Polymeric Packaging Applications for Seafood Products: Packaging-Deterioration Relevance, Technology and Trends
Abstract
:1. Introduction
2. Major Challenges for Seafood Packaging
2.1. Seafood Quality and Freshness
2.2. Fishery Smell in Seafoods
3. Packaging Related Deteriorations of Seafoods
3.1. Microbial Deterioration
3.2. Chemical Deterioration
3.3. Biochemical Deterioration
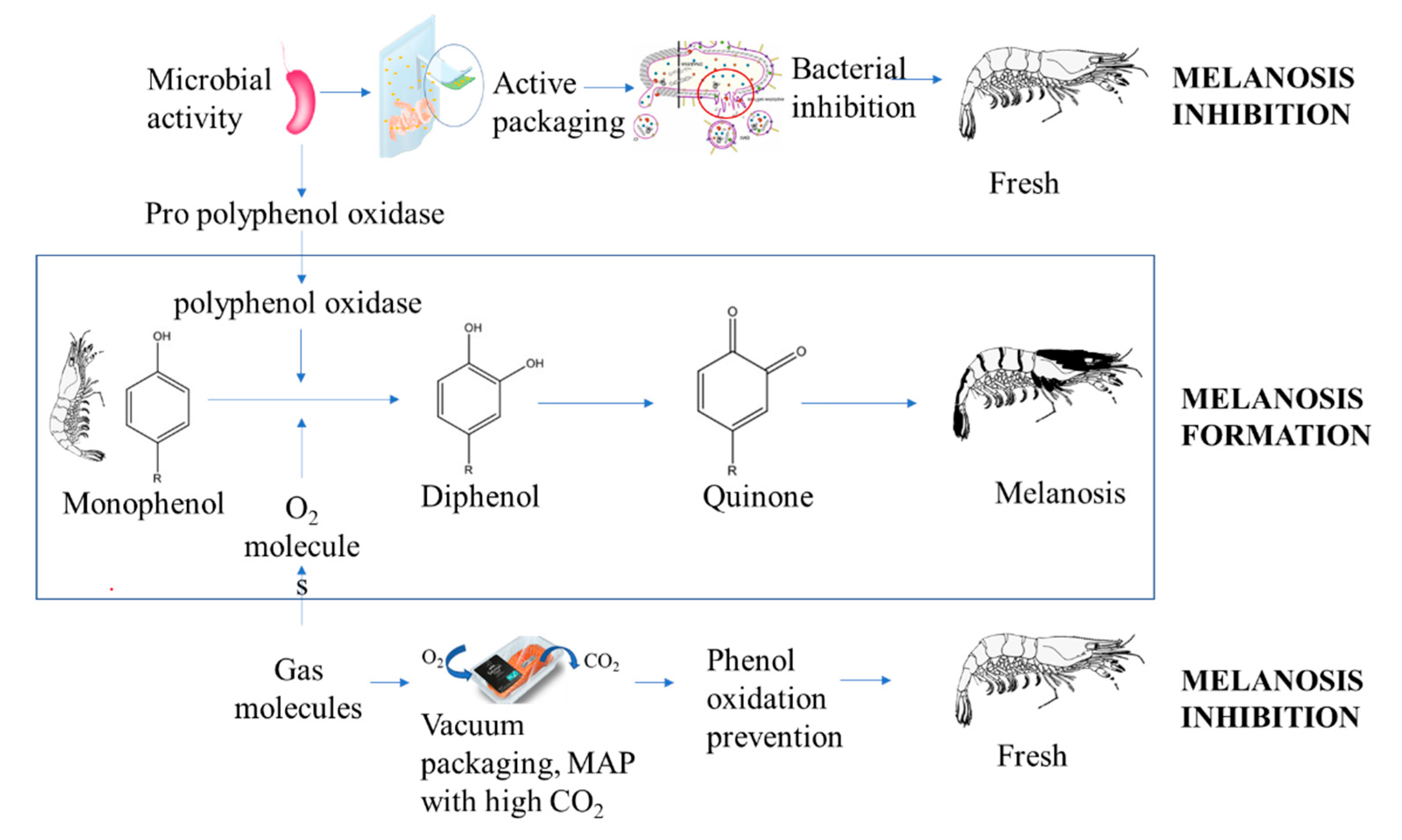
3.4. Melanosis
4. Polymeric Packaging for Seafoods
4.1. Conventional Polymer-Based Packaging
4.2. Bio-Based Packaging
5. Innovative Polymeric Packaging Enhancing and Monitoring Seafood Quality and Freshness
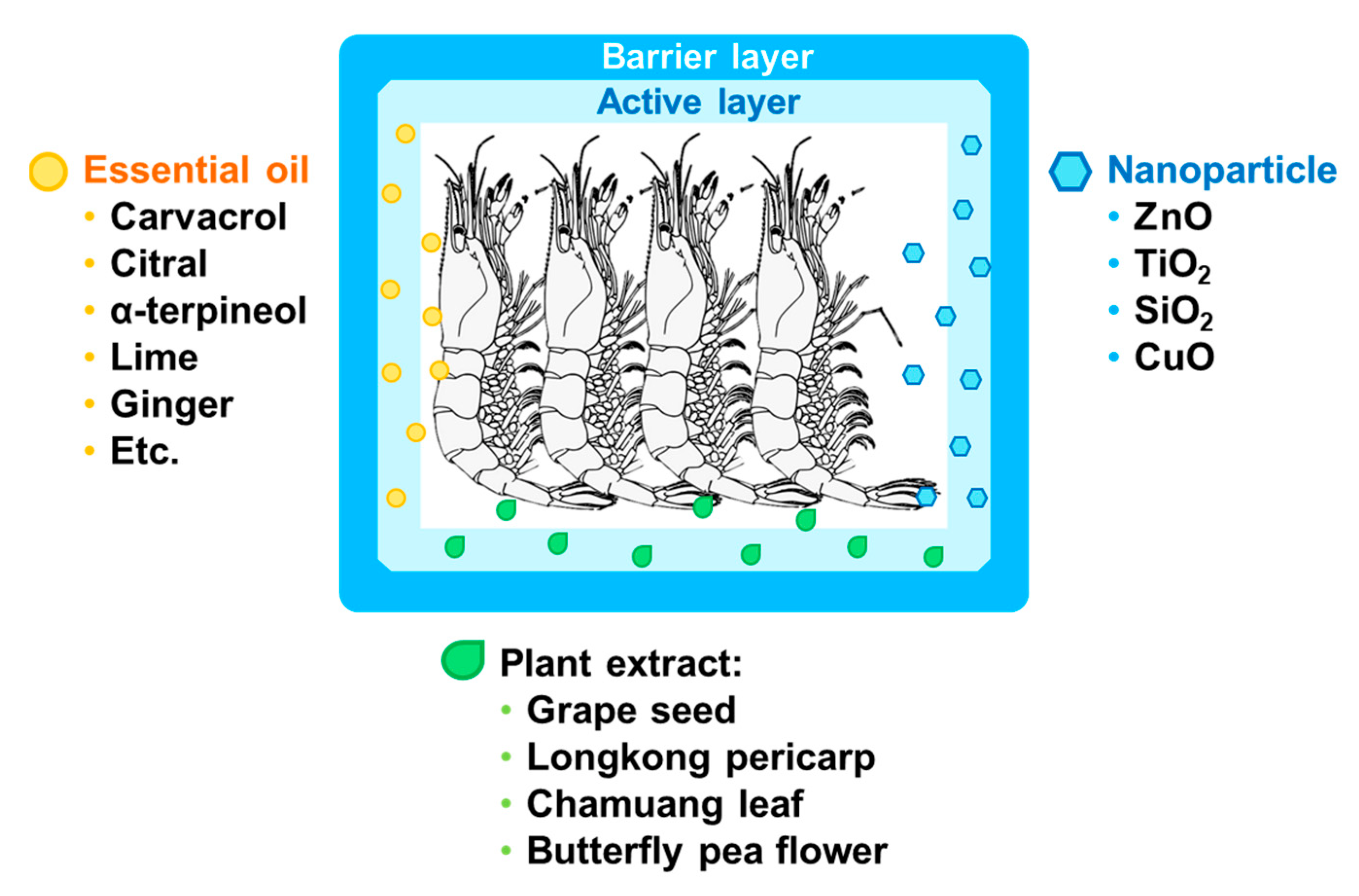
5.1. Active Polymer Technology for Seafoods
5.2. Polymeric Sensors for Seafood
6. Other Combined Technologies with Polymeric Packaging to Preserve Seafood Quality and Freshness
6.1. Modified Atmosphere Packaging for Seafood
6.2. Thermal Insulation Packaging
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Shrimp Prices up Due to High Freight Rates; Food Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Tang, J.; Hong, Y.-K.; Inanoglu, S.; Liu, F. Microwave pasteurization for ready-to-eat meals. Curr. Opin. Food Sci. 2018, 23, 133–141. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Ma, Q.; Lu, X.; Wang, W.; Hubbe, M.A.; Liu, Y.; Mu, J.; Wang, J.; Sun, J.; Rojas, O.J. Recent developments in colorimetric and optical indicators stimulated by volatile base nitrogen to monitor seafood freshness. Food Packag. Shelf Life 2021, 28, 100634. [Google Scholar] [CrossRef]
- Deng, W.; Tian, G.; Wang, Z.; Mao, K.; Liu, X.; Ding, Q.; Sang, Y.; Gao, J. Analysis of volatile components changes of Ruditapes philippinarum during boiling by HS-GC-IMS coupled with multivariate analyses. Aquac. Rep. 2022, 25, 101193. [Google Scholar] [CrossRef]
- Huang, X.H.; Zhang, Y.Y.; Zhu, M.; Zhou, D.Y.; Du, M.; Zhu, B.W.; Qin, L. The effects of different extraction methods on the aroma fingerprint, recombination and visualization of clam soup. Food Funct. 2021, 12, 1626–1638. [Google Scholar] [CrossRef]
- Guo, Q.; Yu, J.; Zhao, Y.; Liu, T.; Su, M.; Jia, Z.; Zhao, Y.; Mu, Z.; Yang, M. Identification of fishy odor causing compounds produced by Ochromonas sp. and Cryptomonas ovate with gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. Sci. Total Environ. 2019, 671, 149–156. [Google Scholar] [CrossRef]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO2 modified atmosphere packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef]
- Ishida, T. Removal of Fish Odors Form Styrofoam Packaging to Improve Recycling Potential Using Hansen Solubility Parameters. Recycling 2020, 5, 30. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Talens Oliag, P.; Hiralt, A. Thermoplastic starch: Improving their barrier properties. Agron. Colomb. 2016, 34 (Suppl. S1), S73–S75. [Google Scholar]
- Bang, G.; Kim, S.W. Biodegradable poly (lactic acid)-based hybrid coating materials for food packaging films with gas barrier properties. J. Ind. Eng. Chem. 2012, 18, 1063–1068. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Yang, Y.; Wang, Q.; Zhang, X.; Hu, H.; Zhu, J. Bio-based poly (butylene diglycolate-co-furandicarboxylate) copolyesters with balanced mechanical, barrier and biodegradable properties: A prospective substitute for PBAT. Polym. Degrad. Stability. 2022, 202, 110010. [Google Scholar] [CrossRef]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Yun Hee, K.; Kgeong Su, L. Method of Removing Fishy Smell Using Peanut Sprouts. Korea Patent No. KR1020180047287, 10 May 2018. [Google Scholar]
- Yoo, H.S. Instant Fish Stew in Which Fishy Smell Is Removed and Manufacturing Method Thereof. Korea Patent No. KR1020140016100, 7 February 2014. [Google Scholar]
- Nielsen, S.M.; Norskov-Lauritsen, N.; Bjarnsholt, T.; Meyer, R.L. Achromobacter Species Isolated from Cystic Fibrosis Patients Reveal Distinctly Different Biofilm Morphotypes. Microorganisms 2016, 4, 33. [Google Scholar] [CrossRef]
- Jalal, K.C.A.; Akbar John, B.; Lyana, N.; Faizul, H.N.; Noor Isma Yanti, M.; Irwandi, J.; Bulbul, M. Comparative study on spoilage and pathogenic bacteria in selected commercial marine and freshwater fishes. Int. Food Res. J. 2017, 24, 298–304. [Google Scholar]
- Gonçalves, A.A.; de Oliveira, A.R.M. Melanosis in crustaceans: A review. LWT—Food Sci. Technol. 2016, 65, 791–799. [Google Scholar] [CrossRef]
- Mohebi, E.; Shahbazi, Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: A novel functional wrapping design. LWT—Food Sci. Technol. 2017, 76, 108–116. [Google Scholar] [CrossRef]
- Shao, J.; Wang, L.; Wang, X.; Ma, J. Enhancing microbial management and shelf life of shrimp Penaeus vannamei by using nanoparticles of metallic oxides as an alternate active packaging tool to synthetic chemicals. Food Packag. Shelf Life 2021, 28, 100652. [Google Scholar] [CrossRef]
- Yuan, D.; Hao, X.; Liu, G.; Yue, Y.; Duan, J. A novel composite edible film fabricated by incorporating W/O/W emulsion into a chitosan film to improve the protection of fresh fish meat. Food Chem. 2022, 385, 132647. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control. 2021, 125, 107944. [Google Scholar] [CrossRef]
- Li, P.; Mei, J.; Xie, J. Chitosan-sodium alginate bioactive coatings containing ε-polylysine combined with high CO2 modified atmosphere packaging inhibit myofibril oxidation and degradation of farmed pufferfish (Takifugu obscurus) during cold storage. LWT 2021, 140, 110652. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques. Am. J. Appl. Sci. 2010, 7, 859. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Post harvest discoloration of dark-fleshed fish muscle: A review. Walailak J. Sci. Technol. (WJST) 2009, 6, 149–166. [Google Scholar]
