Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Quantification of THQ in THQ-LPHNPs
2.3. Box–Behnken Design
2.4. THQ-LPHNPs Production
2.5. THQ-LPHNPs Characterization
2.5.1. Particles Characterization
2.5.2. Encapsulation Efficiency (%EE) and Drug Loading (%DL)
2.6. Stability Experiments
2.6.1. Stability in the Gastrointestinal Milieu
2.6.2. Storage Stability
2.7. THQ Release and Release Kinetics
2.8. Mucoadhesion Study
2.9. Cell Culture Studies
2.9.1. Cell Viability Assay
2.9.2. Lactate Dehydrogenase (LDH) Assay
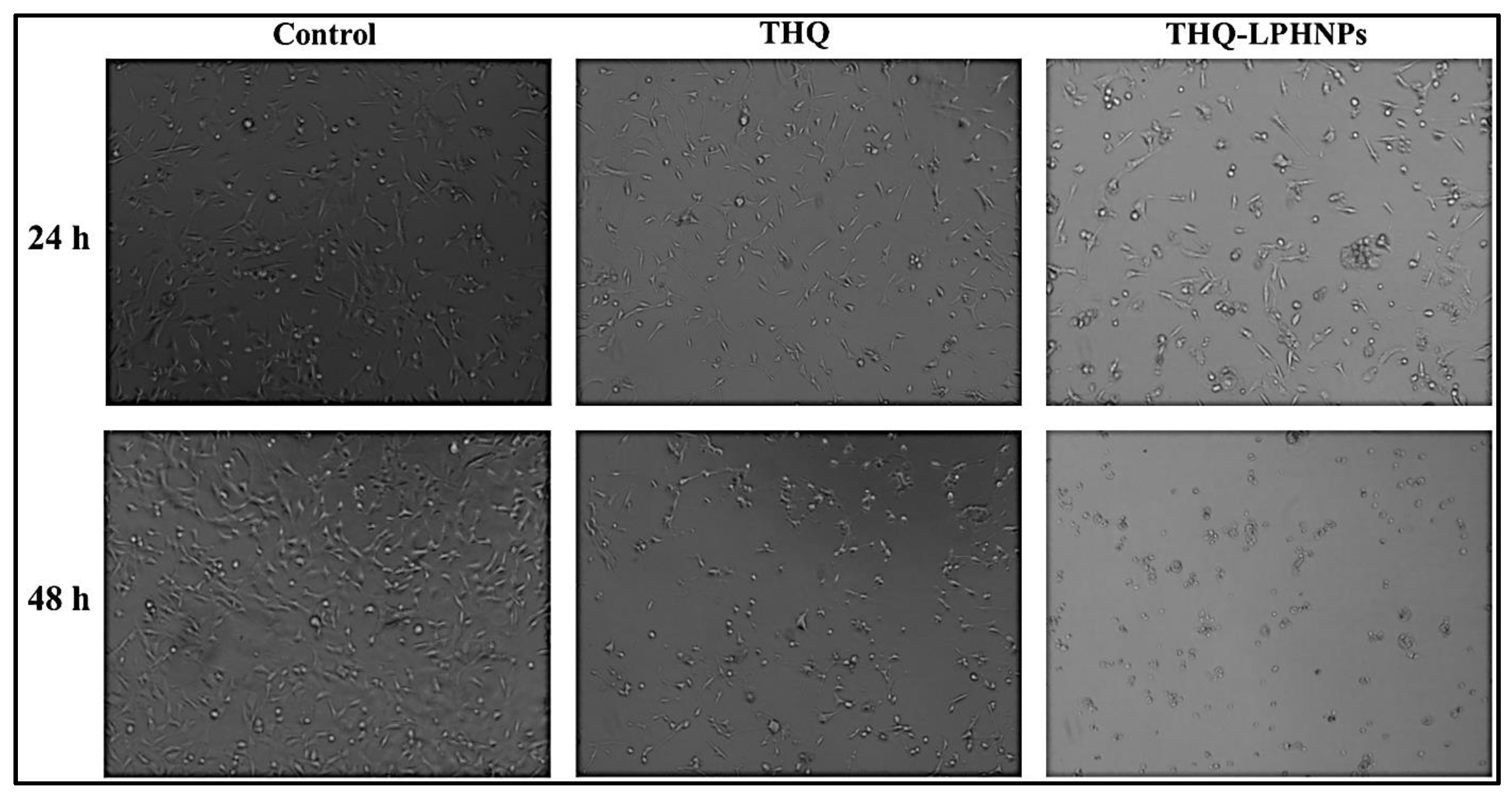
2.9.3. Morphological Examination of Treated Cells
2.10. Ex Vivo THQ Permeation Study
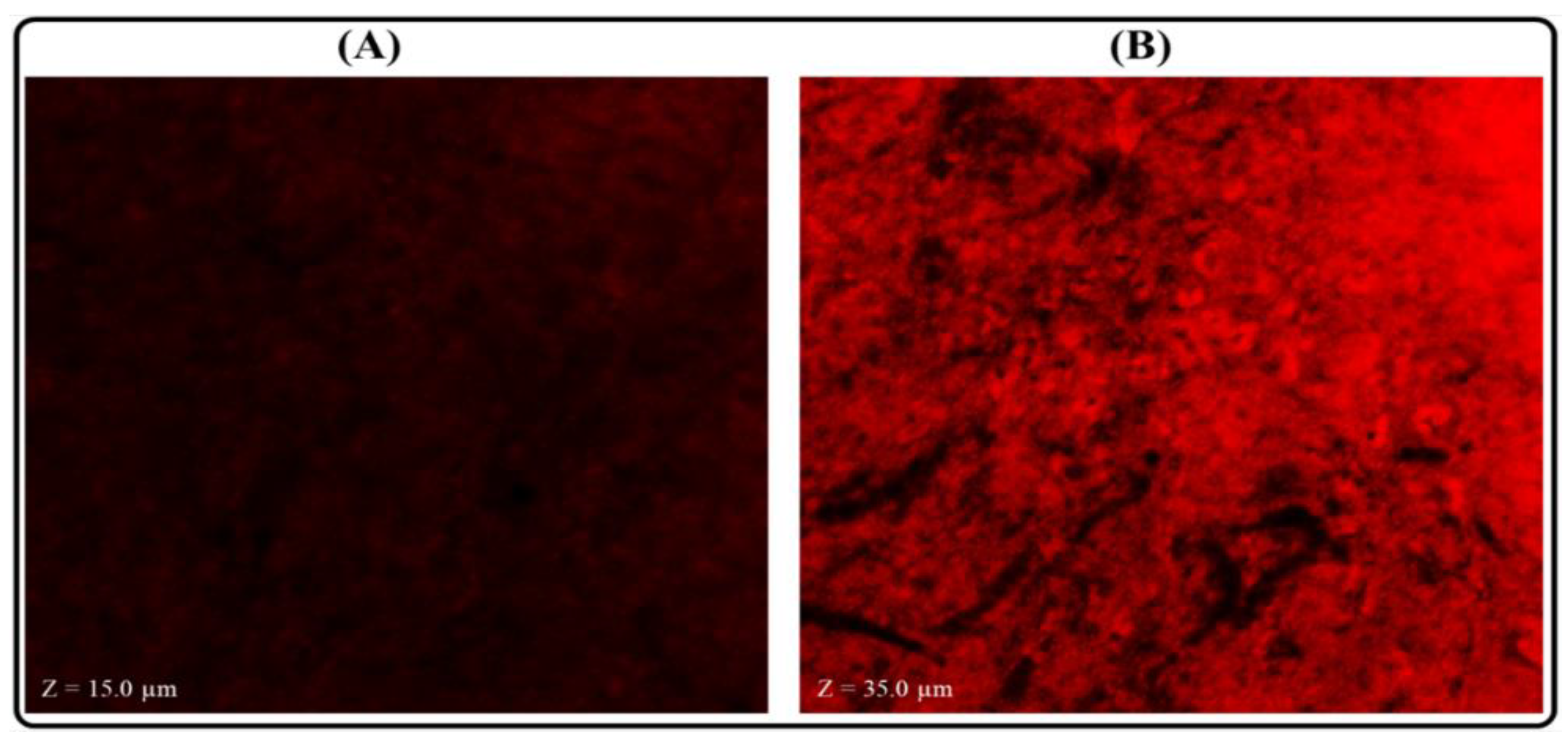
2.11. Measurement of Permeation Depth
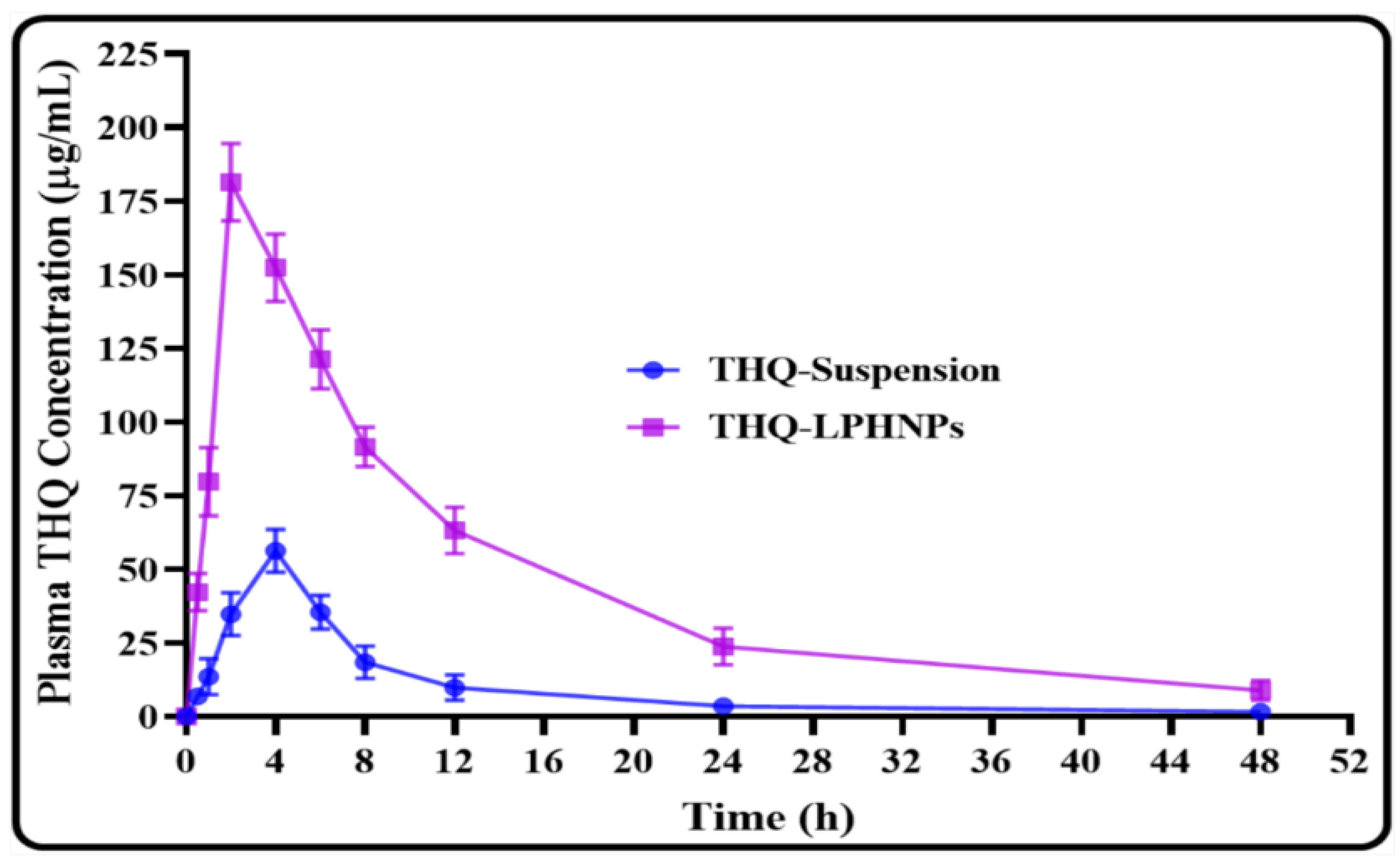
2.12. Relative Bioavailability Study
2.13. Statistical Analysis
3. Results and Discussion
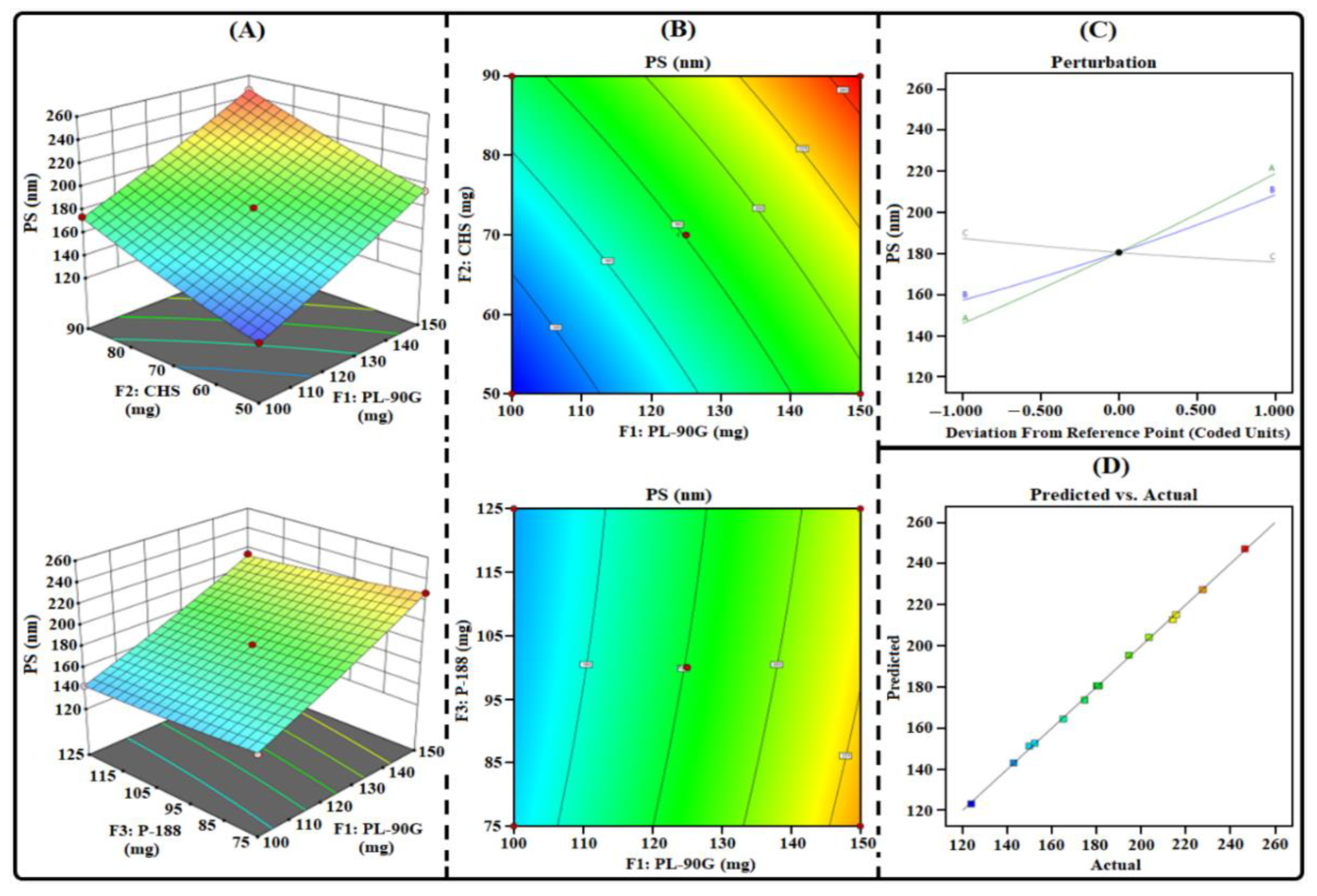
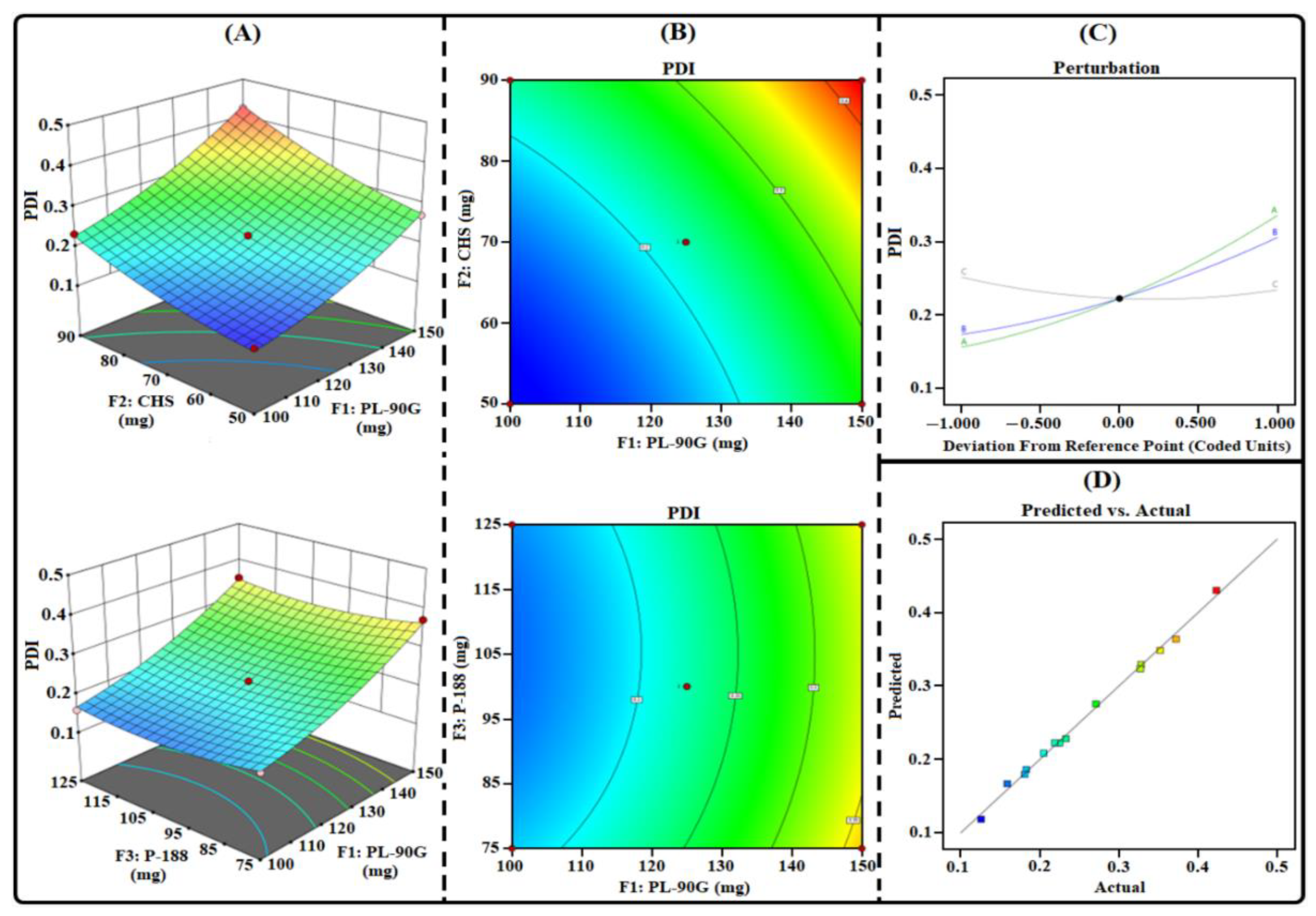
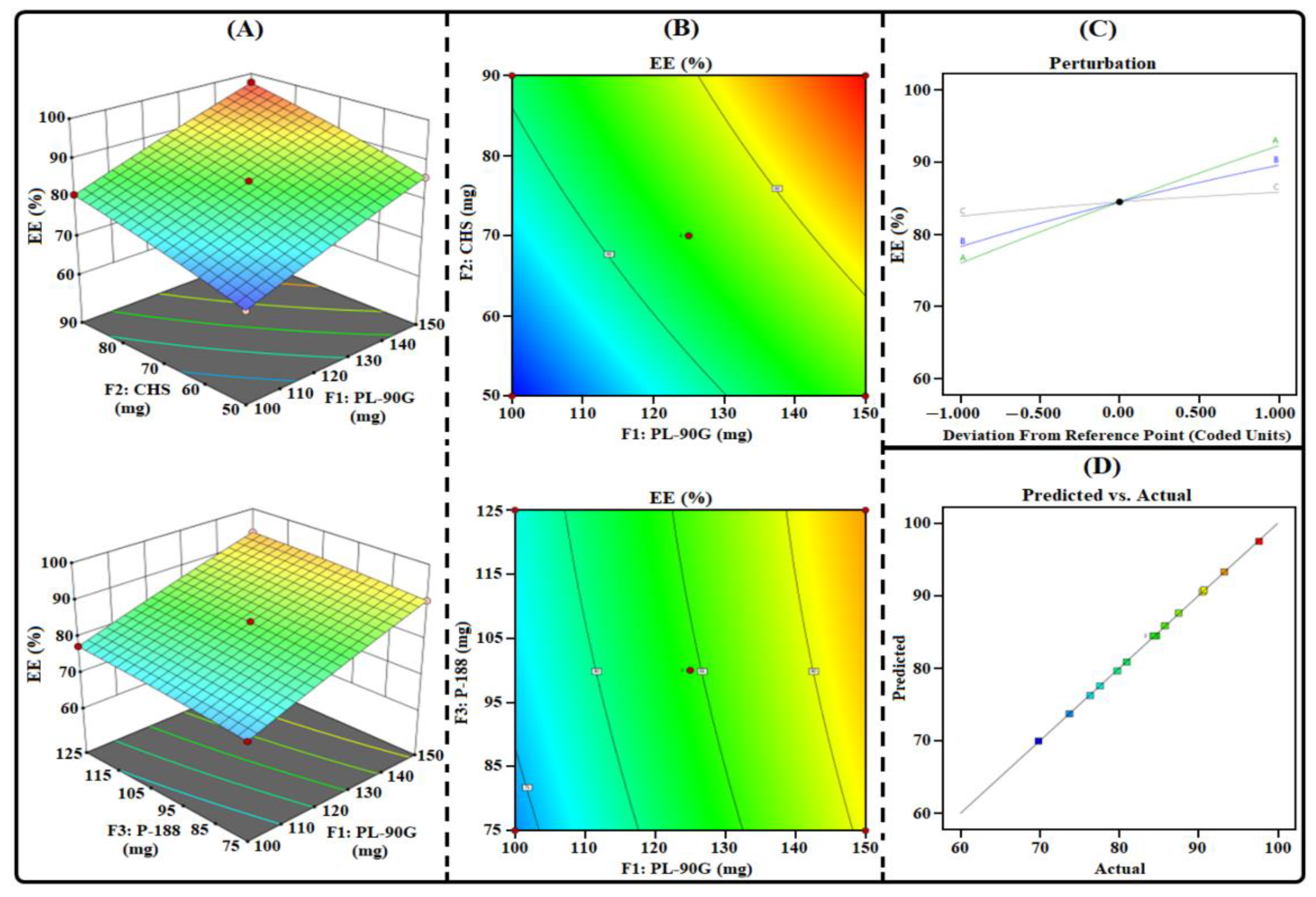
3.1. THQ-LPHNPs Optimization by 33-BBD
3.1.1. Influence of Factors on R1
3.1.2. Influence of Factors on R2
3.1.3. Influence of Factors on R3
3.1.4. Selection of Optimized Composition
3.2. THQ-LPHNPs Characterization
3.2.1. Particles Characterization
3.2.2. %EE and %DL
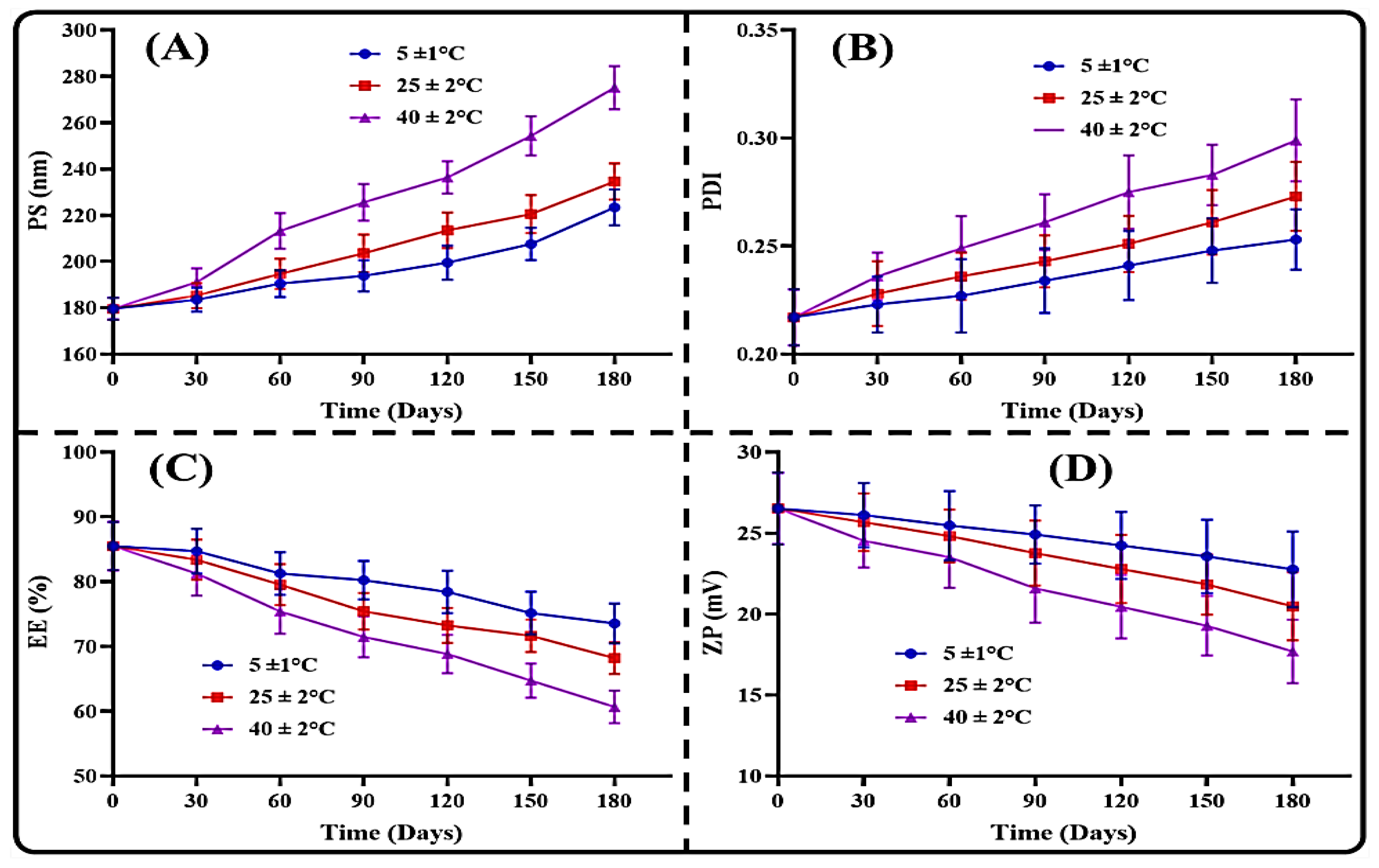
3.3. Stability Results
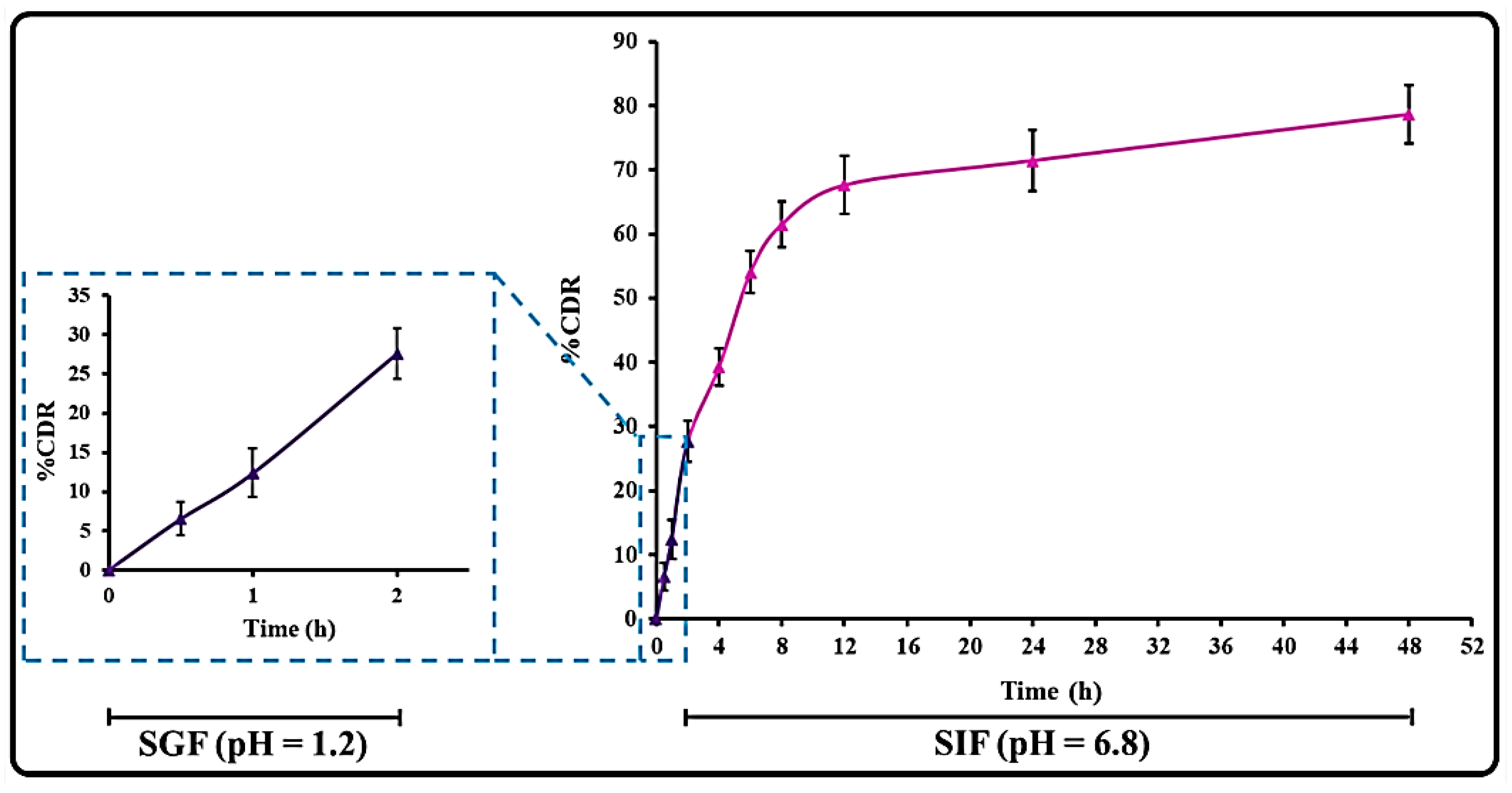
3.3.1. Stability in the Gastrointestinal Milieu
3.3.2. Storage Stability
3.4. THQ Release and Release Kinetics
3.5. Mucoadhesion Study
3.6. Cell Culture Studies
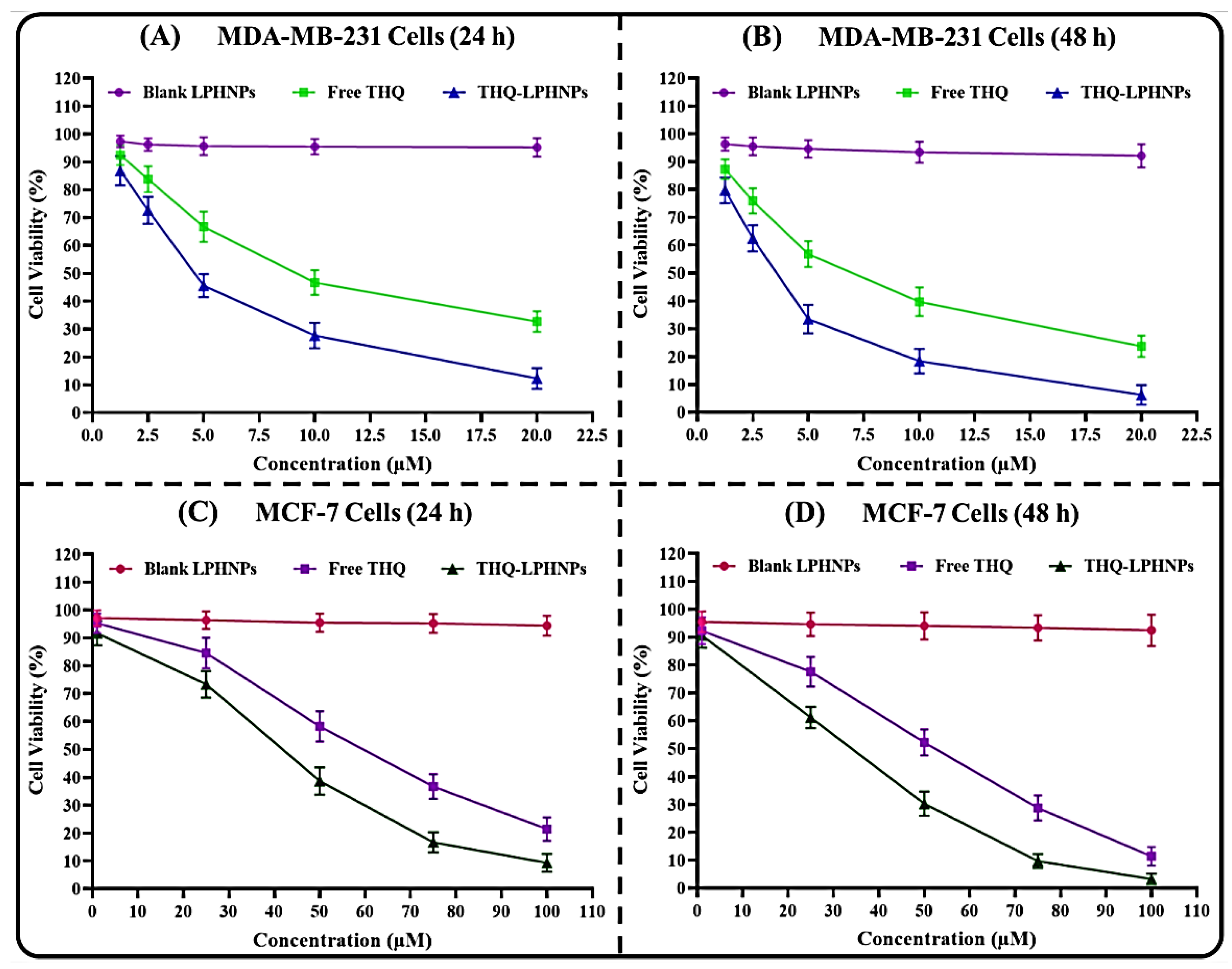
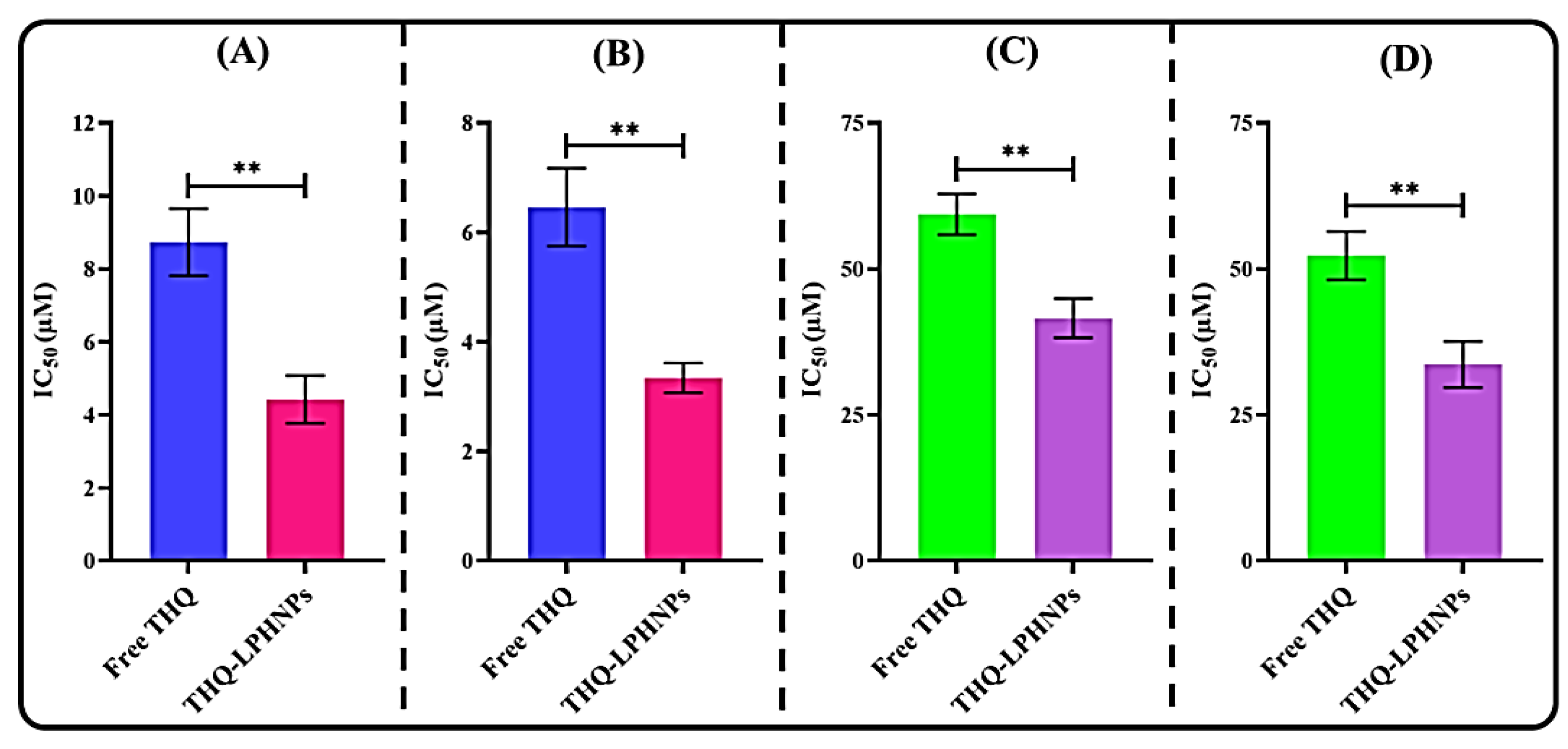
3.6.1. Cell Viability Assay
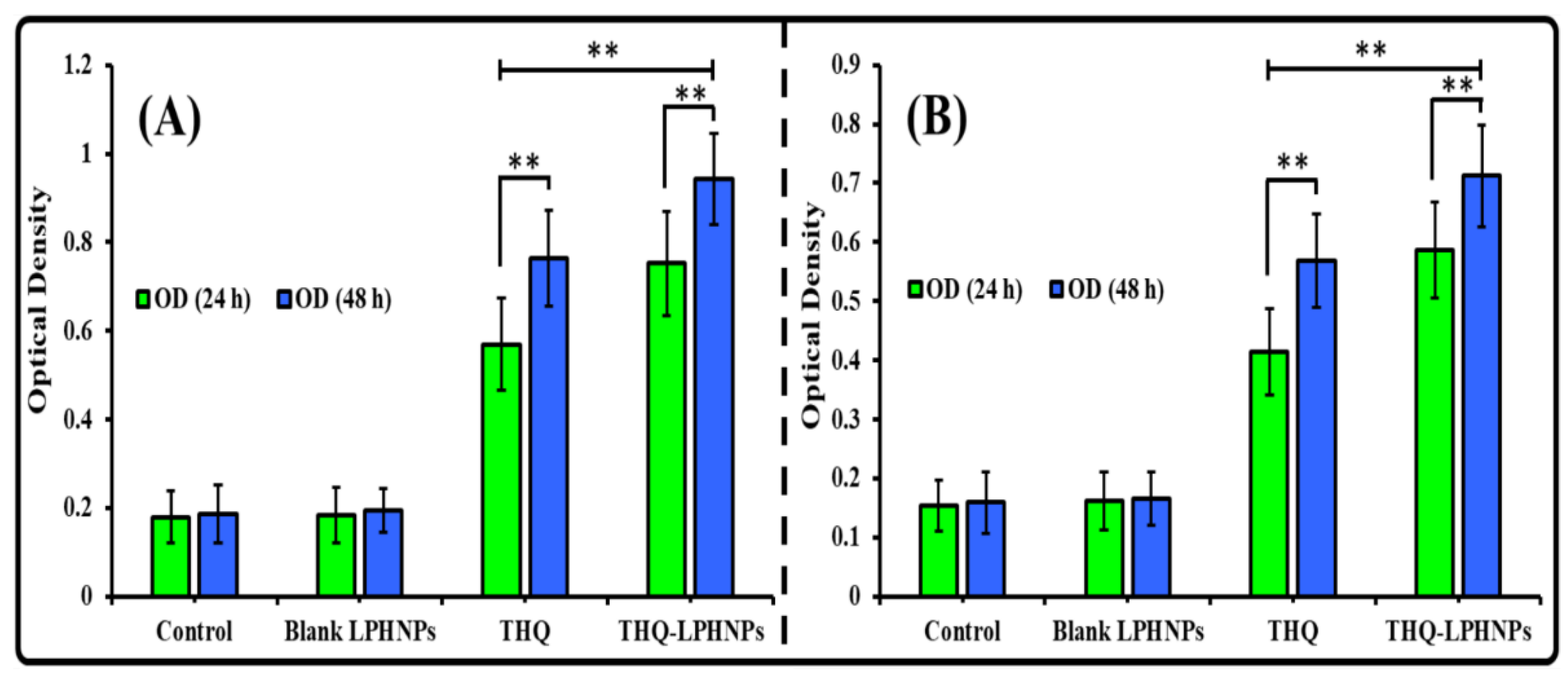
3.6.2. Lactate Dehydrogenase (LDH) Assay
3.6.3. Morphological Examination of Treated Cells
3.7. Ex Vivo THQ Permeation Study
3.8. Measurement of Permeation Depth
3.9. Relative Bioavailability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harwansh, R.K.; Deshmukh, R. Breast cancer: An insight into its inflammatory, molecular, pathological and targeted facets with update on investigational drugs. Crit. Rev. Oncol. Hematol. 2020, 154, 103070. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Davies, E.L. Breast cancer. Medicine 2019, 48, 113–118. [Google Scholar] [CrossRef]
