts. Polymers 2017, 9, 168. [Google Scholar] [CrossRef]
- He, F.; Wang, D.; Jiang, B.; Zhang, Z.; Cheng, Z.; Fu, Z.; Zhang, Q.; Fan, Z. Introducing electron-donating substituents on ligand backbone of α-diimine nickel complex and the effects on catalyst thermal stability in ethylene polymerization. Inorg. Chim. Acta 2018, 486, 704–710. [Google Scholar] [CrossRef]
- Brown, L.A.; Anderson, W.C.; Mitchell, N.E.; Gmernicki, K.R.; Long, B.K. High Temperature, Living Polymerization of Ethylene by a Sterically-Demanding Nickel(II) α-Diimine Catalyst. Polymers 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Z.; Tian, S.-S.; Li, R.-P.; Li, W.-M.; Chen, C.-L. Ligand steric effects on naphthyl-α-diimine nickel catalyzed α-olefin polymerization. Chin. J. Polym. Sci. 2018, 36, 157–162. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Padilla-Vélez, O.; O’Connor, K.S.; Lapointe, A.M.; Macmillan, S.N.; Coates, G.W. Switchable living nickel(ii) α-diimine catalyst for ethylene polymerisation. Chem. Commun. 2019, 55, 7607–7610. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. Unsymmetrical Strategy Makes Significant Differences in α-Diimine Nickel and Palladium Catalyzed Ethylene (Co)Polymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Muhammad, Q.; Pang, W.; Wang, F.; Tan, C. Ortho-functionalized dibenzhydryl substituents in α-diimine Pd catalyzed ethylene polymerization and copolymeriza-tion. Polymers 2020, 12, 2509. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.C.; Patel, H.; Soares, J.B.P.; de Souza, R.F. Polyethylene Made with In Situ Supported Ni-Diimine/SMAO: Replication Phenomenon and Effect of Polymerization Conditions on Polymer Microstructure and Morphology. Macromol. Chem. Phys. 2001, 202, 3237–3247. [Google Scholar] [CrossRef]
- Preishuber-Pflugl, P.; Brookhart, M. Highly Active Supported Nickel Diimine Catalysts for Polymerization of Ethylene. Macromolecules 2002, 35, 6074–6076. [Google Scholar] [CrossRef]
- Alobaidi, F.; Ye, Z.; Zhu, S. Ethylene Polymerization with Silica-Supported Nickel-Diimine Catalyst: Effect of Support and Polymerization Conditions on Catalyst Activity and Polymer Properties. Macromol. Chem. Phys. 2003, 204, 1653–1659. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C.; Van Axel Castelli, V. MgCl2-Based Supports for the Immobilization and Activation of Nickel Diimine Catalysts for Polymerization of Ethylene. Macromolecules 2004, 37, 6258–6259. [Google Scholar] [CrossRef]
- Huang, R.; Koning, C.E.; Chadwick, J.C. Synergetic Effect of a Nickel Diimine in Ethylene Polymerization with Immobilized Fe-, Cr-, and Ti-Based Catalysts on MgCl2 Supports. Macromolecules 2007, 40, 3021–3029. [Google Scholar] [CrossRef]
- Schrekker, H.S.; Kotov, V.; Preishuber-Pflugl, P.; White, P.; Brookhart, M. Efficient Slurry-Phase Homopolymerization of Ethylene to Branched Polyethylenes Using α-Diimine Nickel(II) Catalysts Covalently Linked to Silica Supports. Macromolecules 2006, 39, 6341–6354. [Google Scholar] [CrossRef]
- Li, Y.-G.; Pan, L.; Zheng, Z.-J.; Li, Y.-S. Polymerization of ethylene to branched polyethylene with silica and Merrifield resin supported nickel(II) catalysts with α-diimine ligands. J. Mol. Catal. A Chem. 2008, 287, 57–64. [Google Scholar] [CrossRef]
