3D Printing of Triamcinolone Acetonide in Triblock Copolymers of Styrene–Isobutylene–Styrene as a Slow-Release System
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis
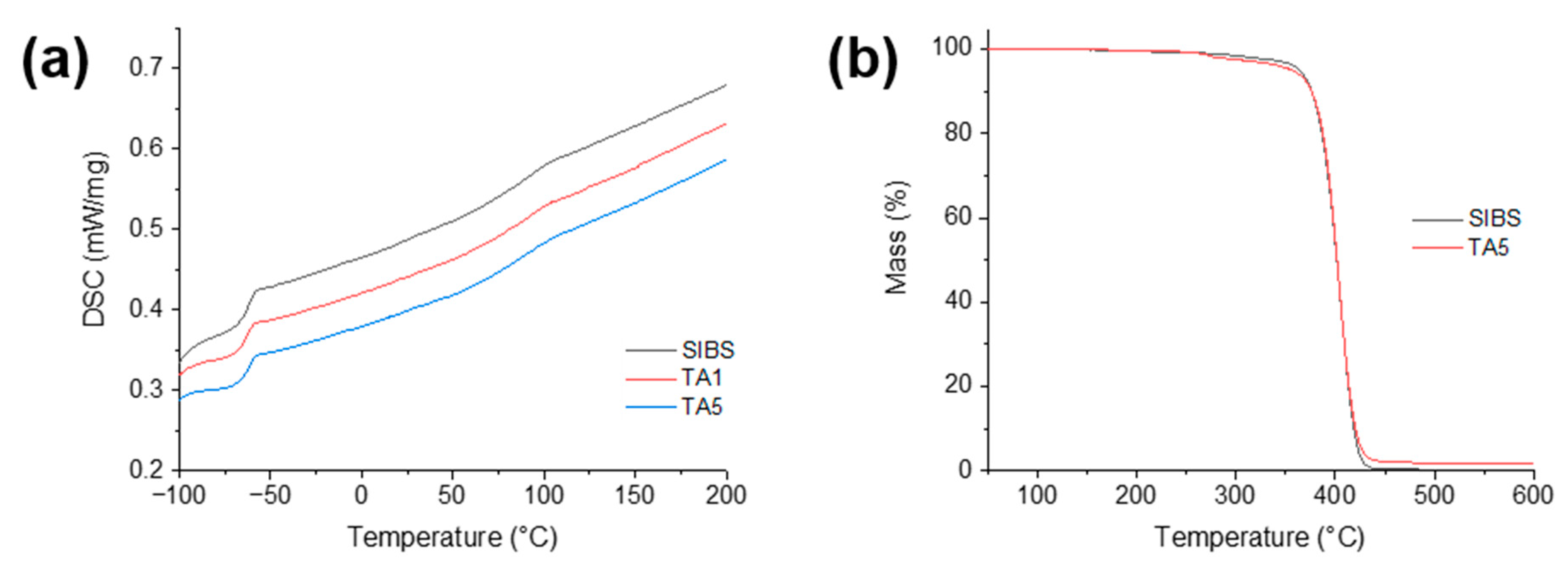
3.2. Thermal Analysis
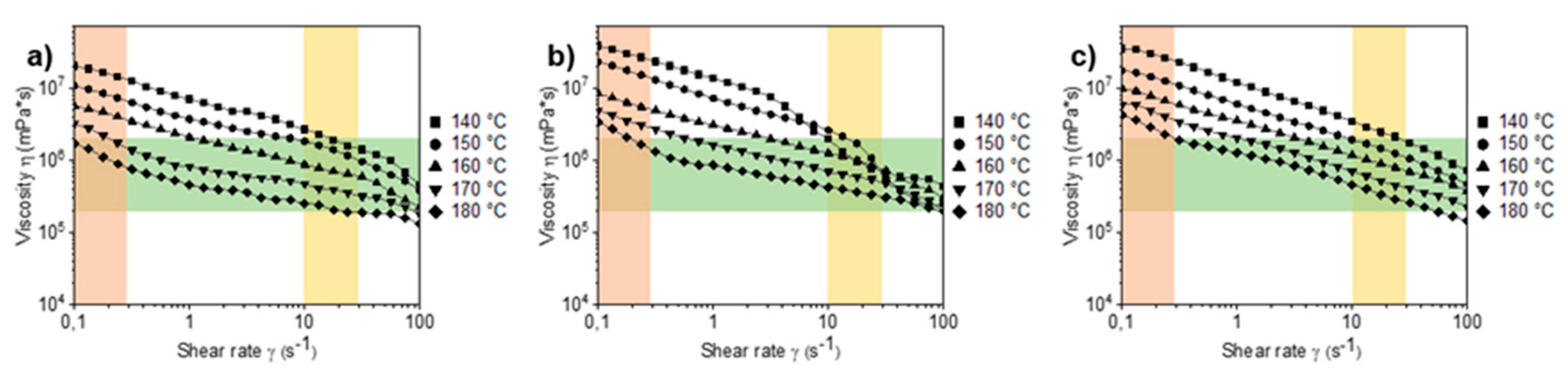
3.3. Rheology
3.4. 3D Printing
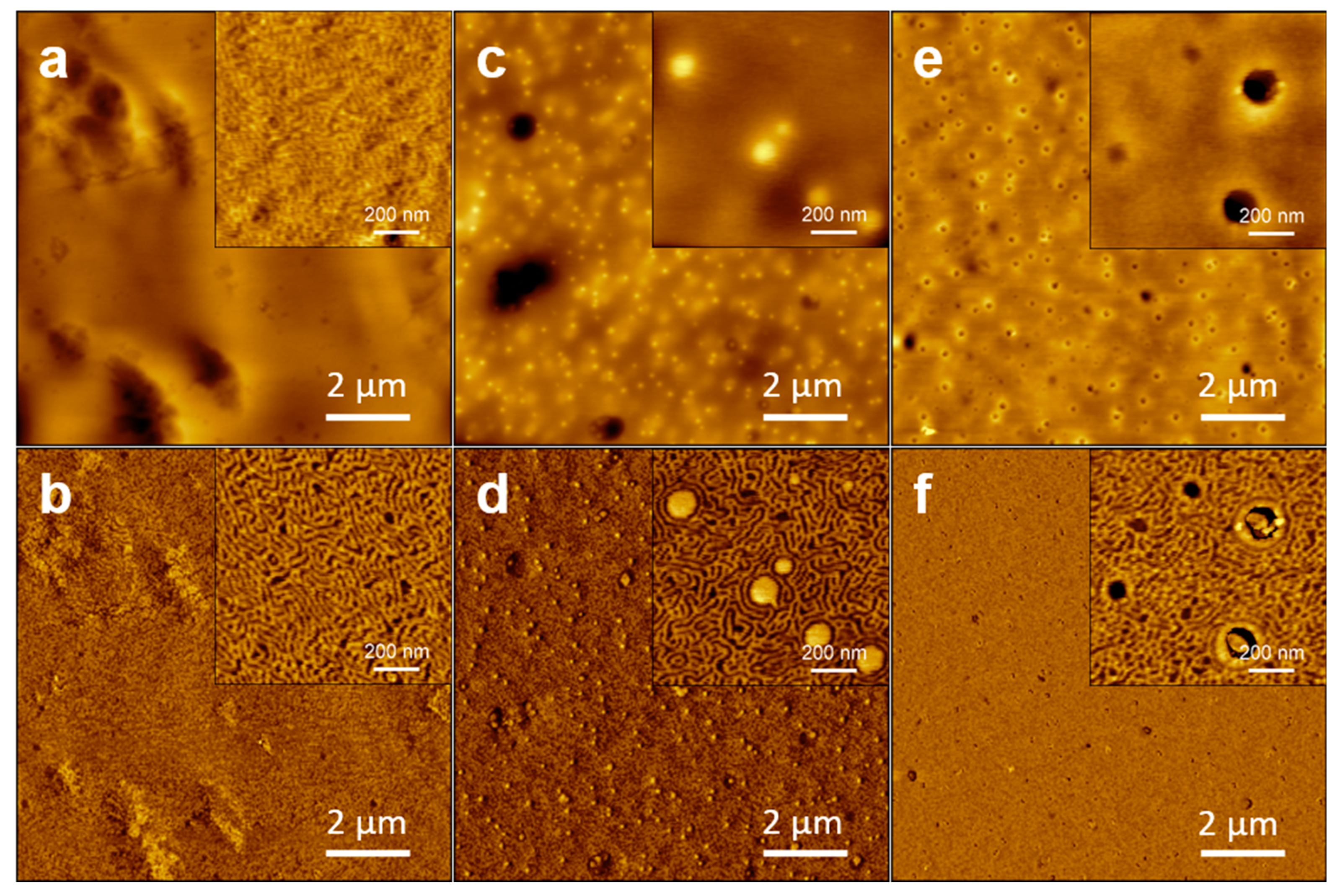
3.5. Atomic Force Microscopy
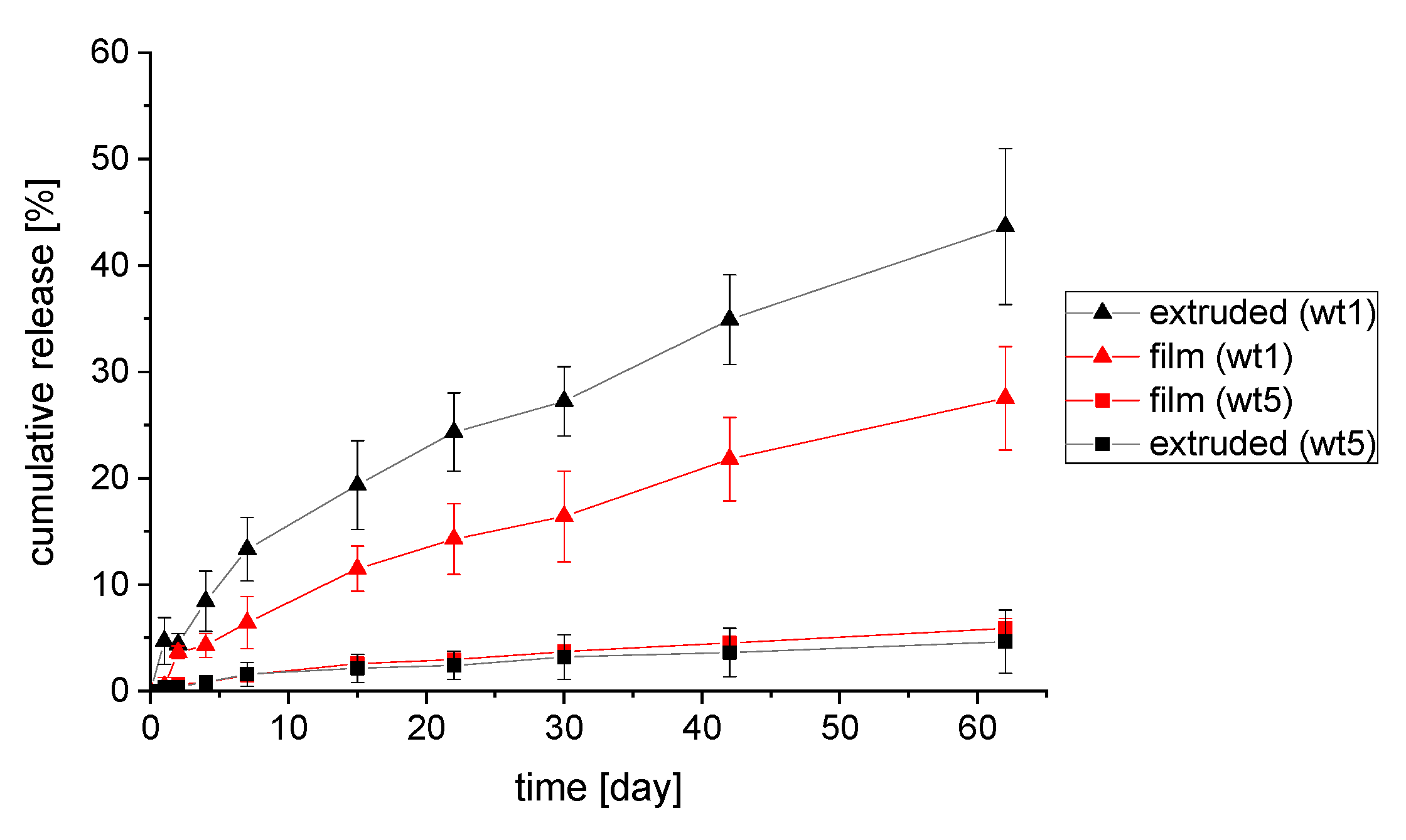
3.6. Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef]
- Smith, D.M.; Kapoor, Y.; Klinzing, G.R.; Procopio, A.T. Pharmaceutical 3D printing: Design and qualification of a single step print and fill capsule. Int. J. Pharm. 2018, 544, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int. J. Pharm. 2018, 544, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Boetker, J.P.; Colombo, S.; Harmankaya, N.; Rantanen, J.; Bohr, A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. J. Control. Release 2017, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef]
- Goyanes, A.; Kobayashi, M.; Martinez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug. Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Suaste-Gómez, E.; Rodríguez-Roldán, G.; Reyes-Cruz, H.; Terán-Jiménez, O. Developing an ear prosthesis fabricated in polyvinylidene fluoride by a 3D printer with sensory intrinsic properties of pressure and temperature. Sensors 