- Jain, V.; Kumar, H.; Anod, H.V.; Chand, P.; Gupta, N.V.; Dey, S.; Kesharwani, S.S. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J. Control. Release 2020, 326, 628–647. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Amin, S.; Mir, S.R.; Fakhri, K.U.; Rizvi, M.M.A. Phytochemical based nanomedicines against cancer: Current status and future prospects. J. Drug Target. 2018, 26, 731–752. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Ballout, F.; Habli, Z.; Rahal, O.N.; Fatfat, M.; Gali-Muhtasib, H. Thymoquinone-based nanotechnology for cancer therapy: Promises and challenges. Drug Discov. Today 2018, 23, 1089–1098. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 319–329. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Ghoneim, M.M.; Alshehri, S.; Imam, S.S.; Md, S.; Alhakamy, N.A.; Jain, K.; Ahmad, J. Receptor-mediated targeted delivery of surface-modified nanomedicine in breast cancer: Recent update and challenges. Pharmaceutics 2021, 13, 2039. [Google Scholar] [CrossRef]
- Rathore, C.; Rathbone, M.J.; Chellappan, D.K.; Tambuwala, M.M.; Pinto, T.J.A.; Dureja, H.; Hemrajani, C.; Gupta, G.; Dua, K.; Negi, P. Nanocarriers: More than tour de force for thymoquinone. Expert Opin. Drug Deliv. 2020, 17, 479–494. [Google Scholar] [CrossRef]
- El-Far, A.H.; Al Jaouni, S.K.; Li, W.; Mousa, S.A. Protective roles of thymoquinone nanoformulations: Potential nanonutraceuticals in human diseases. Nutrients 2018, 10, 1369. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.S.; Saiful Yazan, L.; Ng, W.K.; Abdullah, R.; Mustapha, N.M.; Sapuan, S.; Foo, J.B.; Tor, Y.S.; How, C.W.; Abd Rahman, N.; et al. Thymoquinone loaded in nanostructured lipid carrier showed enhanced anticancer activity in 4T1 tumor-bearing mice. Nanomedicine 2018, 13, 1567–1582. [Google Scholar] [CrossRef]
- Ramachandran, S.; Thangarajan, S. A novel therapeutic application of solid lipid nanoparticles encapsulated thymoquinone (TQ-SLNs) on 3-nitroproponic acid induced Huntington’s disease-like symptoms in wistar rats. Chem. Biol. Interact. 2016, 256, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [PubMed]
- Ganea, G.M.; Fakayode, S.O.; Losso, J.N.; van Nostrum, C.F.; Sabliov, C.M.; Warner, I.M. Delivery of phytochemical thymoquinone using molecular micelle modified poly(D, L lactide-co-glycolide) (PLGA) nanoparticles. Nanotechnology 2010, 21, 285104. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Puvvada, N.; Kumar, B.P.; Sarkar, S.; Konar, S.; Bharti, R.; Dey, G.; Mazumdar, A.; Pathak, A.; Fisher, P.B.; et al. Overcoming Akt induced therapeutic resistance in breast cancer through siRNA and thymoquinone encapsulated multilamellar gold niosomes. Mol. Pharm. 2015, 12, 4214–4225. [Google Scholar] [CrossRef]