- Niederer, M.; Lang, S.; Roux, B.; Stebler, T.; Hohl, C. Identification of nitrite treated tuna fish meat via the determination of nitrous oxide by head space-gas chromatography/mass spectrometry. F1000 Res. 2019, 8, 711. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Srinivasa Gopal, T.K. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- FAO. Quality and Quality Changes in Fresh Fish; Food Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Erkan, N. The Effect of Active and Vacuum Packaging on the Quality of Turkish Traditional Salted Dried Fish “Çİroz”. J. Food Health Sci. 2017, 3, 29–35. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Xie, J.; Yang, S.-P.; Huang, S.; Wu, W.-H.; Li, L. Inhibitory effect of a quercetin-based soaking formulation and modified atmospheric packaging (MAP) on muscle degradation of Pacific white shrimp (Litopenaeus vannamei). LWT—Food Sci. Technol. 2015, 63, 1339–1346. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapıcı, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT—Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and alpha-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.B.; Bui, T.H.; Phan, D.T.A. Persea Americana Mill seed extracts: Understanding insights into the antioxidant and antityrosinase activities and effects on preserving qualities of whiteleg shrimp (Litopenaus vannamei) during refrigerated storage. Food Chem. 2022, 373, 131469. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Benjakul, S.; Gulzar, S. Impact of pulsed electric field and vacuum impregnation with Chamuang leaf extract on quality changes in Pacific white shrimp packaged under modified atmosphere. LWT 2021, 149, 111899. [Google Scholar] [CrossRef]
- Greene, J.P.P. Automotive Plastics and Composites; William Andrew Publishing: Norwich, CT, USA, 2021. [Google Scholar]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control. 2018, 92, 37–46. [Google Scholar] [CrossRef]
- Kumar, P.; Ganguly, S. Role of vacuum packaging in increasing shelf-life in fish processing technology. Asian J. Bio. Sci. 2014, 9, 109–112. [Google Scholar]
- Kan, J.; Liu, J.; Xu, F.; Yun, D.; Yong, H.; Liu, J. Development of pork and shrimp freshness monitoring labels based on starch/polyvinyl alcohol matrices and anthocyanins from 14 plants: A comparative study. Food Hydrocoll. 2022, 124, 107293. [Google Scholar] [CrossRef]
- Wencong, H. Water Absorption Disc Based on PLA Modified Material and Processing Device Thereof. China Patent No. CN213110659, 4 May 2021. [Google Scholar]
- Yukinobu, S. Containing Bag for Fresh Fish or the Like. Japan Patent No. JP1995243741, 19 September 1995. [Google Scholar]
- Takeko, A. Portable Cool Box. Japan Patent No. JP2013085550, 13 May 2013. [Google Scholar]
- Kenichi, M. Intermediate Dish of Foamed Fish Box. Japan Patent No. JP2018135150, 30 August 2018. [Google Scholar]
- Sang Hyung, L.; Deok Whan, C. Cooling Packing Box. Korea Patent No. KR1020180112388, 12 October 2018. [Google Scholar]
- Shang, X. Corrugated Carton Suitable for Transportation and Packaging of Fish Tank. China Patent No. CN209427201, 24 September 2019. [Google Scholar]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control. 2022, 142, 109273. [Google Scholar] [CrossRef]
- Wadaugsorn, K.; Panrong, T.; Wongphan, P.; Harnkarnsujarit, N. Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties. Ind. Crops Prod. 2022, 176, 114311. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Lianwei, W. Starch-Based Degradable Foam Tray. No. CN211309519. 21 August 2020. [Google Scholar]
- Hiroshi, M.; Yutaka, M.; Kiyohiko, S.; Michio, H. Biodegradable Resin Foam and Preparation Thereof. No. JP2001011221. 16 January 2001. [Google Scholar]
- Xiqing, H.; Guoqing, H.; Qingquan, H.; Qianqian, C. Bio-Based Composite Packaging Box Tray and Preparation Method. No. CN112300447. 2 February 2021. [Google Scholar]
- Zhaohui, Z.; Bingjian, L.; Ming, M.; Zhiye, L.; Qingwei, J. Slow-Release and Antibacterial Fresh Food Tray Material and Preparation Method Thereof. No. CN106117624. 16 November 2016. [Google Scholar]
- Mclaughlin, M.J.; Yapp, C.M. A Packaging Container and Process. No. GB2601831. 15 June 2022. [Google Scholar]
- Labuza, T.P.; Breene, W.M. Applications of ‘Active Packaging’ for improvement of shelf-life and nutritional quality of fresh and extended shelf-life foods. J. Food Proc. Preserv. 1989, 13, 1–69. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Pyrogallol loaded thermoplastic cassava starch based films as bio-based oxygen scavengers. Ind. Crops Prod. 2022, 186, 115226. [Google Scholar] [CrossRef]
- Prasad, P.; Kochhar, A. Active packaging in food industry: A review. IOSR J. Environ. Sci. Toxicol. Food Technol. (IOSR-JESTFT) 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Porat, R.; Poverenov, E. Enhancement of agricultural produce quality and storability using citral-based edible coatings; the valuable effect of nano-emulsification in a solid-state delivery on fresh-cut melons model. Food Chem. 2019, 277, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of active films poly (butylene adipate co-terephthalate)—PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind. Crops Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Review of surface treatment methods for polyamide films for potential application as smart packaging materials: Surface structure, antimicrobial and spectral properties. Food Packag. Shelf Life 2020, 24, 100475. [Google Scholar] [CrossRef]