2016, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Rupp, H.; Döhler, D.; Hilgeroth, P.; Mahmood, N.; Beiner, M.; Binder, W.H. 3D Printing of Supramolecular Polymers: Impact of Nanoparticles and Phase Separation on Printability. Macromol. Rapid. Commun. 2019, 40, e1900467. [Google Scholar] [CrossRef]
- Rupp, H.; Binder, W.H. 3D Printing of Core–Shell Capsule Composites for Post—Reactive and Damage Sensing Applications. Adv. Mater. Technol. 2020, 5, 2000509. [Google Scholar] [CrossRef]
- Rupp, H.; Binder, W.H. Multicomponent Stress-Sensing Composites Fabricated by 3D-Printing Methodologies. Macromol. Rapid. Commun. 2021, 42, e2000450. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Li, K.; Xiang, D.; Zhang, M.; Yang, D.; Zhang, J.H.; Mao, J.; Wang, H.; Guo, W.L. Surface immobilization of heparin on functional polyisobutylene-based thermoplastic elastomer as a potential artificial vascular graft. Appl. Surf. Sci. 2018, 445, 8–15. [Google Scholar] [CrossRef]
- Jindal, A.; Puskas, J.E.; McClain, A.; Nedic, K.; Luebbers, M.T.; Baker, J.R.; dos Santo, B.P.; Camassola, M.; Jennings, W.; Einsporn, R.L.; et al. Encapsulation and release of Zafirlukast from electrospun polyisobutylene-based thermoplastic elastomeric fiber mat. Eur. Polym. J. 2018, 98, 254–261. [Google Scholar] [CrossRef]
- Trant, J.F.; McEachran, M.J.; Sran, I.; Turowec, B.A.; de Bruyn, J.R.; Gillies, E.R. Covalent Polyisobutylene-Paclitaxel Conjugates for Controlled Release from Potential Vascular Stent Coatings. ACS Appl. Mater. Interfaces 2015, 7, 14506–14517. [Google Scholar] [CrossRef]