- Goel, S.; Mishra, P. Thymoquinone loaded mesoporous silica nanoparticles retard cell invasion and enhance in vitro cytotoxicity due to ROS mediated apoptosis in HeLa and MCF-7 cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 10988. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent advancement in chitosan-based nanoparticles for improved oral bioavailability and bioactivity of phytochemicals: Challenges and perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Ahmad, J.; Rizwanullah, M.; Suthar, T.; Albarqi, H.A.; Ahmad, M.Z.; Vuddanda, P.R.; Khan, M.A.; Jain, K. Receptor-targeted surface-engineered nanomaterials for breast cancer imaging and theranostic applications. Crit. Rev. Ther. Drug. Carrier Syst. 2022, 39, 1–44. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Alam, M.; Harshita; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Polymer-lipid hybrid nanoparticles: A next-generation nanocarrier for targeted treatment of solid tumors. Curr. Pharm. Des. 2020, 26, 1206–1215. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [Green Version]
- Rizwanullah, M.; Ahmad, J.; Amin, S.; Mishra, A.; Ain, M.R.; Rahman, M. Polymer-lipid hybrid systems: Scope of intravenous-to-oral switch in cancer chemotherapy. Curr. Nanomed. 2020, 10, 164–177. [Google Scholar] [CrossRef]
- Yang, J.M.; Wu, L.J.; Lin, M.T.; Lu, Y.Y.; Wang, T.T.; Han, M.; Zhang, B.; Xu, D.H. Construction and evaluation of chitosan-based nanoparticles for oral administration of exenatide in type 2 diabetic rats. Polymers 2022, 14, 2181. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation of piperine nanoparticles: In vitro breast cancer cell line and in vivo evaluation. Polymers 2022, 14, 1349. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in delivery system. Drug Dev. Ind. Pharm. 2019, 45, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Perwez, A.; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Exemestane encapsulated polymer-lipid hybrid nanoparticles for improved efficacy against breast cancer: Optimization, in vitro characterization and cell culture studies. Nanotechnology 2021, 32, 415101. [Google Scholar] [CrossRef] [PubMed]