- Nair, D.V.; Kiess, A.; Nannapaneni, R.; Schilling, W.; Sharma, C.S. The combined efficacy of carvacrol and modified atmosphere packaging on the survival of Salmonella, Campylobacter jejuni and lactic acid bacteria on turkey breast cutlets. Food Microbiol. 2015, 49, 134–141. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef]
- Lopes, J.; Gonçalves, I.; Nunes, C.; Teixeira, B.; Mendes, R.; Ferreira, P.; Coimbra, M.A. Potato peel phenolics as additives for developing active starch-based films with potential to pack smoked fish fillets. Food Packag. Shelf Life 2021, 28, 100644. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Ghanbarzadeh, B.; Divband, B. Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. Int. J. Food Microbiol. 2020, 312, 108375. [Google Scholar] [CrossRef] [PubMed]
- Azaza, Y.B.; Hamdi, M.; Charmette, C.; Jridi, M.; Li, S.; Nasri, M.; Nasri, R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf Life 2022, 31, 100796. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, N.; Li, L.; Xu, C.; Deng, S.; Wang, Y. Non-migrating active antibacterial packaging and its application in grass carp fillets. Food Packag. Shelf Life 2022, 31, 100786. [Google Scholar] [CrossRef]
- Jamroz, E.; Tkaczewska, J.; Kopec, M.; Cholewa-Wojcik, A. Shelf-life extension of salmon using active total biodegradable packaging with tea ground waste and furcellaran-CMC double-layered films. Food Chem. 2022, 383, 132425. [Google Scholar] [CrossRef]
- Ouahioune, L.A.; Wrona, M.; Nerín, C.; Djenane, D. Novel active biopackaging incorporated with macerate of carob (Ceratonia siliqua L.) to extend shelf-life of stored Atlantic salmon fillets (Salmo salar L.). LWT 2022, 156, 113015. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Méndez, D.A.; Martínez-Abad, A.; Zampini, C.; Torres, S.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Feasibility of active biobased films produced using red chilto wastes to improve the protection of fresh salmon fillets via a circular economy approach. Food Hydrocoll. 2022, 133, 107888. [Google Scholar] [CrossRef]
- Baek, S.-K.; Kim, S.; Song, K.B. Cowpea starch films containing maqui berry extract and their application in salmon packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Cao, T.L.; Song, K.B. Development of bioactive Bombacaceae gum films containing cinnamon leaf essential oil and their application in packaging of fresh salmon fillets. LWT 2020, 131, 109647. [Google Scholar] [CrossRef]
- Cai, M.; Zhong, H.; Li, C.; Aliakbarlu, J.; Zhang, H.; Cui, H.; Lin, L. Application of composite coating of Nostoc commune Vauch polysaccharides and sodium carboxymethyl cellulose for preservation of salmon fillets. Int. J. Biol. Macromol. 2022, 210, 394–402. [Google Scholar] [CrossRef]
- Boyacı, D.; Yemenicioğlu, A. Development of gel-based pads loaded with lysozyme and green tea extract: Characterization of pads and test of their antilisterial potential on cold-smoked salmon. LWT 2020, 128, 109471. [Google Scholar] [CrossRef]
- Loke, X.-J.; Chang, C.-K.; Hou, C.-Y.; Cheng, K.-C.; Hsieh, C.-W. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control. 2021, 125, 108016. [Google Scholar] [CrossRef]
- Zong, L.; Gao, H.; Chen, C.; Xie, J. Effects of starch/polyvinyl alcohol active film containing cinnamaldehyde on the quality of large yellow croaker (Pseudosciaena crocea) proteins during frozen storage. Food Chem. 2022, 389, 133065. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, G.; Yang, Q.; Yi, X.; Fu, L.; Wang, Y. Antibacterial Gelidium amansii polysaccharide-based edible films containing cyclic adenosine monophosphate for bioactive packaging. Int. J. Biol. Macromol. 2022, 212, 324–336. [Google Scholar] [CrossRef]
- Arancibia, M.; Giménez, B.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Release of cinnamon essential oil from polysaccharide bilayer films and its use for microbial growth inhibition in chilled shrimps. LWT—Food Sci. Technol. 2014, 59, 989–995. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, S.Y.; Oh, S.W. Enhancing safety and quality of shrimp by nanoparticles of sodium alginate-based edible coating containing grapefruit seed extract. Int. J. Biol. Macromol. 2021, 189, 84–90. [Google Scholar] [CrossRef]
- Nagarajan, M.; Rajasekaran, B.; Benjakul, S.; Venkatachalam, K. Influence of chitosan-gelatin edible coating incorporated with longkong pericarp extract on refrigerated black tiger Shrimp (Penaeus monodon). Curr. Res. Food Sci. 2021, 4, 345–353. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Harnkarnsujarit, N. Characterisations of cassava starch and poly(butylene adipate-co-terephthalate) blown film with silicon dioxide nanocomposites. Int. J. Food Sci. Technol. 2022, 57, 5078–5089. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- Busi, S.; Rajkumari, J. Chapter 15—Microbially synthesized nanoparticles as next generation antimicrobials: Scope and applications. In Nanoparticles in Pharmacotherapy; Grumezescu, M.A., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 485–524. [Google Scholar]
- Jing, D.; Wen, L.; Xinghe, T.; Xihai, H. Active Packaging Film Based on Essential Oil/Beta-Cyclodextrin Inclusion Compound and Preparation Method for Active Packaging Film. China Patent No. CN102585412, 18 July 2012. [Google Scholar]
- Roberta, O.; Gianni, S.; Veronica, S.; Sauro, V. Material for Packaging Fresh Food of Animal Origin Inhibiting the Development of Biogenic Amines. Europe Patent No. EP2788185, 15 October 2014. [Google Scholar]
- Obaiah, M.C.; Nagarajarao, R.S.C.; Krishnaswamy, S.G.T. Dual Action Active Packaging System Combining Carbon Dioxide Emitter and Oxygen Scavenger for Extending the Shelf Life of Refrigerated Fish Products. India Patent No. IN166/CHE/2010, 25 May 2012. [Google Scholar]
