Synergistic Effect between Piperazine Pyrophosphate and Melamine Polyphosphate in Flame Retardant Coatings for Structural Steel
Abstract
:1. Introduction
2. Methods
2.1. Materials
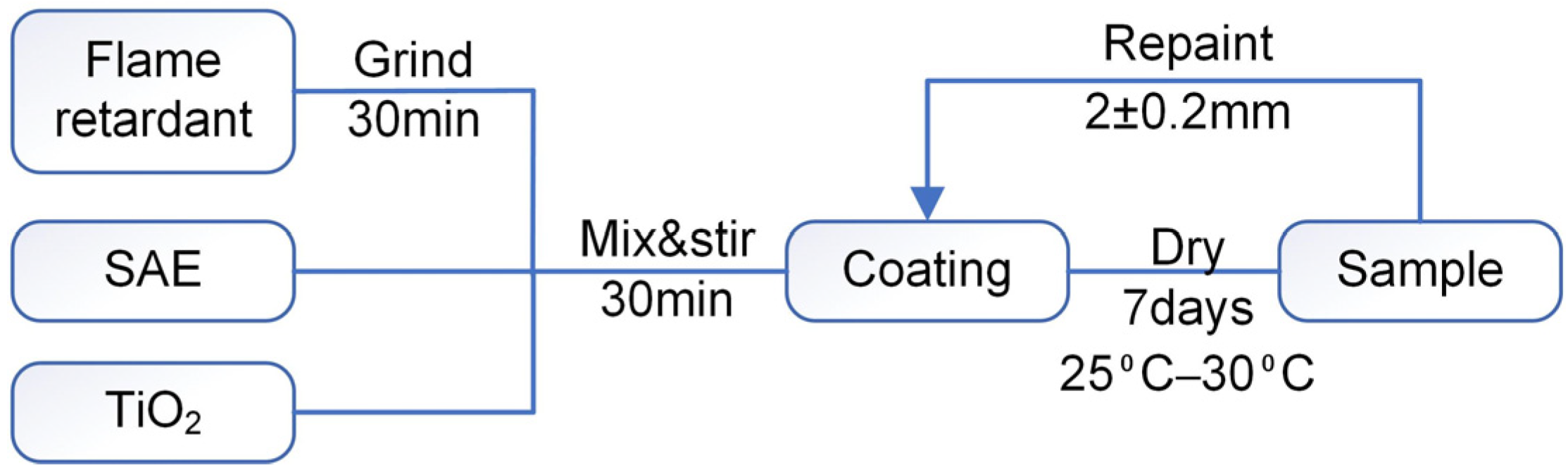
2.2. Sample Preparation
2.3. Fire Performance
2.4. Water Resistance
2.5. Thermal Stability
2.6. Contact Angle Analysis
2.7. Infrared Spectrum Analysis
2.8. Morphology Characterization
3. Results and Discussion
3.1. Fire Protection of the Coatings
3.2. Water Resistance of the Coatings
3.3. Thermal Analysis of the Coatings
3.4. Microstructure of the Intumescent Char Layers
3.5. Analysis of the Flame Retardant Mechanism
4. Conclusions
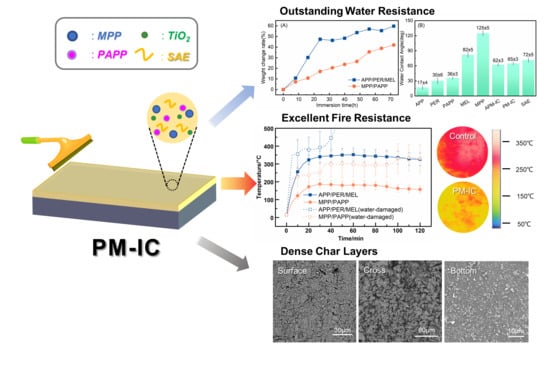
- MPP/PAPP-IC displayed perfect fire resistance and thermal properties. The equilibrium temperature in torch test was only 170 °C in 2 h, which was remarkably lower than APP/PER/MEL-IC and literature reports. The residue reached up to 33.8 wt% in TGA and the initial decomposition temperature was higher, indicating better thermal stability.
- The water-damaged MPP/PAPP-IC could still pass the fire resistance test. The ingredients of MPP/PAPP had better compatibility in SAE binders, and MPP and PAPP were more hydrophobic than APP, leading to the reduction of water absorption. Therefore, the water resistance of the coating was improved.