- Kaithwas, V.; Dora, C.P.; Kushwah, V.; Jain, S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf. B Biointerfaces 2017, 154, 10–20. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Mahvelati, F.; Malaekeh-Nikouei, B.; Hashemi, E.; Oroojalian, F.; Hashemi, M. Bromelain loaded lipid-polymer hybrid nanoparticles for oral delivery: Formulation and characterization. Appl. Biochem. Biotechnol. 2022, 194, 3733–3748. [Google Scholar] [CrossRef]
- Patel, R.R.; Khan, G.; Chaurasia, S.; Kumar, N.; Mishra, B. Rationally developed core–shell polymeric-lipid hybrid nanoparticles as a delivery vehicle for cromolyn sodium: Implications of lipid envelop on in vitro and in vivo behaviour of nanoparticles upon oral administration. RSC Adv. 2015, 5, 76491–76506. [Google Scholar] [CrossRef]
- Ana, R.; Mendes, M.; Sousa, J.; Pais, A.; Falcão, A.; Fortuna, A.; Vitorino, C. Rethinking carbamazepine oral delivery using polymer-lipid hybrid nanoparticles. Int. J. Pharm. 2019, 554, 352–365. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation and evaluation of apigenin-loaded hybrid nanoparticles. Pharmaceutics 2022, 14, 783. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Fakhri, K.U.; Rizvi, M.M.; Mahdi, W.; Kazi, M. Effect of chitosan coating on PLGA nanoparticles for oral delivery of thymoquinone: In vitro, ex vivo, and cancer cell line assessments. Coatings 2020, 11, 6. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Saremi, S.; Ostad, S.N.; Dinarvand, R.; Atyabi, F. Discriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell lines. Nanomedicine 2010, 6, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): In vitro and in vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef] [PubMed]
- Neupane, Y.R.; Srivastava, M.; Ahmad, N.; Kumar, N.; Bhatnagar, A.; Kohli, K. Lipid based nanocarrier system for the potential oral delivery of decitabine: Formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Pharm. 2014, 477, 601–612. [Google Scholar] [CrossRef]
- Solairaj, D.; Rameshthangam, P.; Arunachalam, G. Anticancer activity of silver and copper embedded chitin nanocomposites against human breast cancer (MCF-7) cells. Int. J. Biol. Macromol. 2017, 105, 608–619. [Google Scholar] [CrossRef]