- Karlheinz, H.; Francesca, M. Active Amine Scavenging Film for Fresh Fish Packaging. Europe Patent No. EP1309400, 14 May 2003. [Google Scholar]
- Summers, L. Intelligent Packaging; Centre for Exploitation of Science and Technology: London, UK, 1992. [Google Scholar]
- Day, B.P.F. Active Packaging of Food. In Smart Packaging Technologies for Fast Moving Consumer Goods; Kerry, J., Butler, P., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; Van Boekel, M.A.J.S. (Tiny). Monitoring the quality of perishable foods: Opportunities for intelligent packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhang, H.; Dong, M.; Li, L.; Zhangsun, H.; Wang, L. Dual-functional intelligent gelatin based packaging film for maintaining and monitoring the shrimp freshness. Food Hydrocoll. 2022, 124, 107258. [Google Scholar] [CrossRef]
- Liu, H.; Shi, C.; Sun, X.; Zhang, J.; Ji, Z. Intelligent colorimetric indicator film based on bacterial cellulose and pelargonidin dye to indicate the freshness of tilapia fillets. Food Packag. Shelf Life 2021, 29, 100712. [Google Scholar] [CrossRef]
- Shi, C.; Ji, Z.; Zhang, J.; Jia, Z.; Yang, X. Preparation and characterization of intelligent packaging film for visual inspection of tilapia fillets freshness using cyanidin and bacterial cellulose. Int. J. Biol. Macromol. 2022, 205, 357–365. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, Z.; Zhu, L.; Gao, X.; Zhong, K.; Sun, X.; Li, X.; Li, J. On-package ratiometric fluorescent sensing label based on AIE polymers for real-time and visual detection of fish freshness. Food Chem. 2022, 390, 133153. [Google Scholar] [CrossRef]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- Teymouri, Z.; Shekarchizadeh, H. A colorimetric indicator based on copper nanoparticles for volatile sulfur compounds to monitor fish spoilage in intelligent packaging. Food Packag. Shelf Life 2022, 33, 100884. [Google Scholar] [CrossRef]
- Yabo, F.; Le, L.; Jiazi, S.; Cunxia, H.; Jingrui, L.; Liu, H. Ammonia-Sensitive Flexible Intelligent Package for Detecting Freshness of Fish Meat. China Patent No. CN113960123, 21 January 2022. [Google Scholar]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-based pH-sensitive smart packaging films for monitoring food freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Singh, S. Fabrication of intelligent pH-sensing films with antioxidant potential for monitoring shrimp freshness via the fortification of chitosan matrix with broken Riceberry phenolic extract. Food Chem. 2022, 366, 130574. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control. 2022, 131, 108441. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Lee, M.; Kim, M.; Inthamat, P.; Siripatrawan, U.; Lee, Y.S. Hydroxypropyl methylcellulose/microcrystalline cellulose biocomposite film incorporated with butterfly pea anthocyanin as a sustainable pH-responsive indicator for intelligent food-packaging applications. Food Biosci. 2021, 44, 101392. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Zou, X.; Arslan, M.; Shi, J.; Zhai, X.; Xiao, J.; Wang, X.; Huang, X.; Li, Z.; et al. A high-stable and sensitive colorimetric nanofiber sensor based on PCL incorporating anthocyanins for shrimp freshness. Food Chem. 2022, 377, 131909. [Google Scholar] [CrossRef]
- Ghorbani, M.; Divsalar, E.; Molaei, R.; Ezati, P.; Moradi, M.; Tajik, H.; Abbaszadeh, M. A halochromic indicator based on polylactic acid and anthocyanins for visual freshness monitoring of minced meat, chicken fillet, shrimp, and fish roe. Innov. Food Sci. Emerg. Technol. 2021, 74, 102864. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, J.; Jiang, L.; Zhu, Z.; He, S.; He, S.; Shao, W. Development of intelligent/active food packaging film based on TEMPO-oxidized bacterial cellulose containing thymol and anthocyanin-rich purple potato extract for shelf life extension of shrimp. Food Packag. Shelf Life 2021, 29, 100709. [Google Scholar] [CrossRef]
- Liu, D.; Dang, S.; Zhang, L.; Munsop, K.; Li, X. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, Y.; Han, H.; Geng, H.; Liao, Y.; Han, T. A dual-channel indicator of fish spoilage based on a D-π-A luminogen serving as a smart label for intelligent food packaging. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2022, 266, 120433. [Google Scholar] [CrossRef] [PubMed]
- Embleni, A. Modified atmosphere packaging and other active packaging systems for food, beverages and other fast-moving consumer goods. In Trends in Packaging of Food, Beverages and Other Fast-Moving Consumer Goods (FMCG); Woodhead Publishing: Sawston, UK, 2013; pp. 22–34. [Google Scholar]
- Martinez-Alvarez, O.; Montero, P.; Gomez-Guillen, M.d.C. Controlled atmosphere as coadjuvant to chilled storage for prevention of melanosis in shrimps (Parapenaeus longirostris). Eur. Food Res. Technol. 2004, 220, 125–130. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Hozzein, W.N.; Benjakul, S. Effect of pulsed electric field and modified atmospheric packaging on melanosis and quality of refrigerated Pacific white shrimp treated with leaf extract of Chamuang (Garcinia cowa Roxb.). Food Packag. Shelf Life 2020, 25, 100544. [Google Scholar] [CrossRef]
- Abel, N.; Rotabakk, B.T.; Lerfall, J. Effect of salt on CO2 solubility in salmon (Salmo salar L.) stored in modified atmosphere. J. Food Eng. 2020, 278, 109946. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Yang, X.; Liu, X.; Hong, H.; Luo, Y. Effects of oregano essential oil and nisin on the shelf life of modified atmosphere packed grass carp (Ctenopharyngodon idellus). LWT 2021, 147, 111609. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Yan, Y.; Fang, Z.; Nisar, T.; Sun, L.; Guo, Y.; Xia, N.; Wang, H.; Chen, D.-W. Application of nitric oxide in modified atmosphere packaging of tilapia (Oreschromis niloticus) fillets. Food Control. 2019, 98, 209–215. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Lira Santos, T.C. Improving quality and shelf-life of whole chilled Pacific white shrimp (Litopenaeus vannamei) by ozone technology combined with modified atmosphere packaging. LWT 2019, 99, 568–575. [Google Scholar] [CrossRef]