- MPP/PAPP-IC exhibited great charring capability through the reaction between phosphoric acid and piperazine groups. Reaction between (HPO3)n and TiO2 further enhanced the strength of the surface layer of the char. More uniform and denser char structure was generated, which inhibited the heat transmission, thus improved fire proof properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasir, M.; Ahmad, F.; Yusoff, P.S.M.M.; Ullah, S.; Jimenez, M. Latest trends for structural steel protection by using intumescent fire protective coatings: A review. Surf. Eng. 2019, 36, 334–363. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Anees, S.M.; Dasari, A. A review on the environmental durability of intumescent coatings for steels. J. Mater. Sci. 2017, 53, 124–145. [Google Scholar] [CrossRef]
- Huo, S.; Wang, C.; Hu, Q.; Liu, S.; Zhang, Q.; Liu, Z. A facile strategy to fabricate an intumescent fire-retardant coating with improved fire resistance and water tolerance for steel structure. J. Coat. Technol. Res. 2020, 17, 1401–1411. [Google Scholar] [CrossRef]
- Jimenez, M.; Bellayer, S.; Revel, B.; Duquesne, S.; Bourbigot, S. Comprehensive Study of the Influence of Different Aging Scenarios on the Fire Protective Behavior of an Epoxy Based Intumescent Coating. Ind. Eng. Chem. Res. 2013, 52, 729–743. [Google Scholar] [CrossRef]
- Zhong, S.; Li, J.; Cai, Y.; Yi, L. Novel surfactant-free waterborne acrylic-silicone modified alkyd hybrid resin coatings containing nano-silica for the corrosion protection of carbon steel. Polym. Technol. Mater. 2018, 58, 866–878. [Google Scholar] [CrossRef]
- Cao, K.; Wu, S.-L.; Wang, K.-L.; Yao, Z. Kinetic Study on Surface Modification of Ammonium Polyphosphate with Melamine. Ind. Eng. Chem. Res. 2011, 50, 8402–8406. [Google Scholar] [CrossRef]
- Sun, L.; Qu, Y.; Li, S. Co-microencapsulate of ammonium polyphosphate and pentaerythritol in intumescent flame-retardant coatings. J. Therm. Anal. 2012, 111, 1099–1106. [Google Scholar] [CrossRef]
- Xiao, G.; Yang, Z.; Chen, C.; Chen, C.; Zhong, F.; Wang, M.; Zou, R. Novel carbon nitride@polydopamine/molybdenum disulfide nanoflame retardant improves fire performance of composite coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127575. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, H.; Liu, Y.; Wang, Q. Synergistic effect between piperazine pyrophosphate and melamine polyphosphate in flame retarded glass fiber reinforced polypropylene. Polym. Degrad. Stab. 2020, 184, 109477. [Google Scholar] [CrossRef]
- Yang, R.; Ma, B.; Zhao, H.; Li, J. Preparation, Thermal Degradation, and Fire Behaviors of Intumescent Flame Retardant Polypropylene with a Charring Agent Containing Pentaerythritol and Triazine. Ind. Eng. Chem. Res. 2016, 55, 5298–5305. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G. The novel silicon-containing epoxy/PEPA phosphate flame retardant for transparent intumescent fire resistant coating. Appl. Surf. Sci. 2016, 385, 453–463. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xia, S.-Y.; Liu, C.; Li, B. Preparation of Poly(phosphoric acid piperazine) and Its Application as an Effective Flame Retardant for Epoxy Resin. Chin. J. Polym. Sci. 2018, 36, 655–664. [Google Scholar] [CrossRef]
- Lee, S.; Morgan, A.B.; Schiraldi, D.A.; Maia, J. Improving the flame retardancy of polypropylene foam with piperazine pyrophosphate via multilayering coextrusion of film/foam composites. J. Appl. Polym. Sci. 2019, 137, 48552. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, X.; Wang, J.; Guo, N. Fire, thermal and mechanical properties of TPE composites with systems containing piperazine pyrophosphate (PAPP), melamine phosphate (MPP) and titanium dioxide (TiO2). Plast. Rubber Compos. 2019, 48, 149–159. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Y.; Qian, L.; Chen, Y.; Qiu, Y.; Xu, B.; Xin, F. Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66. Polymers 2019, 11, 1851. [Google Scholar] [CrossRef]