- Silva, D.F.; Vidal, F.C.; Santos, D.; Costa, M.C.; Morgado-Díaz, J.A.; Nascimento, M.D.; de Moura, R.S. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement. Altern. Med. 2014, 14, 175. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Sabir, M.D.; Ahmad, N.; Ali, M.; Kohli, K. Lipid drug conjugate nanoparticle as a novel lipid nanocarrier for the oral delivery of decitabine: Ex vivo gut permeation studies. Nanotechnology 2013, 24, 415102. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Rahat, I.; Rizwanullah, M.; Gilani, S.J.; Bin-Jummah, M.N.; Imam, S.S.; Kala, C.; Asif, M.; Alshehri, S.; Sharma, S.K. Thymoquinone loaded chitosan-Solid lipid nanoparticles: Formulation optimization to oral bioavailability study. J. Drug Deliv. Sci. Technol. 2021, 64, 102565. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Patil, A.B.; Katare, O.P. Chitosan-tailored lipidic nanoconstructs of Fusidic acid as promising vehicle for wound infections: An explorative study. Int. J. Biol. Macromol. 2018, 115, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakur, K.; Sharma, G.; Kush, P.; Jain, U.K. Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 137, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Singh, B.; Sharma, G.; Kushwah, V.; Tyagi, R.K.; Jain, S.; Katare, O.P. Development and characterization of single step self-assembled lipid polymer hybrid nanoparticles for effective delivery of methotrexate. RSC Adv. 2015, 5, 62989–62999. [Google Scholar] [CrossRef]
- Mahmood, S.; Kiong, K.C.; Tham, C.S.; Chien, T.C.; Hilles, A.R.; Venugopal, J.R. PEGylated lipid polymeric nanoparticle-encapsulated acyclovir for in vitro controlled release and ex vivo gut sac permeation. AAPS PharmSciTech 2020, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef]
- Gajra, B.; Dalwadi, C.; Patel, R.R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using Box Behnken design. DARU J. Pharm. Sci. 2015, 23, 3. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Balasubramanian, V.; Rehman, M.; Correia, A.; Kashif, P.M.; Mäkilä, E.; Salonen, J.; Santos, H.A. Development and optimization of methotrexate-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery applications. Int. J. Pharm. 2017, 533, 156–168. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Mitragotri, S.; Rai, B. Permeation of nanoparticles across the intestinal lipid membrane: Dependence on shape and surface chemistry studied through molecular simulations. Nanoscale 2020, 12, 6318–6333. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Granja, S.; Boni, F.I.; Prezotti, F.G.; Ferreira, L.; Cury, B.S.; Reis, R.M.; Baltazar, F.; Gremiao, M.P.D. Modulating chitosan-PLGA nanoparticle properties to design a co-delivery platform for glioblastoma therapy intended for nose-to-brain route. Drug Deliv. Transl. Res. 2020, 10, 1729–1747. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Z.; Liao, Y.; Li, S.; Xue, Y.; Firempong, M.A.; Xu, Y.; Yu, J.; Smyth, H.D.; Xu, X. Improved intestinal absorption and oral bioavailability of astaxanthin using poly (ethylene glycol)-graft-chitosan nanoparticles: Preparation, in vitro evaluation and pharmacokinetics in rats. J. Sci. Food Agric. 2022, 102, 1002–1011. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible Colon-targeted delivery. Pharm. Dev. Technol. 2021, 26, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Docler, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.J.; Costa Lima, S.A.; Afonso, C.M.M.; Reis, S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol. 2020, 158, 180–188. [Google Scholar] [CrossRef]
- de Lázaro, I.; Mooney, D.J. A nanoparticle’s pathway into tumours. Nat. Mater. 2020, 19, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Geethakumari, D.; Sathyabhama, A.B.; Sathyan, K.R.; Mohandas, D.; Somasekharan, J.V.; Puthiyedathu, S.T. Folate functionalized chitosan nanoparticles as targeted delivery systems for improved anticancer efficiency of cytarabine in MCF-7 human breast cancer cell lines. Int. J. Biol. Macromol. 2022, 199, 150–161. [Google Scholar] [CrossRef]
- Taghipour-Sabzevar, V.; Sharifi, T.; Moghaddam, M.M. Polymeric nanoparticles as carrier for targeted and controlled delivery of anticancer agents. Ther. Deliv. 2019, 10, 527–550. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Fakhria, A.; Gilani, S.J.; Imam, S.S. Formulation of thymoquinone loaded chitosan nano vesicles: In-vitro evaluation and in-vivo anti-hyperlipidemic assessment. J. Drug Deliv. Sci. Technol. 2019, 50, 339–346. [Google Scholar] [CrossRef]
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zheng, X.; Bai, R.; Yang, Y.; Jian, L. Bioadhesive poly(methyl vinyl ether-co-maleic anhydride)-TPGS copolymer modified PLGA/lipid hybrid nanoparticles for improving intestinal absorption of cabazitaxel. Int. J. Pharm. 2022, 611, 121301. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, H.; Xu, E.; Zhang, X.; Guan, J.; Zhao, R.; Mao, S. Exploring the potential of functional polymer-lipid hybrid nanoparticles for enhanced oral delivery of paclitaxel. Asian J. Pharm. Sci. 2021, 16, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gao, N.; Song, X. Bioadhesive polymer/lipid hybrid nanoparticles as oral delivery system of raloxifene with enhancive intestinal retention and bioavailability. Drug Deliv. 2021, 28, 252–260. [Google Scholar] [CrossRef] [PubMed]