- Sezer, Y.Ç.; Bulut, M.; Boran, G.; Alwazeer, D. The effects of hydrogen incorporation in modified atmosphere packaging on the formation of biogenic amines in cold stored rainbow trout and horse mackerel. J. Food Compos. Anal. 2022, 112, 104688. [Google Scholar] [CrossRef]
- Jing, X.; Peiyun, L.; Jinfeng, W.; Jun, M. Method for Keeping Puffer Fish Fresh through Combination of Combined Coating and Modified Atmosphere Packaging. China Patent No. CN111066874, 28 April 2020. [Google Scholar]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Modelling approaches for designing and evaluating the performance of modified atmosphere packaging (MAP) systems for fresh produce: A review. Food Packag. Shelf Life 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Chan, S.S.; Skare, M.; Rotabakk, B.T.; Sivertsvik, M.; Lerfall, J.; Løvdal, T.; Roth, B. Evaluation of physical and instrumentally determined sensory attributes of Atlantic salmon portions packaged in modified atmosphere and vacuum skin. LWT 2021, 146, 111404. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Cold plasma combined with liposomal ethanolic coconut husk extract: A potential hurdle technology for shelf-life extension of Asian sea bass slices packaged under modified atmosphere. Innov. Food Sci. Emerg. Technol. 2020, 65, 102448. [Google Scholar] [CrossRef]
- Sørensen, J.S.; Boknaes, N.; Mejlholm, O.; Dalgaard, P. Superchilling in combination with modified atmosphere packaging resulted in long shelf-life and limited microbial growth in Atlantic cod (Gadus morhua L.) from capture-based-aquaculture in Greenland. Food Microbiol. 2020, 88, 103405. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S.; Zhang, B.; Deng, S.; Mittal, A. Effect of squid pen chitooligosaccharide in conjugation with different modified atmospheric packaging conditions on color and storage stability of tuna slices. Food Control. 2021, 125, 108013. [Google Scholar] [CrossRef]
- Lerfall, J.; Shumilina, E.; Jakobsen, A.N. The significance of Shewanella sp. strain HSO12, Photobacterium phosphoreum strain HS254 and packaging gas composition in quality deterioration of fresh saithe fillets. LWT 2022, 154, 112636. [Google Scholar] [CrossRef]
- Tagrida, M.; Benjakul, S. Liposomes loaded with betel leaf (Piper betle L.) extract: Antibacterial activity and preservative effect in combination with hurdle technologies on tilapia slices. Food Control. 2022, 138, 108999. [Google Scholar] [CrossRef]
- Dieckmann, E.; Nagy, B.; Yiakoumetti, K.; Sheldrick, L.; Cheeseman, C. Thermal insulation packaging for cold-chain deliveries made from feathers. Food Packag. Shelf Life 2019, 21, 100360. [Google Scholar] [CrossRef]
- Sormin, R.B.D.; Pattipeilohy, F.; Koritelu, N. The Effect of Cool Box Insulator Type on the Temperature Characteristics and Quality of Decapterus Russelly (Rüppell, 1830) During Chilling Preservation. Aquat. Procedia 2016, 7, 195–200. [Google Scholar] [CrossRef]
- Ariany, Z.; Said, S.D.; Hartono. The Development of Thermal Insulation System on Fish Cargo Hold by Utilizing Paper Waste (Cellulose) for Improving Catch Quality. Adv. Sci. Lett. 2018, 24, 9886–9889. [Google Scholar] [CrossRef]
- Navaranjan, N.; Fletcher, G.C.; Summers, G.; Parr, R.; Anderson, R. Thermal insulation requirements and new cardboard packaging for chilled seafood exports. J. Food Eng. 2013, 119, 395–403. [Google Scholar] [CrossRef]
- Haiying, W.; Qian, W. Circular Fish Tank Buffering Packaging Box Formed by One Piece of Paper. China Patent No. CN209720191, 3 December 2019. [Google Scholar]
- Xiaogu, Z.; Huahao, Z.; Lie, C.; Yuanxing, D.; Yixhi, L.; Huiquan, X.; Zhou, C. Packaging Box for Refrigerating Fresh Fish Meat. China Patent CN215246117, 21 December 2021. [Google Scholar]
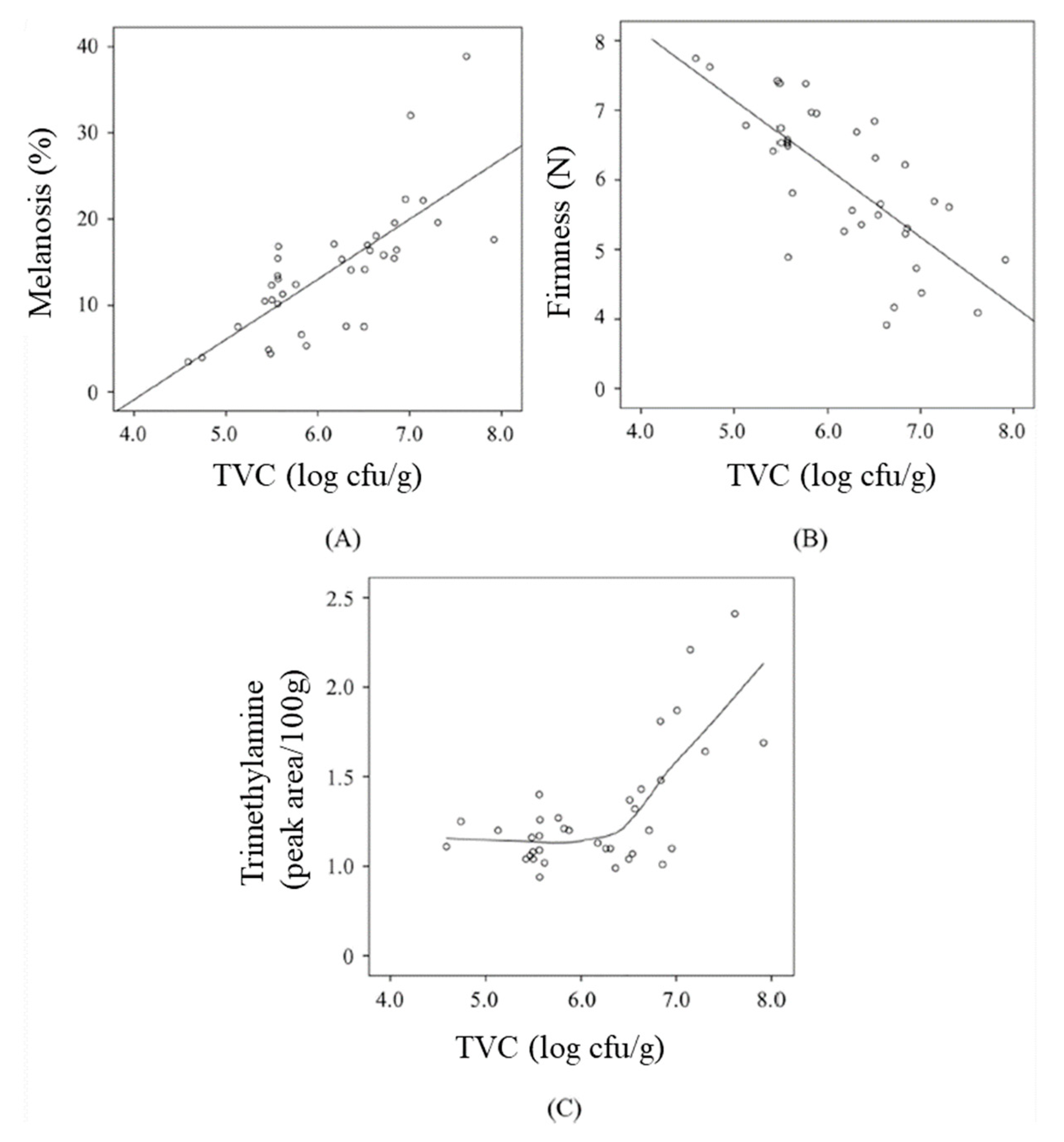
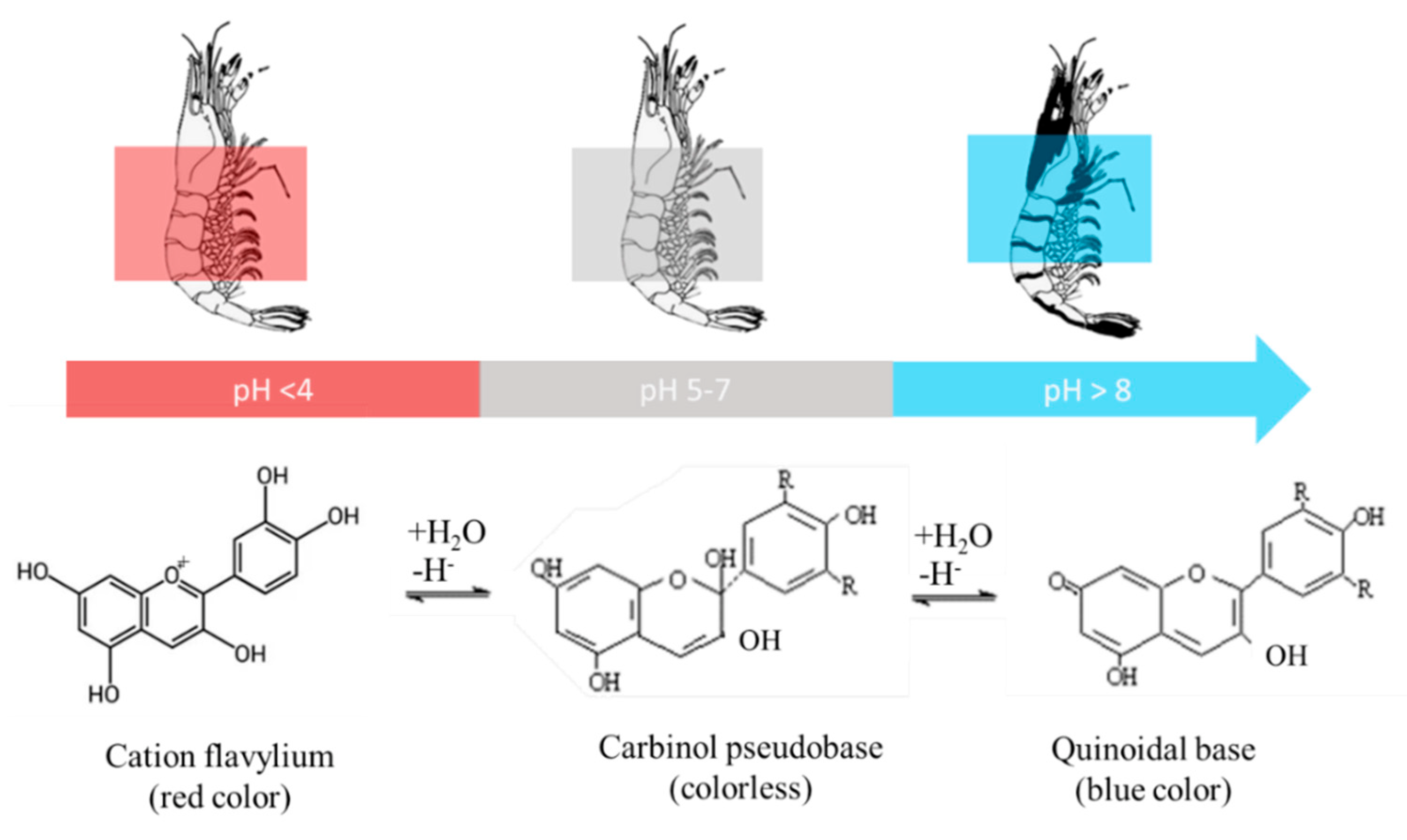
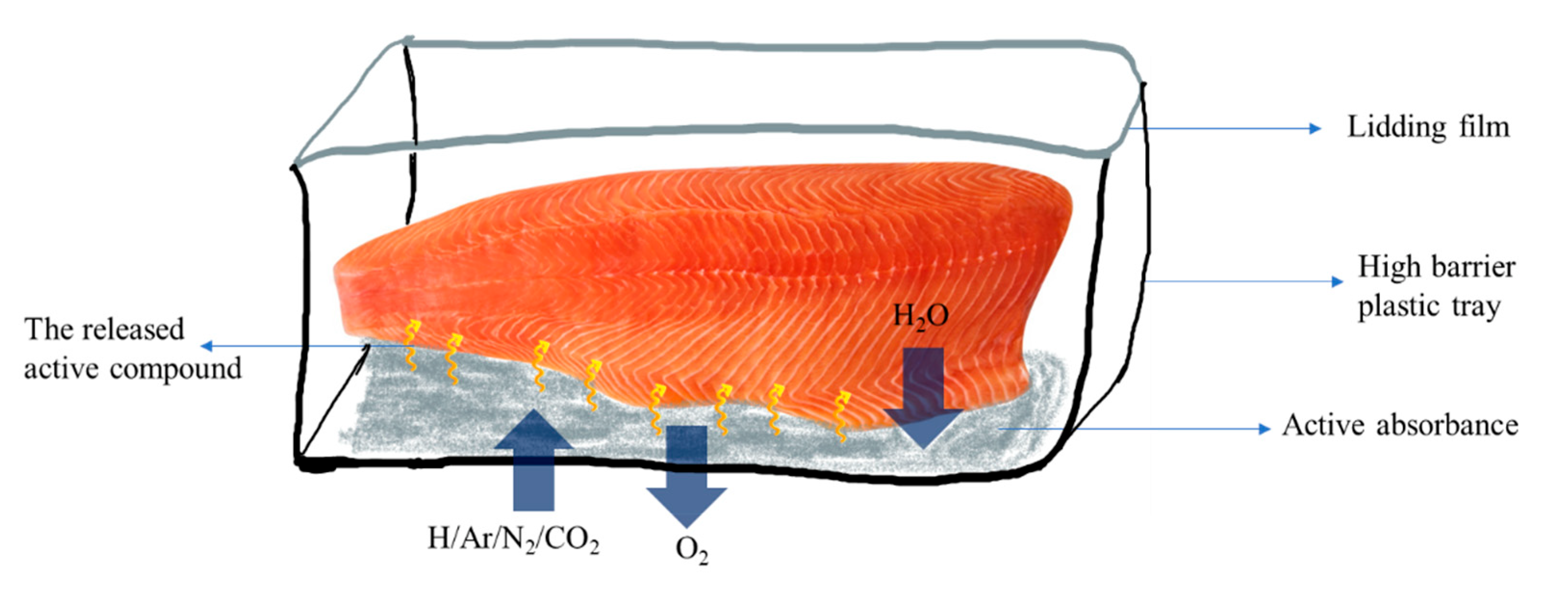
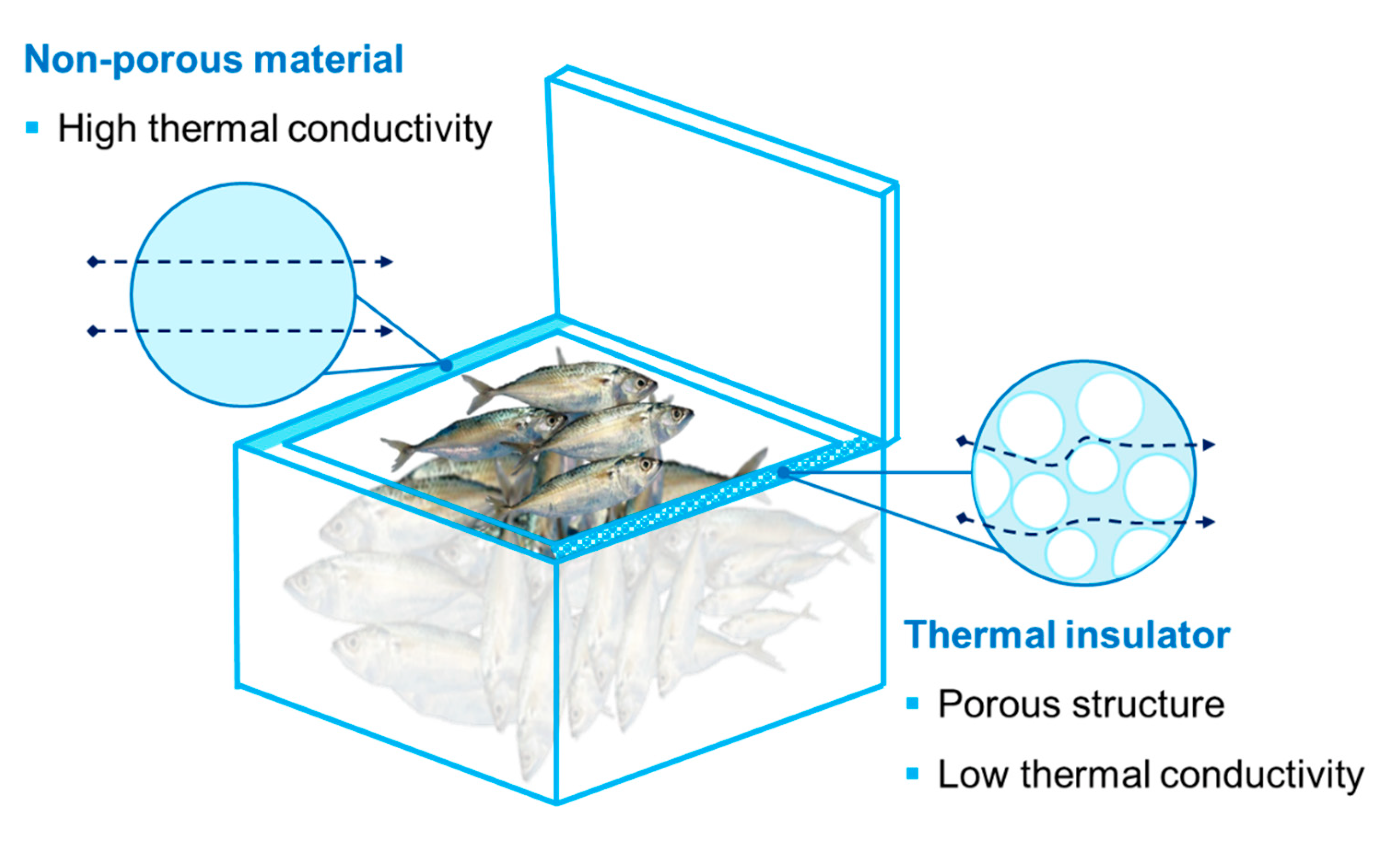
| Active Agent | Polymer Materials | Seafood Product | Packaging Properties | Seafood Qualities | Source |
|---|---|---|---|---|---|
| Nanoparticles SiO, ZnO, CuO, SiO-ZnO, Si-CuO and ZnO-CuO. | Gelatin/ polyvinyl alcohol | Shrimp (Penaeus vannamei) | Nanoparticles exhibited antimicrobial properties against S. aureus, L. monocytogenes, E. coli, P. fluorescens, Vibrio and Aeromonas, with inhibition zone 20.1–22.1 mm and 7.2–12 mm for SiO-ZnO and CuO, respectively. | SiO-ZnO was more effective than CuO in controlling TVB-N, Shewanella putrefaciens, Enterobacteriaceae, and Pseudomonas spp. of shrimp. | [24] |
| ZnO, TiO2, or ZnO/TiO2 | Gelatin/ polyvinyl alcohol | White shrimp | ZnO + TiO2 was more effective against Gram-negative bacteria than Gram-positive bacteria with the inhibition zone of 8.11 to 12.63 mm. | Active film improved shrimp shelf-life (12 days) compared to the control (6 days). | [68] |
| Sardinella protein isolate (SPI) | Chitosan | Shrimp | Structural and thermal properties of chitosan were improved with SPI incorporation, but it exhibited lower mechanical properties. | Shrimp packed in SPI film had psychotropic and mesophilic bacteria of 0.49 and 0.44 log CFU/g, respectively, lower than control (2.21 and 5.79 log CFU/g) on day 9. | [69] |