- Liang, C.; Du, Y.; Wang, Y.; Ma, A.; Huang, S.; Ma, Z. Intumescent fire-retardant coatings for ancient wooden architectures with ideal electromagnetic interference shielding. Adv. Compos. Hybrid Mater. 2021, 4, 979–988. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, G.; Yang, J. Influences of silicone emulsion on fire protection of waterborne intumescent fire-resistive coating. J. Coat. Technol. Res. 2013, 11, 231–237. [Google Scholar] [CrossRef]
- Mariappan, T.; Agarwal, A.; Ray, S. Influence of titanium dioxide on the thermal insulation of waterborne intumescent fire protective paints to structural steel. Prog. Org. Coat. 2017, 111, 67–74. [Google Scholar] [CrossRef]
- Yew, M.C.; Ramli Sulong, N.H.; Yew, M.K. Influences of flame-retardant fillers on fire protection and mechanical properties of intumescent coatings. Prog. Org. Coat. 2015, 78, 59–66. [Google Scholar] [CrossRef]
- Mustapa, S.; Sulong, N.R. Performance of Palm Oil Clinker as a Bio-Filler with Hybrid Fillers in Intumescent Fire Protective Coatings for Steel. Sains Malays. 2017, 46, 2489–2496. [Google Scholar]
- Nasir, K.M.; Sulong, N.R.; Johan, M.R.; Afifi, A.M. An investigation into waterborne intumescent coating with different fillers for steel application. Pigment Resin Technol. 2018, 47, 142–153. [Google Scholar] [CrossRef]
- Beh, J.H.; Yew, M.C.; Saw, L.H. Fire Resistance and Mechanical Properties of Intumescent Coating Using Novel BioAsh for Steel. Coatings 2020, 10, 1117. [Google Scholar] [CrossRef]
- Wang, C.; Huo, S.; Liu, S.; Hu, Q.; Zhang, Q.; Liu, Z. Recycle of magnesium alloy scrap for improving fire resistance, thermal stability, and water tolerance of intumescent fire-retardant coatings. J. Coat. Technol. Res. 2020, 18, 447–458. [Google Scholar] [CrossRef]
- Yew, M.C.; Ramli Sulong, N.H.; Yew, M.K. Investigation on solvent-borne intumescent flame-retardant coatings for steel. Mater. Res. Innov. 2014, 18 (Suppl. 6), S6-384–S6-388. [Google Scholar] [CrossRef]
- Xie, W.; Chen, H.; He, D.; Zhang, Y.; Fu, L.; Ouyang, J.; Yang, H. An emerging mineral-based composite flame retardant coating: Preparation and enhanced fireproof performance. Surf. Coat. Technol. 2019, 367, 118–126. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Shariff, A.M.; Bustam, M. Synergistic effects of kaolin clay on intumescent fire retardant coating composition for fire protection of structural steel substrate. Polym. Degrad. Stab. 2014, 110, 91–103. [Google Scholar] [CrossRef]
- Beheshti, A.; Heris, S.Z. Experimental investigation and characterization of an efficient nanopowder-based flame retardant coating for atmospheric-metallic substrates. Powder Technol. 2015, 269, 22–29. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Yusoff, P.S.M.M. Effect of boric acid and melamine on the intumescent fire-retardant coating composition for the fire protection of structural steel substrates. J. Appl. Polym. Sci. 2012, 128, 2983–2993. [Google Scholar] [CrossRef]
- Zhou, G.; Li, S.; Zhang, X.; Liu, Z.; He, M.; Chen, X.; Yang, W. Synthesis and properties of a fire-retardant coating based on intercalated expandable graphite-modified cellulose for steel structures. J. Build. Eng. 2022, 51, 104270. [Google Scholar] [CrossRef]
- Tang, B.; Feng, W.; Guo, J.; Sun, J.; Zhang, S.; Gu, X.; Li, H.; Yang, W. Hydrophobic modification of pentaerythritol and its application in fire-retardant coatings for steel structures. Prog. Org. Coat. 2019, 138, 105391. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Zhang, S.; Gu, X.; Wang, H.; Jiang, P.; Zhao, Q. Effects of titanium dioxide on the flammability and char formation of water-based coatings containing intumescent flame retardants. Prog. Org. Coat. 2014, 78, 318–324. [Google Scholar] [CrossRef]