| Factors | Levels | ||
|---|---|---|---|
| Independent variables | Low (−1) | Medium (0) | High (+1) |
| F1 = PL-90G concentration (mg) | 100 | 125 | 150 |
| F2 = CHS concentration (mg) | 50 | 70 | 90 |
| F3 = P-188 concentration (mg) | 75 | 100 | 125 |
| Responses (Dependent variables) | Goal | ||
| R1 = Particle size (PS; nm) | Minimize | ||
| R2 = Polydispersity index (PDI) | Minimize | ||
| R3 = Entrapment efficiency (EE; %) | Maximize | ||
| Runs | Independent Factors | Dependent Factors (Responses) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 (mg) | F2 (mg) | F3 (mg) | R1 (PS in nm) | R2 (PDI) | R3 (EE in %) | ||||
| Actual | Predicted | Actual | Predicted | Actual | Predicted | ||||
| NP1 | 100 | 50 | 100 | 123.79 | 121.10 | 0.126 | 0.118 | 69.86 | 69.99 |
| NP2 | 125 | 50 | 125 | 152.26 | 154.67 | 0.181 | 0.179 | 79.78 | 79.67 |
| NP3 | 150 | 70 | 125 | 214.19 | 212.81 | 0.352 | 0.348 | 93.27 | 93.30 |
| NP4 | 125 | 90 | 125 | 203.48 | 204.18 | 0.327 | 0.323 | 90.72 | 90.83 |
| NP5 | 125 | 70 | 100 | 180.52 | 183.58 | 0.219 | 0.222 | 84.37 | 84.48 |
| NP6 | 150 | 70 | 75 | 227.67 | 224.39 | 0.372 | 0.363 | 90.53 | 90.55 |
| NP7 | 100 | 70 | 125 | 142.78 | 143.06 | 0.159 | 0.167 | 77.61 | 77.59 |
| NP8 | 150 | 50 | 100 | 194.47 | 195.44 | 0.271 | 0.275 | 85.79 | 85.88 |
| NP9 | 125 | 50 | 75 | 165.09 | 164.39 | 0.205 | 0.208 | 76.37 | 76.26 |
| NP10 | 125 | 70 | 100 | 181.14 | 180.58 | 0.224 | 0.222 | 84.71 | 84.48 |
| NP11 | 100 | 70 | 75 | 149.85 | 151.24 | 0.183 | 0.186 | 73.76 | 73.73 |
| NP12 | 125 | 70 | 100 | 180.09 | 180.58 | 0.225 | 0.222 | 84.37 | 84.48 |
| NP13 | 125 | 90 | 75 | 215.63 | 215.22 | 0.328 | 0.329 | 87.52 | 87.63 |
| NP14 | 150 | 90 | 100 | 246.53 | 247.22 | 0.423 | 0.430 | 97.65 | 97.52 |
| NP15 | 100 | 90 | 100 | 174.63 | 173.66 | 0.233 | 0.228 | 80.97 | 80.88 |
| Model | R2 | Adjusted R2 | Predicted R2 | SD | Press | Remark |
|---|---|---|---|---|---|---|
| Response-1 (R1) | ||||||
| Linear | 0.9967 | 0.9958 | 0.9944 | 2.21 | 89.93 | - |
| 2F1 | 0.9973 | 0.9953 | 0.9924 | 2.32 | 122.58 | - |
| Quadratic | 0.9995 | 0.9985 | 0.9919 | 1.32 | 131.55 | Suggested |
