Bio-Nanocomposite Based on Edible Gelatin Film as Active Packaging from Clarias gariepinus Fish Skin with the Addition of Cellulose Nanocrystalline and Nanopropolis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Cellulose Nanocrystal (CNC)
2.1.2. Preparation of Dumbo Catfish Skin Nanogelatin (NGC)
2.1.3. Preparation of Bio-Nanocomposites Edible Film
2.2. Characterization of NEF
2.2.1. Thickness
2.2.2. Water Content and Water Solubility
2.2.3. Swelling
2.2.4. pH
2.2.5. Mechanical Property
2.2.6. Morphology
2.2.7. Water Vapor Permeability (WVP)
2.2.8. Oxygen Permeability
2.2.9. Thermal Stability
2.2.10. Transparency
2.2.11. Color
2.3. Statistical Analysis
3. Results and Discussion
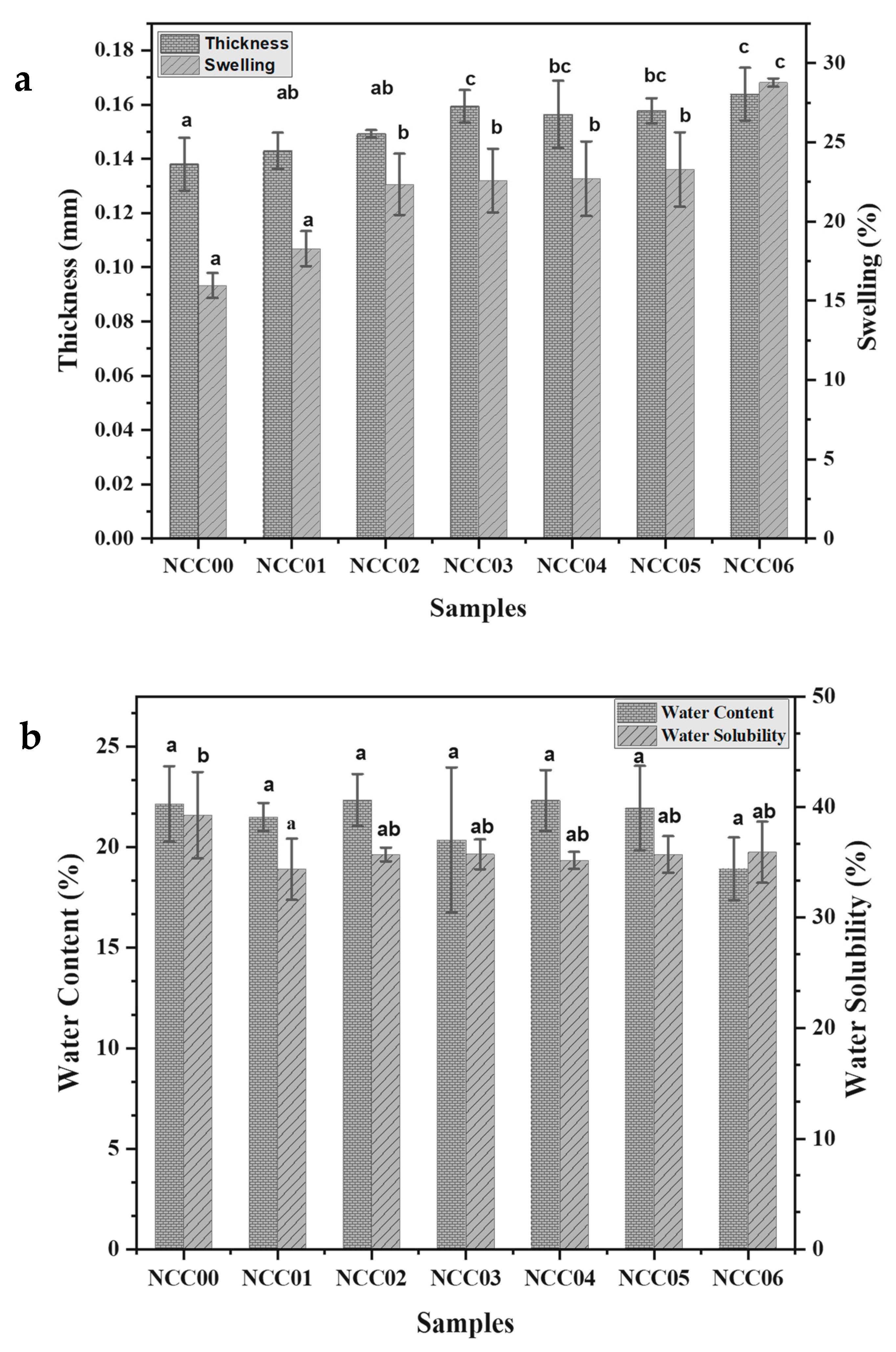
3.1. Thickness of NEF
3.2. Swelling of NEF
3.3. Water Content
3.4. Water Solubility
3.5. pH of NEF
3.6. Water Vapor Permeability
3.7. Oxygen Permeability
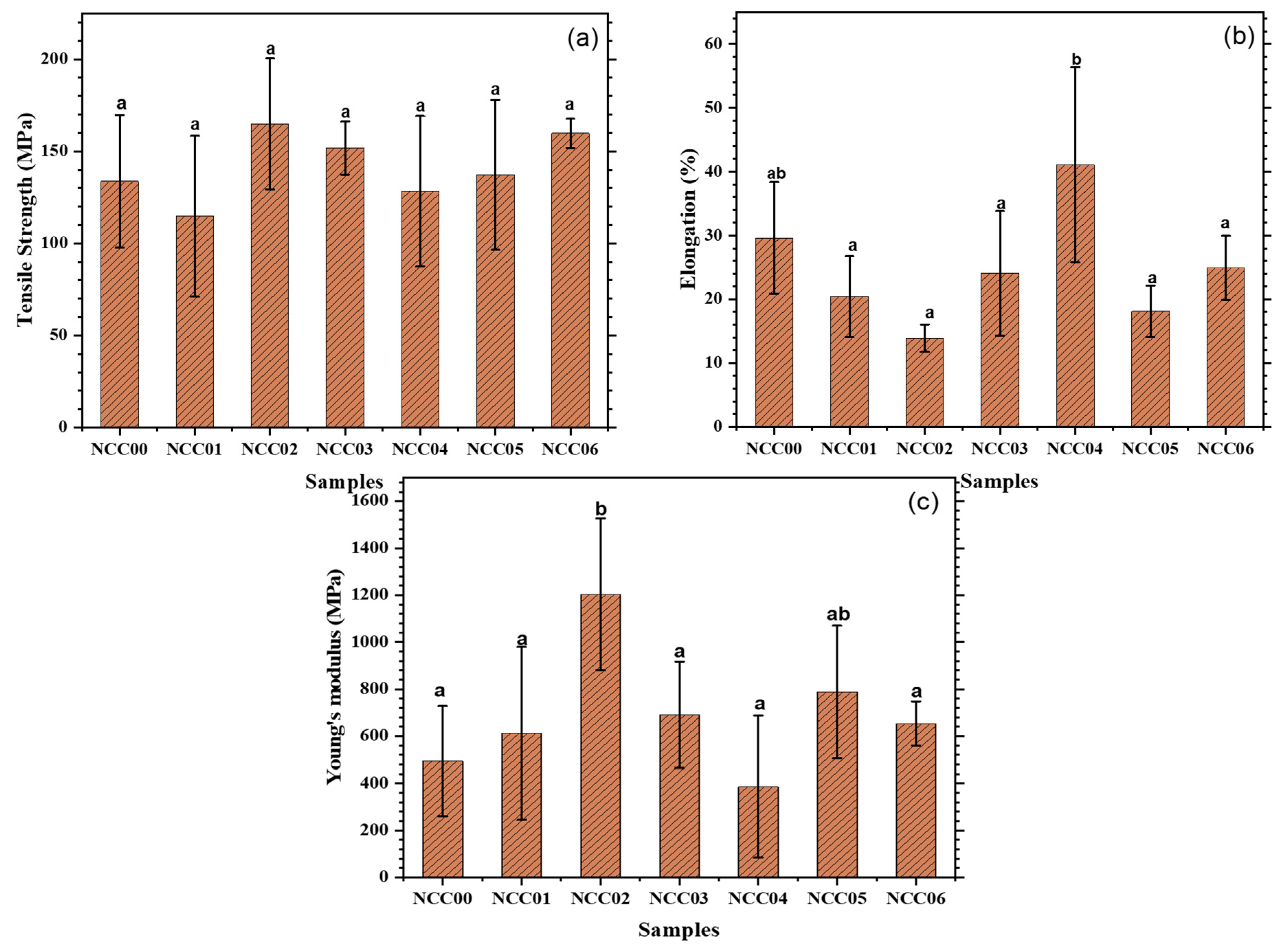
3.8. Mechanical Properties of NEF
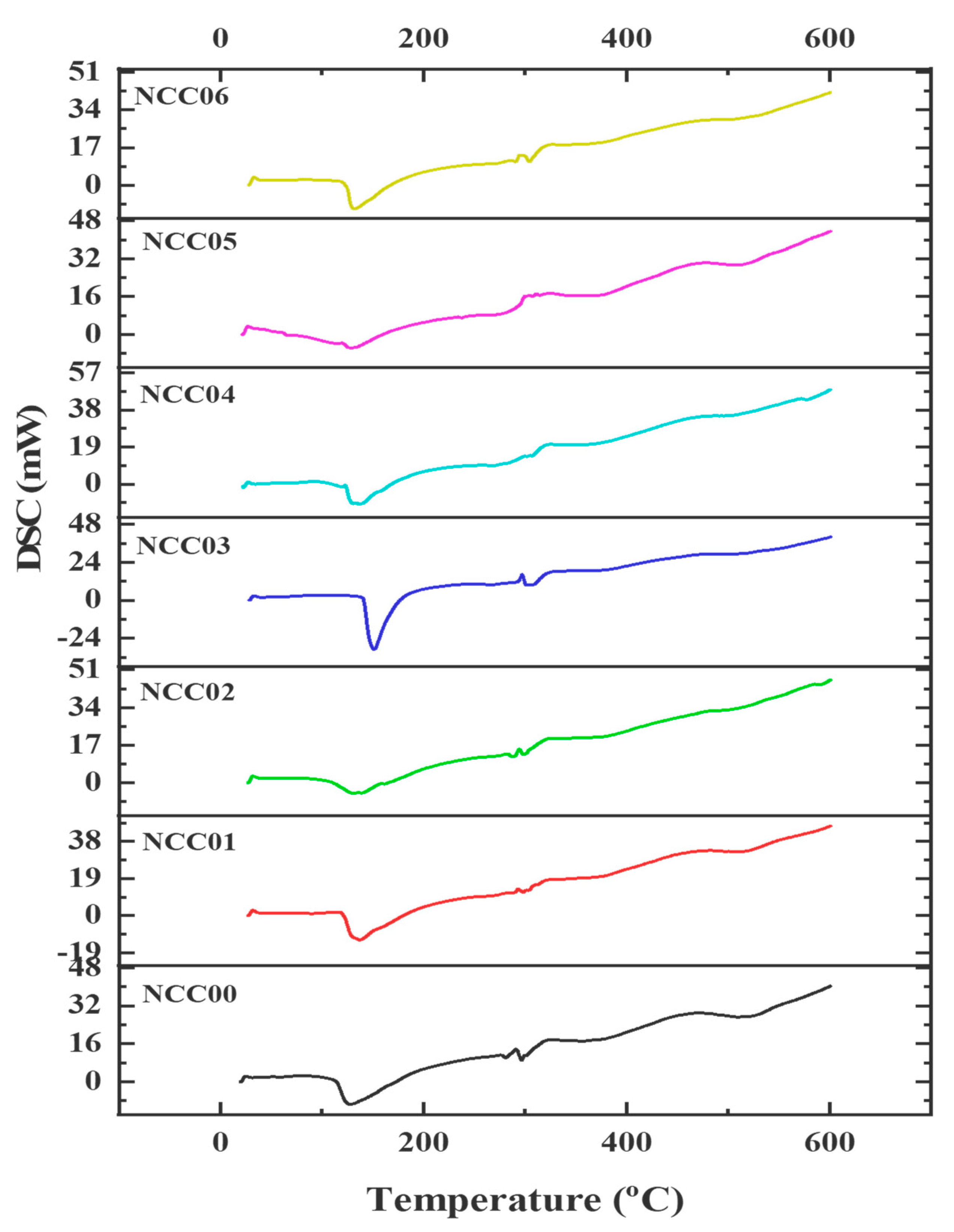
3.9. Thermal Resistance Properties of NEF
3.10. Morphological Properties of NEF
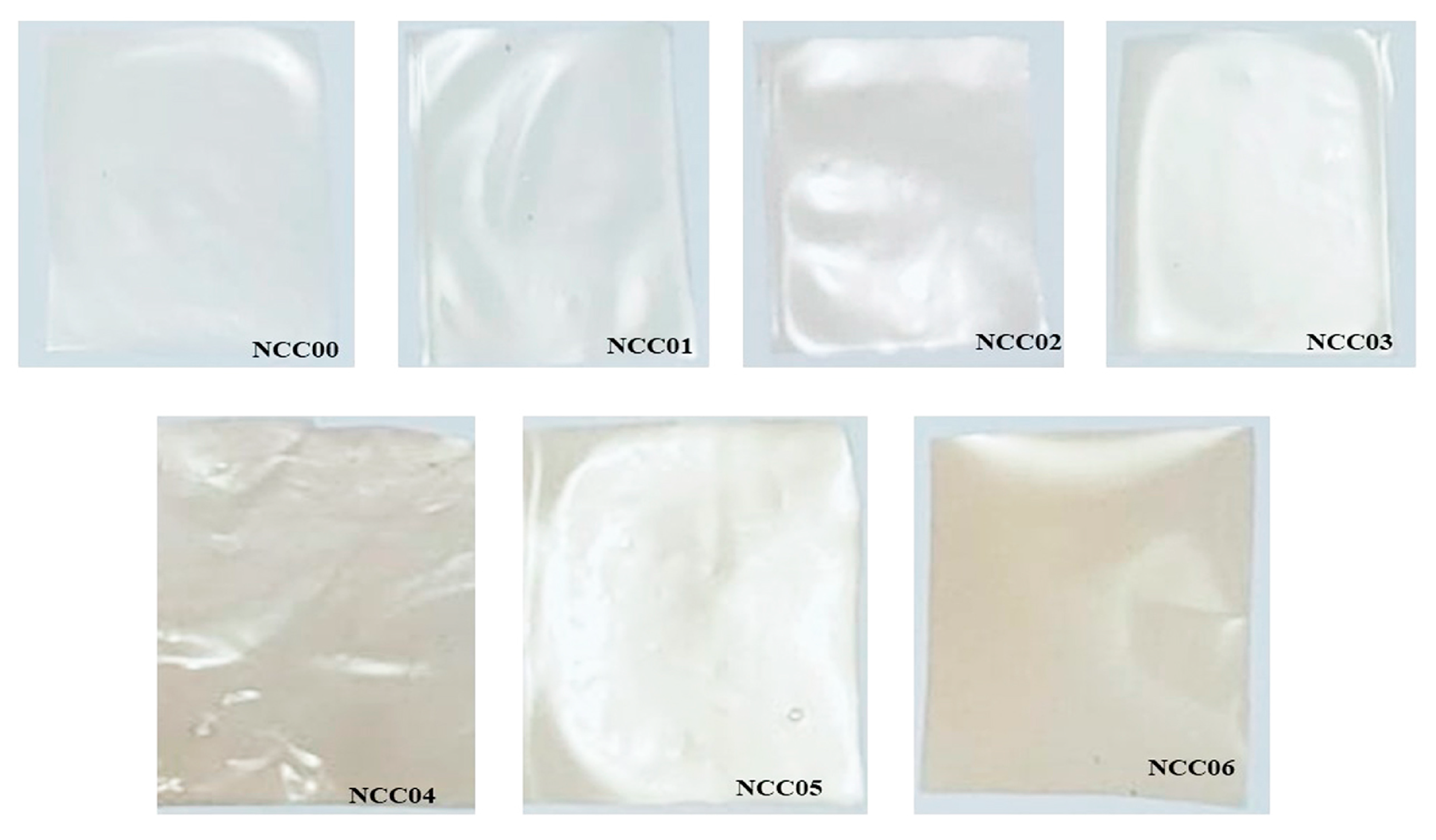
3.11. Transparency Properties of NEF
3.12. Color of NEF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, S.; Rhim, J.-W. Preparation of Antimicrobial and Antioxidant Gelatin/Curcumin Composite Films for Active Food Packaging Application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef] [PubMed]
- Nor Adilah, A.; Gun Hean, C.; Nur Hanani, Z.A. Incorporation of Graphene Oxide to Enhance Fish Gelatin as Bio-Packaging Material. Food Packag. Shelf Life 2021, 28, 100679. [Google Scholar] [CrossRef]
- Nurul Syahida, S.; Ismail-Fitry, M.R.; Ainun, Z.M.A.; Nur Hanani, Z.A. Effects of Palm Wax on the Physical, Mechanical and Water Barrier Properties of Fish Gelatin Films for Food Packaging Application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Khodaei, D.; Oltrogge, K.; Hamidi-Esfahani, Z. Preparation and Characterization of Blended Edible Films Manufactured Using Gelatin, Tragacanth Gum and, Persian Gum. LWT 2020, 117, 108617. [Google Scholar] [CrossRef]
- Huang, T.; Fang, Z.; Zhao, H.; Xu, D.; Yang, W.; Yu, W.; Zhang, J. Physical Properties and Release Kinetics of Electron Beam Irradiated Fish Gelatin Films with Antioxidants of Bamboo Leaves. Food Biosci. 2020, 36, 100597. [Google Scholar] [CrossRef]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up Difficulties and Commercial Aspects of Edible Films for Food Packaging: A Review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Deng, H.; Guo, Y.; Xue, J. Gelatin Films Incorporated with Thymol Nanoemulsions: Physical Properties and Antimicrobial Activities. Int. J. Biol. Macromol. 