- Yew, M.C.; Ramli Sulong, N.H.; Yew, M.K. Fire propagation performance of intumescent fire protective coatings using eggshells as a novel biofiller. Sci. World J. 2014, 2014, 805094. [Google Scholar] [CrossRef] [PubMed]
- Sut, A.; Metzsch-Zilligen, E.; Großhauser, M.; Pfaendner, R.; Schartel, B. Synergy between melamine cyanurate, melamine polyphosphate and aluminum diethylphosphinate in flame retarded thermoplastic polyurethane. Polym. Test. 2019, 74, 196–204. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J. Influences of glass flakes on fire protection and water resistance of waterborne intumescent fire resistive coating for steel structure. Prog. Org. Coat. 2011, 70, 150–156. [Google Scholar] [CrossRef]
- Hu, Z.; Zhong, Z.Q.; Gong, X.D. Flame retardancy, thermal properties, and combustion behaviors of intumescent flame-retardant polypropylene containing(poly)piperazine pyrophosphate and melamine polyphosphate. Polym. Adv. Technol. 2020, 31, 2701–2710. [Google Scholar] [CrossRef]





| Sample | IFR (wt%) |
|---|---|
| APP/PER/MEL | 15/6/9 |
| MPP/PAPP | 10/20 |
| Fire Retardant | Binder | Filler | Time/Min | Equil. Temp./°C | Ref |
|---|---|---|---|---|---|
| MPP/PAPP (30 wt%) | SAE | TiO2 (5 wt%) | 120 | 170 | This work |
| MF-APP/PER/MEL # (54.2 wt%) | Acrylic resin | MF-BZ # (6 wt%) | 100 | 212 | Huo [4] |
| APP/PER/MEL (37 wt%) | VAC * | CaCO3 (10 wt%) | 100 | 264 | Md Nasir [22] |
| APP/PER/MEL (40 wt%) | VAC * | TiO2/BioAsh (10 wt%) | 60 | 113 | Beh [23] |
| APP/PER/MEL (40 wt%) | Acrylic resin | TiO2 (2.4 wt%) | 60 | 171 | Wang [24] |
| MPP/DPER/MEL (25 wt%) | Epoxy | CNP@Mo ‡ (2 wt%) | 60 | 180 | Xiao [9] |
| APP/PER/MEL (37 wt%) | Acrylic resin | TiO2/Mg(OH)2 (7.4 wt%) | 60 | 188 | Yew [25] |
| APP/PER/MEL (45 wt%) | VAE * | Na-REC ‡/TiO2 (15 wt%) | 60 | 202 | Xie [26] |
| APP/EG/MEL # (23 wt%) | Epoxy | Boric Acid/Kaolin clay (16.5 wt%) | 60 | 257 | Ullah [27] |
| APP/PER/MEL (50 wt%) | Acrylic resin | Nano-TiO2 (20 wt%) | 60 | 289 | Beheshti [28] |
| APP/EG/MEL # (23 wt%) | Epoxy | Boric acid (11 wt%) | 60 | 337 | Ullah [29] |
| APP/PER/EG # (27 wt%) | SAE | Al (OH)3 (3 wt%) | 60 | 350 | Zhou [30] |
| APP/PER/MEL (46 wt%) | Epoxy | TiO2 (10 wt%) | 46 | 417 | Tang [31] |
| APP/PER/MEL | MPP/PAPP | |
|---|---|---|
| Char thickness | 14 mm | 6 mm |
| Intumescent factor | 7 | 3 |
| Coating | Td,1% (°C) | R800°C (wt%) |
|---|---|---|
| APP/PER/MEL | 222 | 29.9 |
| MPP/PAPP | 296 | 33.8 |
| MPP | 379 | 28.9 |
| PAPP | 313 | 22.9 |
| Band Position (cm−1) | |||
|---|---|---|---|
| Assignment | 25 °C | 400 °C | 500 °C |
| N-H stretching mode | - | 3433 | 3433 |
| C-H stretching mode | 3027, 699 | 3027, 699 | - |
| C=H stretching mode | 2854, 2925, 1453 | 2854, 2925, 1453 | - |
| C=O stretching mode | 1731 | 1731 | - |
| C=C stretching mode | - | 1631 | 1631 |
| PO2/PO3 stretching mode | - | 1010 | 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Huang, Y.; Tang, W.; Zhang, Y.; Qian, L. Synergistic Effect between Piperazine Pyrophosphate and Melamine Polyphosphate in Flame Retardant Coatings for Structural Steel. Polymers 2022, 14, 3722. https://doi.org/10.3390/polym14183722
Li L, Huang Y, Tang W, Zhang Y, Qian L. Synergistic Effect between Piperazine Pyrophosphate and Melamine Polyphosphate in Flame Retardant Coatings for Structural Steel. Polymers. 2022; 14(18):3722. https://doi.org/10.3390/polym14183722
Chicago/Turabian StyleLi, Lianliang, Yating Huang, Wei Tang, Yi Zhang, and Lijun Qian. 2022. "Synergistic Effect between Piperazine Pyrophosphate and Melamine Polyphosphate in Flame Retardant Coatings for Structural Steel" Polymers 14, no. 18: 3722. https://doi.org/10.3390/polym14183722
APA StyleLi, L., Huang, Y., Tang, W., Zhang, Y., & Qian, L. (2022). Synergistic Effect between Piperazine Pyrophosphate and Melamine Polyphosphate in Flame Retardant Coatings for Structural Steel. Polymers, 14(18), 3722. https://doi.org/10.3390/polym14183722