- Bahuleyan, B.K.; Oh, J.M.; Chandran, D.; Ha, J.Y.; Hur, A.Y.; Park, D.-W.; Ha, C.S.; Suh, H.; Kim, I. Highly Efficient Supported Diimine Ni(II) and Iminopyridyl Fe(II) Catalysts for Ethylene Polymerizations. Top. Catal. 2010, 53, 500–509. [Google Scholar] [CrossRef]
- Jiang, H.; He, F.; Wang, H. A new strategy to prepare branching polyethylene by using an α-diimine nickel(II) complex covalently supported on MgCl2/AlRn(OEt)3-n. J. Polym. Res. 2008, 16, 183–189. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, J.; Wang, F. Polymerization of ethylene using a nickel α-diimine complex covalently supported on SiO2–MgCl2 bisupport. Polym. Bull. 2010, 65, 767–777. [Google Scholar] [CrossRef]
- Choi, Y.; Soares, J.B.P. Synthesis of Supported Nickel Diimine Catalysts for Ethylene Slurry Polymerization. Macromol. Chem. Phys. 2009, 210, 1979–1988. [Google Scholar] [CrossRef]
- Choi, Y.; Soares, J.B. Ethylene slurry polymerization using nickel diimine catalysts covalently-attached onto MgCl2-based supports. Polymer 2010, 51, 2271–2276. [Google Scholar] [CrossRef]
- Huang, C.; Zakharov, V.A.; Semikolenova, N.V.; Matsko, M.A.; Mahmood, Q.; Talsi, E.P.; Sun, W.-H. Comparisons between homogeneous and immobilized 1-(2,6-dibenzhydryl-4-nitrophenylimino)-2-mesityliminoacenaphthylnickel bromide as a precatalyst in ethylene polymerization. J. Catal. 2019, 372, 103–108. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, G.-X. Radical co-polymerization of diiminedibromidenickel(II)-functionalized olefin with styrene: Synthesis of polymer-incorporated nickelII α-diimine catalysts for ethylene polymerization. Appl. Catal. A Gen. 2004, 262, 13–18. [Google Scholar] [CrossRef]
- Jiang, T. Preparation of spherical MgCl2/SiO2/THF-supported late-transition metal catalysts for ethylene polymerization. China Pet. Processing Petrochem. Technol. 2014, 16, 77. [Google Scholar]
- Bahuleyan, B.K.; Jermy, B.R.; Ahn, I.Y.; Suh, H.; Park, D.-W.; Ha, C.S.; Kim, I. One-pot synthesis of spherical periodic mesoporous organosilica supported catalyst bearing Ni(II) α-diimine complexes for ethylene polymerization. Catal. Commun. 2009, 11, 252–256. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Z.; Cui, Q.; Gu, Z.; Mercier, L. Surface-initiated catalytic ethylene polymerization within nano-channels of ordered mesoporous silicas for synthesis of hybrid silica composites containing covalently tethered polyethylene. Polymer 2011, 52, 5961–5974. [Google Scholar] [CrossRef]
- Favero, C.; Closs, M.B.; Galland, G.B.; Stieler, R.; Rossetto, E.; Bernardo-Gusmão, K. A binary nickel diimine-MCM-41 supported catalyst and its application in ethylene polymerization. J. Catal. 2019, 377, 63–71. [Google Scholar] [CrossRef]
- Zhang, R.-F.; Hou, Y.-H.; Wei, X.-L.; Zhao, D.-D.; Cui, M.-M.; Zhai, F.-F.; Li, X.-L.; Liu, B.-Y.; Yang, M. Thermostable α-Diimine Nickel Complexes with Substituents on Acenaphthequinone-backbone for Ethylene Polymerization. Chin. J. Polym. Sci. 2020, 6, 134–136. [Google Scholar] [CrossRef]
- Clas, S.-D.; Heyding, R.D.; McFaddin, D.C.; Russell, K.E.; Scammell-Bullock, M.V.; Kelusky, E.C.; St-Cyr, D. Crystallinities of copolymers of ethylene and 1-alkenes. J. Polym. Sci. Pol. Phys. 1988, 26, 1271–1286. [Google Scholar] [CrossRef]