2020, 150, 161–168. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Karaś, M.; Zięba, E.; Mężyńska, M.; Wiącek, A.E. Release Kinetics and Antimicrobial Properties of the Potassium Sorbate-Loaded Edible Films Made from Pullulan, Gelatin and Their Blends. Food Hydrocoll. 2020, 101, 105539. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Ghorbani, M.; Tabibiazar, M. A Gelatin-Based Film Reinforced by Covalent Interaction with Oxidized Guar Gum Containing Green Tea Extract as an Active Food Packaging System. Food Bioprocess Technol. 2020, 13, 1633–1644. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and Protein Hydrolysates as Food Preservatives and Bioactive Components of Edible Films and Coatings—A Review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Bilatto, S.; Paschoalin, R.T.; Soares, A.C.; Moreira, F.K.V.; Oliveira, O.N.; Mattoso, L.H.C.; Bras, J. Eco-Friendly Gelatin Films with Rosin-Grafted Cellulose Nanocrystals for Antimicrobial Packaging. Int. J. Biol. Macromol. 2020, 165, 2974–2983. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning Structure and Properties of Gelatin Edible Films through Pullulan Dialdehyde Crosslinking. LWT 2021, 138, 110607. [Google Scholar] [CrossRef]
- Khedri, S.; Sadeghi, E.; Rouhi, M.; Delshadian, Z.; Mortazavian, A.M.; de Toledo Guimarães, J.; fallah, M.; Mohammadi, R. Bioactive Edible Films: Development and Characterization of Gelatin Edible Films Incorporated with Casein Phosphopeptides. LWT 2021, 138, 110649. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of Anthocyanin as a Color Indicator in Gelatin Films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging Films Formulated with Gelatin and Anthocyanins Nanocomplexes: Physical Properties, Antioxidant Activity and Its Application for Olive Oil Protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Etxabide, A.; Maté, J.I.; Kilmartin, P.A. Effect of Curcumin, Betanin and Anthocyanin Containing Colourants Addition on Gelatin Films Properties for Intelligent Films Development. Food Hydrocoll. 2021, 115, 106593. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Properties and Application of Bilayer Films Based on Poly (lactic acid) and Fish Gelatin Containing Epigallocatechin Gallate Fabricated by Thermo-Compression Molding. Food Hydrocoll. 2020, 105, 105792. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Ghorbani, M.; Mahmoodzadeh, F.; Nikzad, B. Development of Reinforced Aldehyde-Modified Kappa-Carrageenan/Gelatin Film by Incorporation of Halloysite Nanotubes for Biomedical Applications. Int. J. Biol. Macromol. 2020, 160, 669–676. [Google Scholar] [CrossRef]