| Potato peel phenolic | Starch | Smoked sea bream fillet | Films showed a yellowish color and improvement in water tolerance, elasticity, and antioxidant activity (56–85% of ABTS inhibition). | Fillets packed in active film had a pleasant smell and flavor, an increase in golden color, and higher stiffness than fillets packed in control film. | [67] |
| Chitosan or lysozyme | PLA | Grass carp fillet | The film provides an antimicrobial agent to form an amine bond to inhibit E. coli and S. aureus. | Active film prolonged the fillet up to 3 days. PLA/chitosan was more effective in inhibiting bacterial growth than PLA/lysozyme. | [70] |
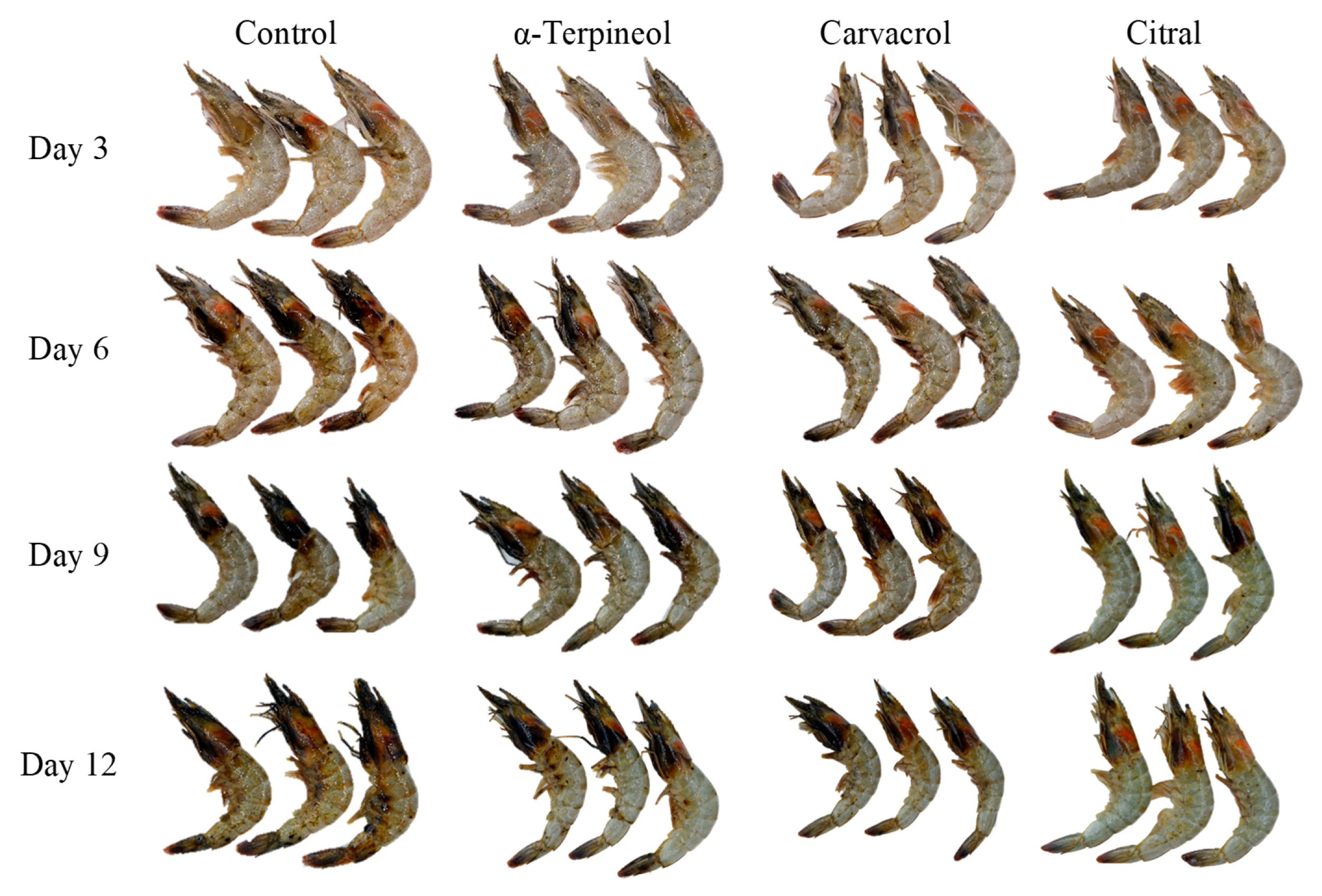
| Essential oil (Carvacrol, citral and α-terpineol) | PLA/PBAT | Pacific white shrimp | Essential oil modified barrier properties and microstructure affected by polymer-essential oil interacted via hydrogen bonding and carbonyl groups. | Shrimp deterioration was prevented by active film. Citral and carvacrol more effectively stabilized protein conformation in muscle tissues, retained drip loss and adhesion between the cephalothorax and abdomen. | [37] |
| Green tea ground waste | Potato starch, gelatin, carboxymethyl cellulose (CMC) | Salmon | The film had high water vapor permeability (WVP) but limited germination due to a low pH. The DPPH radical scavenging of the tray containing tea waste was 80.75%. | The active tray + film provided potential inhibition against biogenic amine accumulation, 19% lower spoilage bacteria of salmon than the control after 6 days of storage. | [71] |
| Carob (Ceratonia siliqua L.) seed macerate | Cellulose and water-based biodegradable adhesive | Atlantic salmon fillets | Salmon packaged in active film had a lower pH, drip-loss, TBARS and TVB-N up to 5 days of storage compared to the control. | [72] | |
| Solanum betaceum (chilto) seed and peel extract | Pectin enriched extract | Atlantic salmon fillets | The active agent reduced mechanical properties and WVP. | Pectin containing phenolic extract showed better salmon lipid and protein oxidation protection than anthocyanin during 10 days of storage. | [73] |
| Maqui berry extract (MBE) | Cowpea starch | Salmon | MBE decreased rigidity, increased flexibility, UV-light blocking and antioxidant properties of cowpea starch film. | Cowpea starch film with 20% MBE retarded lipid oxidation of salmon. | [74] |
| Cinnamon leaf essential oil (CLE) | Bombacaceae gum | Salmon | CLE decreased tensile strength and WVP, while 1.25% CLE increased radical scavenging 1.8 times compared to the control. | Active film retarded lipid oxidation, malonaldehyde and hydroperoxide generation in salmon. | [75] |
| Nostoc commune Vauch polysaccharides (NVP) | sodium CMC | Salmon | Ratio NVP:CMC at 1:3 showed the strongest hydrogen bond and denser structure. | Salmon coated with NVP + CMC had a lower pH value, lipid and protein oxidation, preserved color and texture during 8 days of chilling storage. | [76] |
| Lysozyme (LYS) and green tea extract (GTE) | Gelatine/rice starch g-pads | Smoked salmon | Gelatin/rice starch-based g-pads mechanically improved after LYS and GTE addition, showing 1.8 and 1.7 log Listeria innocua reduction, respectively. | Salmon packaged with g-pads containing LYS and GTE had 1.5–1.9 log lower Listeria load than the control. | [77] |
| Cinnamaldehyde (CIN) | CMC or collagen (COL) | Tilapia | Collagen/6% CIN delayed TPC and Vibrio parahaemolyticus by up to 1.82 log cfu/g than control at day 14 of storage, with lower TVB-N and TBARS. Shelf-life of tilapia was 3 days longer than the control. | [78] | |
| Cinnamaldehyde | Corn starch/polyvinyl alcohol | Large yellow croaker | Fish packaged in active film exhibited lower myofibril secondary and tertiary oxidation, water loss, water migration, and lipid oxidation. | [79] | |
| Cyclic adenosine monophosphate (cAMP) | Red seaweed polysaccharide | Large yellow croaker | Barrier properties, surface wettability, mechanical strength, and antimicrobial activity against Gram positive and negative bacteria were promoted. | Shelf-life of large yellow croaker packaged in active film was 2 days longer than control, with lower microbial growth and TVB-N. | [80] |
| Colorimetric Indicator | Packaging Material | Seafood Product | Function of Detection | Detection Response | Source |
|---|---|---|---|---|---|
| Riceberry phenolic extract | Chitosan | Shrimp | pH-sensitive, ammonia detector | Color changed from orange-red to yellow as shrimp spoilage response. | [107] |
| Betacyanin from paper flower | Potato starch | Caspian sprat | pH-sensitive, ammonia detector | Color changed from light pink to yellow in response to pH between 2–13 and ammonia 0.01–0.1 mg ammonia/mL water. | [108] |
| Betalains Amaranthus leaf extract | Polyvinyl alcohol and gelatin | Fish or chicken | pH-sensitive, antimicrobial activity | Color changed from red to yellow, corroborated by increased pH, TVB-N and microbial growth of meat. | [109] |
| Black currant anthocyanin | Konjac glucomannan and methyl cellulose | Tilapia fish | pH-sensitive | Color changed from pink-purple to yellow in response to tilapia spoilage. | [102] |
| Blueberry anthocyanin | Potato starch and chondroitin sulfate | shrimp | pH-sensitive | Color changed from originally pink to light grey and finally to grayish-green correlated to TVB-N, pH values and microbial profile in shrimp. | [110] |
| Butterfly pea (Clitoria ternatea) anthocyanin | Hydroxypropyl methylcellulose/microcrystalline cellulose | Mackerel (Scomber scombrus) | NH3-sensitive | Deep or light purple (fresh mackerel), violet color (mackerel suitable to eat), green to blue ocean or colonial blue (spoilage mackerel). | [111] |