| Response-2 (R2) | ||||||
| Linear | 0.9518 | 0.9387 | 0.9272 | 0.0214 | 0.0076 | - |
| 2F1 | 0.9580 | 0.9265 | 0.9037 | 0.0235 | 0.0101 | - |
| Quadratic | Suggested | |||||
| Response-3 (R3) | ||||||
| Linear | 0.9970 | 0.9962 | 0.9953 | 0.4684 | 3.78 | - |
| 2F1 | 0.9976 | 0.9958 | 0.9944 | 0.4942 | 4.55 | - |
| Quadratic | 0.9998 | 0.9994 | 0.9978 | 0.1887 | 1.79 | Suggested |
| Parameters | SGF (pH = 1.2) | SIF (pH = 6.8) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Particles size (nm) | 179.63 ± 4.77 | 193.31 ± 6.44 | 179.63 ± 4.77 | 187.82 ± 5.39 |
| Polydispersity index | 0.217 ± 0.013 | 0.272 ± 0.016 | 0.217 ± 0.013 | 0.241 ± 0.013 |
| Entrapment efficiency (%) | 85.49 ± 3.73 | 78.54 ± 3.57 | 85.49 ± 3.73 | 81.46 ± 3.39 |
| Zeta potential (mV) | +26.52 ± 2.21 | +21.18 ± 2.83 | +26.52 ± 2.21 | +23.53 ± 1.94 |
| Model | Model Equation | Equation | R2 | Release Exponent (‘n’) |
|---|---|---|---|---|
| Zero-order model | Mt = M0 + k0 t | y = 0.0127x + 0.3162 | 0.5585 | - |
| First-order model | ln Mt = ln M0 + k1 t | y = –0.0124x + 1.8232 | 0.7214 | - |
| Higuchi-matrix model | Mt = M0 + k t1/2 | y = 0.1162x + 0.1339 | 0.7824 | - |
| Korsmeyer–Peppas model | Mt/M∞ = k tn | y = 0.5522x − 0.8337 | 0.8736 | 0.2397 |
| Parameters | THQ-Suspension | THQ-LPHNPs |
|---|---|---|
| (µg/mL) | 56.23 | 181.49 * |
| (h) | 4 | 2 |
| (µ·h/mL) | 466.215 | 2213.807 * |
| (µ·h/mL) | 492.789 | 2376.788 * |
| (µ·h2/mL) | 4830.007 | 26897.355 * |
| (µ·h2/mL) | 6552.578 | 37725.244 * |
| MRT (h) | 10.360 | 12.149 |
| (h) | 9.658 | 12.779 * |
| (h−1) | 0.059 | 0.047 |
| – | 4.748 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imam, S.S.; Gilani, S.J.; Bin Jumah, M.N.; Rizwanullah, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S. Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments. Polymers 2022, 14, 3705. https://doi.org/10.3390/polym14183705
Imam SS, Gilani SJ, Bin Jumah MN, Rizwanullah M, Zafar A, Ahmed MM, Alshehri S. Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments. Polymers. 2022; 14(18):3705. https://doi.org/10.3390/polym14183705
Chicago/Turabian StyleImam, Syed Sarim, Sadaf Jamal Gilani, May Nasser Bin Jumah, Md. Rizwanullah, Ameeduzzafar Zafar, Mohammed Muqtader Ahmed, and Sultan Alshehri. 2022. "Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments" Polymers 14, no. 18: 3705. https://doi.org/10.3390/polym14183705