- Loo, C.P.Y.; Sarbon, N.M. Chicken Skin Gelatin Films with Tapioca Starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The Effect of Plasticizers on the Functional Properties of Biodegradable Gelatin-Based Film: A Review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-Based Composite Films and Their Application in Food Packaging: A Review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible Films from Chitosan-Gelatin: Physical Properties and Food Packaging Application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Sánchez, J.T.; García, A.V.; Martínez-Abad, A.; Vilaplana, F.; Jiménez, A.; Garrigós, M.C. Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel. Foods 2020, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Barapatre, A.; Jha, H. Synthesis and Characterization of Acacia Lignin-Gelatin Film for Its Possible Application in Food Packaging. Bioresour. Bioprocess. 2016, 3, 27. [Google Scholar] [CrossRef]
- Theerawitayaart, W.; Prodpran, T.; Benjakul, S.; Nilsuwan, K.; de la Caba, K. Storage Stability of Fish Gelatin Films by Molecular Modification or Direct Incorporation of Oxidized Linoleic Acid: Comparative Studies. Food Hydrocoll. 2021, 113, 106481. [Google Scholar] [CrossRef]
- Ji, M.; Wu, J.; Sun, X.; Guo, X.; Zhu, W.; Li, Q.; Shi, X.; Tian, Y.; Wang, S. Physical Properties and Bioactivities of Fish Gelatin Films Incorporated with Cinnamaldehyde-Loaded Nanoemulsions and Vitamin, C. LWT 2021, 135, 110103. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of Lipids with Different Physical State on the Physicochemical Properties of Starch/Gelatin Edible Films Prepared by Extrusion Blowing. Int. J. Biol. Macromol. 2021, 185, 1005–1014. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Fish Gelatin Films Laminated with Emulsified Gelatin Film or Poly(lactic) Acid Film: Properties and Their Use as Bags for Storage of Fried Salmon Skin. Food Hydrocoll. 2021, 111, 106199. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti khiabani, M.; Akhondzadeh basti, A.; Abdulkhani, A. The Effects of Gelatin-CMC Films Incorporated with Chitin Nanofiber and Trachyspermum Ammi Essential Oil on the Shelf Life Characteristics of Refrigerated Raw Beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Łupina, K.; Biendl, M. Edible Films Based on Gelatin, Carboxymethyl Cellulose, and Their Blends as Carriers of Potassium Salts of Iso-α-Acids: Structural, Physicochemical and Antioxidant Properties. Food Hydrocoll. 2021, 115, 106574. [Google Scholar] [CrossRef]