| Clitoria ternatea anthocyanin | Polycaprolactone | Shrimp | pH-sensitive and ammonia detector | Color change from pale-blue to yellow-green in response to shrimp spoilage. | [112] |
| Malva sylvestris anthocyanins | PLA, polyethylene glycol (PEG), and calcium bentonite (CB) | Shrimp, fish roe, meat and chicken fillet | pH-sensitive | Color change from light red (pH 2) to green (pH 11) and more sensitive to shrimp and fish roe rather than chicken and meat correlated to TVB-N value. | [113] |
| Anthocyanin-rich purple potato extract | 2,2 6,6-tetramethylpiperidine-1-oxyradical, oxidized bacterial cellulose and thymol | shrimp | Volatile ammonia detector | Color changed to dark purple in response to shrimp spoilage after 32 h. | [114] |
| Curcumin nano capsules | Soy protein isolate and cellulose nanocrystals | shrimp | pH-sensitive, ammonia detector, anti-radical scavenging | The yellow (pH 3–7) color becomes reddish-brown (pH 8–11) in response to TVB-N changes in shrimp during storage. | [103] |
| Curcumin | Corn starch, polyvinyl alcohol | Pangasius bocourti (catfish) | pH-sensitive | Color changed from yellow to orange in the range acidic (pH 3) to neutral (pH 7), and turned to red at pH 8–10 in response to TVB-N changes. | [115] |
| Copper nanoparticles | Salmon trout | Volatile sulfur compound | The color of white, yellow and brown as a colorimetric indicator related to fresh, semi-fresh, and spoiled salmon, respectively. | [104] | |
| Alizarin | Gelatin and lavender essential oil | shrimp | pH-sensitive and ammonia detector, antimicrobial activity | The color changed from yellow to red-brown in response to increasing TVB-N in shrimp after 3 days of storage. | [98] |
| Donor-π-acceptor (D-π-A) | Cellulose | Fish | Amine detector | The color changed from red to yellow in response to putrid fish, while the emission changed to bright cyan. | [116] |
| Pelargonidin | Bacterial cellulose | Tilapia fillet | pH-sensitive | The color change from red to colorless in response to the TVB-N value and sensory changes of tilapia fillets. | [99] |
| Cyanicin-3-glucoside | Bacterial cellulose | Tilapia fillets | pH-sensitive | Color changed from red to green in pH range 3–10. During application, rose-red fresh tilapia turned to purple (acceptable) and lavender (spoilage). | [100] |
| Rhodamine B | AIE-stimuli-responsive polymer tetraphenylethylene (TPA) and polymethacrylic acid (PMA) | Salmon | pH-sensitive | Color change from pink (fresh) to dark blue (spoilage) was linearly correlated with TVB-N, indicating that the sensing label was feasible and non-destructive for quantitative TVB-N. | [101] |
| Gas Composition | Supplementary Material | Seafood Product | Outcome | Source |
|---|---|---|---|---|
| Argon (Ar), nitrogen (N2) and carbon dioxide (CO2) | 1% chamuang leaf extract (CLE) | Pacific white shrimp | Pulse electric field-CLE-CO2 treated shrimp showed the lowest pH value, carbonyl content, TVB-N, peroxide value and TBARS, and melanosis. | [119] |
| CO2:O2:N2 ratio 1) 80:5:15, 2) 60:5:35, 3) 40:5:55, 4) 20:5:75, 5) 80:15:5, 6) 60:12:25, 7) 40:15:45, 8) 20:15:65 | - | Pacific white shrimp | Increased CO2 to 60–80% effectively reduced microbial growth, melanosis, and lipid oxidation. O2 decreased with increasing CO2 during storage due to microbial growth. O2 and CO2 decreased due to consumption by microbials and dissolved in shrimp meat, respectively. | [10] |
| 75% CO2 and 25% N2 | Oregano 0.1%, nisin 0.2%, oregano 0.1% + nisin 0.2% | Grass carp (Ctenopharyngodon idellus) | Nisin and oregano EO showed synergistic antimicrobial effects, which extended the shelf-life of fish fillets up to 28 days, with lower microbial growth and tyramine levels and increased pH values. | [121] |
| 60% CO2/40% N2 | Skin vacuum packaging: Skintite HB 125 alu/pet (PE/EVOH combination) | Atlantic salmon portions | MAP showed comparable results with skin packaging in terms of drip loss, water holding capacity, texture, and microbial count. Salmon packaged in MAP and skin packaging extended microbial shelf-life by 1.5 times compared to the control. | [127] |
| 60% CO2/5% O2/ 35% N2 | ε-polylysine (0.1%, 0.2%, 0.3%) | Pufferfish (Takifugu obscurus) | ε-polylysine, chitosan and sodium alginate coatings and MAP delayed myofibril oxidation, preserved Ca2+-ATPase activity, α-helix and β-sheet contents, and stabilized tertiary structure during cold storage. | [27] |
| MAP1: 50% CO2/50% N2 MAP2: 60% CO2/40% N2 RAP1: 50% CO2/46% N2/4% H2A RAP2: 60% CO2/36% N2/4% H2 | - | Rainbow trout and horse mackerel | Reduction rates of biogenic amine in fish packaged following the reducing atmosphere packaging (RAP) technique were 2 times higher than in MAP, indicating the efficiency of hydrogen incorporation to prevent biogenic amine formation. | [124] |
| 60% CO2/40% N2 | Non-carbonated 10% NaCl-brine Carbonated water Carbonated 10% NaCl-brine | Salmon (Salmo salar L.) | Increasing NaCl concentration reduced CO2 solubility in salmon according to Henry’s constant and CO2 absorption within the salmon. | [120] |
| 60% CO2/30% Ar/10% O2 (cold plasma/CP) | Ethanolic coconut husk extract (ECHE) Liposomal encapsulated ECHE (LE-ECHE) | Asian sea bass (Lates calcalifer) | Cold plasma-treated fish enriched with ECHE and LE-ECHE were 1 log cfu/g lower than control. ECHE and LE-ECHE treated fish exhibited lower protein and lipid oxidation compared to CP only. | [128] |
| 40% CO2/60% N2 | Superchilling condition | Atlantic cod (Gadus morhua L.) | A 1.7 °C superchilling temperature with MAP 35% CO2 condition effectively extended the shelf-life of Atlantic cod by up to 32 days. | [129] |