- Galland, G.B.; de Souza, R.F.; Mauler, R.S.; Nunes, F.F. 13C NMR Determination of the Composition of Linear Low-Density Polyethylene Obtained with [η3-Methallyl-nickel-diimine]PF6 Complex. Macromolecules 1999, 32, 1620–1625. [Google Scholar] [CrossRef]
- He, X.; Guo, Y.; Chen, X.; Wu, B.; Zou, J.; Wen, Y.; Chen, D. Synthesis of MWNTs/SiO2 Supported Nickel and Palladium Complexes and their Application as Catalysts for Cyclic Olefins Polymerization. J. Organomet. Chem. 2021, 949, 121953. [Google Scholar] [CrossRef]










| Silica Gel or Supported Catalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Nickel Content (%) |
|---|---|---|---|---|
| 955 | 280 | 1.50 | 18.20 | - |
| 2408D | 318 | 1.10 | 15.10 | - |
| S-C1-a | 158 | 1.00 | 16.40 | 1.25 |
| S-C1-b | 220 | 0.90 | 14.10 | 1.06 |
| S-C2-a | 206 | 0.90 | 16.40 | 0.54 |
| S-C2-b | 212 | 0.80 | 14.20 | 0.53 |
| Entry | Catalyst | T (°C) | A1 b | A2 c | Tmd (°C) | χcd (%) | Mwe (kg/mol) | PDI e | Branches f (1000 C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | S-C1-a | 30 | 1.64 | 349 | 120 | 33 | n.d. | n.d. | n.d. |
| 2 | S-C1-a | 50 | 2.08 | 443 | 116 | 31 | 563 | 3.9 | 67 |
| 3 | S-C1-a | 70 | 1.11 | 236 | 114 | 16 | 417 | 4.0 | 78 |
| 4 | S-C1-b | 10 | 0.24 | 44 | 123 | 56 | n.d. | n.d. | n.d. |
| 5 | S-C1-b | 30 | 1.42 | 258 | 118 | 38 | 774 | 4.0 | 56 |
| 6 | S-C1-b | 50 | 1.83 | 333 | 116 | 22 | 482 | 4.2 | n.d. |
| 7 | S-C1-b | 70 | 1.00 | 182 | 113 | 13 | 261 | 3.2 | 82 |
| Entry | Catalyst | T (°C) | A1 b | A2 c | Tmd (°C) | χcd (%) | Mwe (kg/mol) | PDI e |
|---|---|---|---|---|---|---|---|---|
| 8 | S-C2-a | 30 | 1.02 | 94 | 80/123 | 16 | n.d. | n.d. |
| 9 | S-C2-a | 50 | 1.28 | 107 | 74 | 13 | 969 | 2.5 |
| 10 | S-C2-a | 70 | 1.19 | 109 | 60 | 15 | 911 | 2.3 |
| 11 | S-C2-a | 80 | 0.90 | 82 | 58 | 13 | n.d. | n.d. |
| 12 | S-C2-b | 30 | 0.87 | 78 | 79 | 20 | n.d. | n.d. |
| 13 | S-C2-b | 50 | 1.07 | 96 | 75 | 14 | 825 | 2.8 |
| 14 | S-C2-b | 70 | 1.01 | 91 | 61 | 11 | 725 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, K.; Hou, Y.; Zhao, X.; Sun, Y.; Liu, B.; Yang, M. Slurry Homopolymerization of Ethylene Using Thermostable α-Diimine Nickel Catalysts Covalently Linked to Silica Supports via Substituents on Acenaphthequinone-Backbone. Polymers 2022, 14, 3684. https://doi.org/10.3390/polym14173684
Zong K, Hou Y, Zhao X, Sun Y, Liu B, Yang M. Slurry Homopolymerization of Ethylene Using Thermostable α-Diimine Nickel Catalysts Covalently Linked to Silica Supports via Substituents on Acenaphthequinone-Backbone. Polymers. 2022; 14(17):3684. https://doi.org/10.3390/polym14173684
Chicago/Turabian StyleZong, Kening, Yanhui Hou, Xiaobei Zhao, Yali Sun, Binyuan Liu, and Min Yang. 2022. "Slurry Homopolymerization of Ethylene Using Thermostable α-Diimine Nickel Catalysts Covalently Linked to Silica Supports via Substituents on Acenaphthequinone-Backbone" Polymers 14, no. 17: 3684. https://doi.org/10.3390/polym14173684
APA StyleZong, K., Hou, Y., Zhao, X., Sun, Y., Liu, B., & Yang, M. (2022). Slurry Homopolymerization of Ethylene Using Thermostable α-Diimine Nickel Catalysts Covalently Linked to Silica Supports via Substituents on Acenaphthequinone-Backbone. Polymers, 14(17), 3684. https://doi.org/10.3390/polym14173684