- Park, J.; Nam, J.; Yun, H.; Jin, H.-J.; Kwak, H.W. Aquatic Polymer-Based Edible Films of Fish Gelatin Crosslinked with Alginate Dialdehyde Having Enhanced Physicochemical Properties. Carbohydr. Polym. 2021, 254, 117317. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; de la Caba, K. The Effect of Cross-Linking with Citric Acid on the Properties of Agar/Fish Gelatin Films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Moreira, F.K.V.; Mattoso, L.H.C.; Bras, J. Electrostatic Interactions Regulate the Physical Properties of Gelatin-Cellulose Nanocrystals Nanocomposite Films Intended for Biodegradable Packaging. Food Hydrocoll. 2021, 113, 106424. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physicochemical Properties of Gelatin Films Containing Tea Polyphenol-Loaded Chitosan Nanoparticles Generated by Electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, Antioxidant and Antibacterial Properties of Fish Gelatin-Based Edible Films Enriched with Orange Peel Pectin: Wrapping Application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat Sealable Soluble Soybean Polysaccharide/Gelatin Blend Edible Films for Food Packaging Applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Nur Amila Najwa, I.S.; Guerrero, P.; de la Caba, K.; Nur Hanani, Z.A. Physical and Antioxidant Properties of Starch/Gelatin Films Incorporated with Garcinia Atroviridis Leaves. Food Packag. Shelf Life 2020, 26, 100583. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Pourfathi, B.; Ranjbaryan, S. Gelatin Films Activated by Cinnamon Essential Oil and Reinforced with 1D, 2D and 3D Nanomaterials: Physical and Release Controlling Properties. J. Polym. Environ. 2021, 29, 3068–3078. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin/Agar-Based Functional Film Integrated with Pickering Emulsion of Clove Essential Oil Stabilized with Nanocellulose for Active Packaging Applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A Comprehensive Review on the Nanocomposites Loaded with Chitosan Nanoparticles for Food Packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef]
- Tessaro, L.; Lourenço, R.V.; Martelli-Tosi, M.; do Amaral Sobral, P.J. Gelatin/Chitosan Based Films Loaded with Nanocellulose from Soybean Straw and Activated with “Pitanga” (Eugenia Uniflora L.) Leaf Hydroethanolic Extract in W/O/W Emulsion. Int. J. Biol. Macromol. 2021, 186, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Whiteside, W.S. Nano-Cellulose Reinforced Starch Bio Composite Films—A Review on Green Composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.-J. Chemical and Physical Reinforcement of Hydrophilic Gelatin Film with Di-Aldehyde Nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef]