| 80% Ar/20% O2 | Chitooligosaccharide (COS) from squid pen | Yellowfin tuna | COS 400 ppm combined with MAP preserved the redness of tuna with lowest metmyoglobin content during storage, oxygen-based MAP showed highest lipid oxidation. | [130] |
| 67% CO2/ 33% O2 or N2 | - | Saithe fillets | Combination CO2/O2 with inoculated P. phosphoreum and Shewanella sp. exhibited the highest H-value, hypoxanthine, and TMA level of saithe fillets. | [131] |
| 60% CO2/30%Ar/10% O2 | Betel (Piper betle L.) leaf ethanolic extract (BLEE) Liposome loaded BLEE (L/BLEE) | Tilapia slices | L/BLEE at 400 ppm/MAP/non-thermal plasma treatment effectively extended the shelf-life of tilapia slices up to 12 days, related to deformation and perforation of the cell wall of bacteria observed via SEM. | [132] |
| Patent Title | Invention Details | Application | Patent Source |
|---|---|---|---|
| Containing bag for fresh fish | A bag contained waterproof material at the outer most portion, equipped with outer heat insulating material made from glass wool and nonwoven fabric. The cooling system, prepared from nitrogen gas sealed in the hollow layer reached cooling temperature of −1 °C to −5 °C. The mouth of the bag was equipped with fastener material (Velcro fastener or waterproof fastener). | Transportation and storage bag for high-economical commodity, particularly tuna and camellia in cold insulation state. | JP1995243741 [45] |
| Portable cool box | The component of the cooling box was hollow with a rectangular parallelepiped case body, a lid, cold insulator, and support mechanism. The insulator material contained highly water-absorbing polymer sodium polyacrylate sealed in a hollow plastic case to maintain high water absorbency by forming a gel structure. | Portable cool box for retaining the freshness of fish or other objects which can be easily used in fishing or camping. | JP2013085550 [46] |
| Intermediate dish of foamed fish box | The overlapped box containing two outer boxes and an upper layer made from cold resistant olefin sheet-based raw material. The depth of the main container was adjusted by calculating the amount of ice required. An edge over the upper layer created an overlapped insulator box. | Container for fresh fish at processing site or for transportation. | JP2018135150 [47] |
| Cooling packing box | A Styrofoam-based insulator box equipped with an intermediate lid attached to the upper end of the main body. The intermediate lid had storage space for frozen food products in the middle, with holes to allow cold air to pass through the storage space in the intermediate lid. | Non-direct contact cooling system using cold air for fresh products. | KR1020180112388 [48] |
| Corrugated carton suitable for transportation and packaging of fish tank | A corrugated box for transporting fish tanks equipped with an inner and outer groove on the foam base. The inner groove is equipped with a rubber sleeve and the fish tank is placed on the outside of the rubber sleeve on the outer groove. Between the fish tank and rubber sleeve, rubber supporting feet are attached with a soft rubber cushion, EPE pads protect the fish tank and are reusable. | Corrugated paper box for fish tank transportation and packaging. | CN209427201 [49] |
| Circular fish tank buffering packaging box formed by one piece of paper | A fish box formed by one piece of paper packaging as the main body, with linings and a handle, comprising a bottom plate, four end plates, a top plate, four lining plates and a bottom plate. The handle comprises two triangular top plates. Prevents fish transportation damage with reduced amounts of foam, reduced production cost, and material saving. | Paper box for fish transportation to minimize damage. | CN209720191 [137] |
| Packaging box for refrigerating fresh fish meat | A box with an inside structure, including an inflatable bag with an inflatable interface on the top, foam plastic board, absorbent paper made from wood pulp layer and a non-woven layer on both sides, and a wave-shaped convex strip in the inner wall. A sealing gasket is attached between the box cover and box body, with a support pad placed in the bottom of the box. | Packaging box for refrigerating fresh fish meat. | CN215246117 [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laorenza, Y.; Chonhenchob, V.; Bumbudsanpharoke, N.; Jittanit, W.; Sae-tan, S.; Rachtanapun, C.; Chanput, W.P.; Charoensiddhi, S.; Srisa, A.; Promhuad, K.; et al. Polymeric Packaging Applications for Seafood Products: Packaging-Deterioration Relevance, Technology and Trends. Polymers 2022, 14, 3706. https://doi.org/10.3390/polym14183706
Laorenza Y, Chonhenchob V, Bumbudsanpharoke N, Jittanit W, Sae-tan S, Rachtanapun C, Chanput WP, Charoensiddhi S, Srisa A, Promhuad K, et al. Polymeric Packaging Applications for Seafood Products: Packaging-Deterioration Relevance, Technology and Trends. Polymers. 2022; 14(18):3706. https://doi.org/10.3390/polym14183706
Chicago/Turabian StyleLaorenza, Yeyen, Vanee Chonhenchob, Nattinee Bumbudsanpharoke, Weerachet Jittanit, Sudathip Sae-tan, Chitsiri Rachtanapun, Wasaporn Pretescille Chanput, Suvimol Charoensiddhi, Atcharawan Srisa, Khwanchat Promhuad, and et al. 2022. "Polymeric Packaging Applications for Seafood Products: Packaging-Deterioration Relevance, Technology and Trends" Polymers 14, no. 18: 3706. https://doi.org/10.3390/polym14183706
APA StyleLaorenza, Y., Chonhenchob, V., Bumbudsanpharoke, N., Jittanit, W., Sae-tan, S., Rachtanapun, C., Chanput, W. P., Charoensiddhi, S., Srisa, A., Promhuad, K., Wongphan, P., & Harnkarnsujarit, N. (2022). Polymeric Packaging Applications for Seafood Products: Packaging-Deterioration Relevance, Technology and Trends. Polymers, 14(18), 3706. https://doi.org/10.3390/polym14183706