- Dogaru, B.-I.; Stoleru, V.; Mihalache, G.; Yonsel, S.; Popescu, M.-C. Gelatin Reinforced with CNCs as Nanocomposite Matrix for Trichoderma harzianum KUEN 1585 Spores in Seed Coatings. Molecules 2021, 26, 5755. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Guo, Y.; Long, J.; Mu, R.-J.; Pang, J. Enhanced Functional Properties of Nanocomposite Film Incorporated with EGCG-Loaded Dialdehyde Glucomannan/Gelatin Matrix for Food Packaging. Food Hydrocoll. 2020, 108, 105863. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, L.S.F.; Ferreira, C.M.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H.C. Scaled-up Production of Gelatin-Cellulose Nanocrystal Bionanocomposite Films by Continuous Casting. Carbohydr. Polym. 2020, 238, 116198. [Google Scholar] [CrossRef] [PubMed]
- Yavari Maroufi, L.; Ghorbani, M.; Tabibiazar, M.; Mohammadi, M.; Pezeshki, A. Advanced Properties of Gelatin Film by Incorporating Modified Kappa-Carrageenan and Zein Nanoparticles for Active Food Packaging. Int. J. Biol. Macromol. 2021, 183, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of Bio-Nanocomposite Films Based on Gelatin/Polyvinyl Alcohol Blend Reinforced with Bacterial Cellulose Nanowhiskers for Food Packaging Applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A.; Milan, P.B.; Amoupour, M. In Vitro and in Vivo Studies of Biaxially Electrospun Poly(caprolactone)/Gelatin Nanofibers, Reinforced with Cellulose Nanocrystals, for Wound Healing Applications. Cellulose 2020, 27, 5179–5196. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, Mechanical and Wound Healing Properties of Chitosan/Gelatin Blend Films Containing Tannic Acid and/or Bacterial Nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and Degradability Properties of Polyvinyl Alcohol/Gelatin Nanocomposite Films Filled Water Hyacinth Cellulose Nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Khan, N.; Kumar, D.; Kumar, P. Silver Nanoparticles Embedded Guar Gum/Gelatin Nanocomposite: Green Synthesis, Characterization and Antibacterial Activity. Colloid Interface Sci. Commun. 2020, 35, 100242. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Gelatin Films: Study Review of Barrier Properties and Implications for Future Studies Employing Biopolymer Films. Food Packag. Shelf Life 2021, 29, 100688. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/Starch/Propolis/Anthocyanins Rosemary Extract Composite Films as Active and Intelligent Food Packaging Materials. J. Food Saf. 2020, 40, e12725. [Google Scholar] [CrossRef]
- Jonaidi Jafari, N.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The Effect of Chitosan Coating Incorporated with Ethanolic Extract of Propolis on the Quality of Refrigerated Chicken Fillet. J Food Process. Preserv. 2018, 42, e13336. [Google Scholar] [CrossRef]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez-Cervera, C.E.; Andrade-Pizarro, R.D. Development and Characterization of Edible Films Based on Native Cassava Starch, Beeswax, and Propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; de Ramos-García, M.L.; del Martínez-González, M.C.; Hernández-Romano, J. Physicochemical Characterization and Antimicrobial Activity of Edible Propolis-Chitosan Nanoparticle Films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.; Rachtanapun, C.; Rachtanapun, P. Biodegradable Rice Starch/Carboxymethyl Chitosan Films with Added Propolis Extract for Potential Use as Active Food Packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Coban, M.Z.; Coban, O.E. Potency and Use of Chia Mucilage Coating Containing Propolis Liquid Extract for Improves Shelf-Life of Sea Bass Fillets. Acta Sci. Pol. Technol. Aliment. 2020, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Shaltouki, P.; Mohamadi, E.; Moghaddasi, M.A.; Farahbakhsh, A.; Bahmanpour, H. Synthesis and Characterization of Nanoparticles Propolis Using Beeswax. Iran. J. Chem. Chem. Eng. 2019, 38, 9–19. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced Antibacterial Performance of Gelatin/Chitosan Film Containing Capsaicin Loaded MOFs for Food Packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Divband, B.; Ehsani, A.; McClements, D.J. Nanocomposite Films Consisting of Functional Nanoparticles (TiO2 and ZnO) Embedded in 4A-Zeolite and Mixed Polymer Matrices (Gelatin and Polyvinyl Alcohol). Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef]
- Rulaningtyas, R.; Suksmono, A.B.; Mengko, T.L.R.; Putri Saptawati, G.A. Segmentasi Citra Berwarna Dengan Menggunakan Metode Clustering Berbasis Patch Untuk Identifikasi Mycobacterium Tuberculosis. JBP 2015, 17, 19. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional Biocompatible Nanocomposite Films Consisting of Selenium and Zinc Oxide Nanoparticles Embedded in Gelatin/Cellulose Nanofiber Matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Yuwawech, K.; Wootthikanokkhan, J.; Wanwong, S.; Tanpichai, S. Polyurethane/Esterified Cellulose Nanocrystal Composites as a Transparent Moisture Barrier Coating for Encapsulation of Dye Sensitized Solar Cells. J. Appl. Polym. Sci. 2017, 134, 45010. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Chen, T.; Liu, Y. The Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan–Gelatin Edible Films Incorporated with the Extract from Hop Plant. Polym. Bull. 2021, 78, 3607–3624. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the Recent Developments in All-Cellulose Nanocomposites: Properties and Applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Oksman, K. Crosslinked Poly(Vinyl Alcohol) Composite Films with Cellulose Nanocrystals: Mechanical and Thermal Properties. J. Appl. Polym. Sci. 2018, 135, 45710. [Google Scholar] [CrossRef]


| Sample Code | Gelatin (% w/v) | Carboxymethyl Cellulose (% w/v) | Glycerol % (v/v) | Cellulose Nanocrystal (% w/w) | Nanopropolis (% w/v) |
|---|---|---|---|---|---|
| NCC00 | 1 | 1 | 0.25 | - | - |
| NCC01 | 1 | 1 | 0.25 | 1 | - |
| NCC02 | 1 | 1 | 0.25 | 3 | - |
| NCC03 | 1 | 1 | 0.25 | 5 | - |
| NCC04 | 1 | 1 | 0.25 | 1 | 0.1 |
| NCC05 | 1 | 1 | 0.25 | 3 | 0.1 |
| NCC06 | 1 | 1 | 0.25 | 5 | 0.1 |
| Samples | pH | WVP × 107 (g m−1 Pa−1 h−1) | OPTR (g m−1 d−1) | OP × 109 (g m−1 Pa−1 d−1) |
|---|---|---|---|---|
| NCC00 | 6 ± 0.0 | 3.6 ± 0.2 b | 3.1 ± 0.2 b | 4.6 ± 0.3 b |
| NCC01 | 6 ± 0.0 | 2.9 ± 0.1 a | 2.3 ± 1.1 ab | 3.1 ± 1.4 ab |
| NCC02 | 6 ± 0.0 | 3.0 ± 0.2 a | 2.2 ± 0.6 ab | 3.2 ± 0.8 ab |
| NCC03 | 6 ± 0.0 | 2.9 ± 0.3 a | 2.0 ± 1.7 ab | 3.1 ± 2.7 ab |
| NCC04 | 6 ± 0.0 | 3.0 ± 0.3 ab | 1.6 ± 1.4 ab | 2.6 ± 2.3 ab |
| NCC05 | 6 ± 0.0 | 3.4 ± 0.4 ab | 0.9 ± 0.1 a | 1.4 ± 0.2 a |
| NCC06 | 6 ± 0.0 | 3.1 ± 0.3 ab | 1.0 ± 0.5 a | 1.5 ± 0.8 a |
| Samples | Tg (°C) | Tm (°C) | Enthalpy (×103 J/kg) |
|---|---|---|---|
| NCC00 | 127.76 | 325.11 | 44.20 |
| NCC01 | 137.06 | 353.92 | 26.58 |
| NCC02 | 130.34 | 354.68 | 76.16 |
| NCC03 | 151.39 | 352.89 | 62.25 |
| NCC04 | 137.24 | 325.58 | 42.92 |
| NCC05 | 128.63 | 311.13 | 16.76 |
| NCC06 | 131.79 | 304.40 | 77.19 |
| Samples | Transmittance (%) at a Different Wavelength (nm) | Transparency (mm−1) | |||||
|---|---|---|---|---|---|---|---|
| 300 | 400 | 500 | 600 | 700 | 800 | ||
| NCC00 | 13.82 ± 2.06 b | 48.27 ± 2.03 e | 63.05 ± 1.58 d | 69.60 ± 1.21 d | 72.73 ± 1.00 d | 74.46 ± 0.81 d | 11.04 ± 0.20 a |
| NCC01 | 12.61 ± 1.50 b | 43.31 ± 1.14 de | 57.45 ± 1.18 c | 64.29 ± 1.71 c | 68.00 ± 1.89 c | 70.28 ± 1.91 c | 12.49 ± 0.62 ab |
| NCC02 | 11.85 ± 3.46 b | 41.76 ± 5.29 cd | 55.78 ± 3.98 bc | 62.57 ± 2.82 bc | 66.23 ± 2.12 bc | 68.49 ± 1.75 c | 13.09 ± 1.31 b |
| NCC03 | 10.94 ± 2.42 b | 38.54 ± 3.46 bc | 51.56 ± 3.38 ab | 58.76 ± 1.70 a | 61.39 ± 2.88 a | 63.67 ± 2.54 a | 14.19 ± 0.73 c |
| NCC04 | 6.32 ± 2.76 a | 36.81 ± 3.30 ab | 55.63 ± 1.40 bc | 64.54 ± 2.85 c | 67.99 ± 2.04 c | 70.43 ± 2.24 c | 12.21 ± 1.34 ab |
| NCC05 | 4.59 ± 1.96 a | 32.66 ± 1.76 ab | 51.57 ± 0.80 ab | 60.01 ± 1.99 ab | 64.87 ± 2.41 ab | 67.92 ± 1.93 bc | 14.06 ± 0.66 c |
| NCC06 | 3.91 ± 1.97 a | 30.91 ± 4.23 a | 49.87 ± 2.39 a | 57.94 ± 1.47 a | 62.56 ± 0.93 ab | 65.07 ± 0.81 ab | 14.48 ± 0.24 c |
| Samples | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|
| NCC00 | 16.079 ± 1.276 a | 2.368 ± 1.266 ab | 4.852 ± 0.725 d | 17.002 ± 1.293 a |
| NCC01 | 18.990 ± 2.397 ab | 2.650 ± 0.838 b | 4.065 ± 0.711 cd | 19.624 ± 2.355 ab |
| NCC02 | 18.897 ± 1.107 ab | 2.293 ± 0.026 ab | 2.501 ± 1.281 c | 19.221 ± 1.250 ab |
| NCC03 | 19.340 ± 0.402 b | 1.983 ± 0.203 ab | 2.556 ± 0.758 c | 19.618 ± 0.485 ab |
| NCC04 | 20.754 ± 2.490 bc | 1.290 ± 0.636 ab | −3.565 ± 1.556 a | 21.139 ± 2.540 bc |
| NCC05 | 23.573 ± 1.916 bc | 1.054 ± 0.394 a | −4.177 ± 1.051 a | 23.974 ± 2.050 bc |
| NCC06 | 20.761 ± 1.046 c | 1.132 ± 0.846 a | −1.302 ± 1.611 b | 20.886 ± 1.023 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratna; Aprilia, S.; Arahman, N.; Bilad, M.R.; Suhaimi, H.; Munawar, A.A.; Nasution, I.S. Bio-Nanocomposite Based on Edible Gelatin Film as Active Packaging from Clarias gariepinus Fish Skin with the Addition of Cellulose Nanocrystalline and Nanopropolis. Polymers 2022, 14, 3738. https://doi.org/10.3390/polym14183738
Ratna, Aprilia S, Arahman N, Bilad MR, Suhaimi H, Munawar AA, Nasution IS. Bio-Nanocomposite Based on Edible Gelatin Film as Active Packaging from Clarias gariepinus Fish Skin with the Addition of Cellulose Nanocrystalline and Nanopropolis. Polymers. 2022; 14(18):3738. https://doi.org/10.3390/polym14183738
Chicago/Turabian StyleRatna, Sri Aprilia, Nasrul Arahman, Muhammad Roil Bilad, Hazwani Suhaimi, Agus Arip Munawar, and Indera Sakti Nasution. 2022. "Bio-Nanocomposite Based on Edible Gelatin Film as Active Packaging from Clarias gariepinus Fish Skin with the Addition of Cellulose Nanocrystalline and Nanopropolis" Polymers 14, no. 18: 3738. https://doi.org/10.3390/polym14183738
APA StyleRatna, Aprilia, S., Arahman, N., Bilad, M. R., Suhaimi, H., Munawar, A. A., & Nasution, I. S. (2022). Bio-Nanocomposite Based on Edible Gelatin Film as Active Packaging from Clarias gariepinus Fish Skin with the Addition of Cellulose Nanocrystalline and Nanopropolis. Polymers, 14(18), 3738. https://doi.org/10.3390/polym14183738