- Trant, J.F.; Sran, I.; de Bruyn, J.R.; Ingratta, M.; Borecki, A.; Gillies, E.R. Synthesis and properties of arborescent polyisobutylene derivatives and a paclitaxel conjugate: Towards stent coatings with prolonged drug release. Eur. Polym. J. 2015, 72, 148–162. [Google Scholar] [CrossRef]
- Trant, J.F.; Abd Rabo Moustafa, M.M.; Sran, I.; Gillies, E.R. Polyisobutylene—Paclitaxel conjugates with pendant carboxylic acids and polystyrene chains: Towards multifunctional stent coatings with slow drug release. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2209–2219. [Google Scholar] [CrossRef]
- Schulz, M.; Binder, W.H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid. Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, M.; He, J.; Wu, Y.; Ni, P. Preparation of Polymeric Prodrug Paclitaxel-Poly(lactic acid)-b-Polyisobutylene and Its Application in Coatings of a Drug Eluting Stent. ACS Appl. Mater. Interfaces 2015, 7, 11263–11271. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Werner, S.; Bacia, K.; Binder, W.H. Controlling molecular recognition with lipid/polymer domains in vesicle membranes. Angew. Chem. Int. Edit. 2013, 52, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Stone, G.W.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, T.; Turco, M.; Caputo, R.; Bergin, P.J.; Bowman, T.S.; et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc. Interv. 2009, 2, 1248–1259. [Google Scholar] [CrossRef]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef]
- Puskas, J.E.; Chen, Y. Biomedical application of commercial polymers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement. Biomacromolecules 2004, 5, 1141–1154. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Rosenthal, K.S.; Kashibhatla, B. Two generations of synthetic membranes for biological/medical applications. Des. Monomers Polym. 2004, 7, 485–494. [Google Scholar] [CrossRef]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Chapter Three—Paclitaxel. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2019; Volume 44, pp. 205–238. [Google Scholar]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Rizvi, A.; Newman, W.; Mastali, K.; Wang, J.C.; Caputo, R.; Doostzadeh, J.; Cao, S.; Simonton, C.A.; Sudhir, K. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 2010, 362, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773. [Google Scholar] [CrossRef]
- Parker, T.; Dave, V.; Falotico, R. Polymers for drug eluting stents. Curr. Pharm. Des. 2010, 16, 3978–3988. [Google Scholar] [CrossRef]
- Shen, N.F.; Liu, S.; Kasbe, P.; Khabaz, F.; Kennedy, J.P.; Xu, W.N. Macromolecular Engineering and Additive Manufacturing of Poly(styrene-b-isobutylene-b-styrene). ACS Appl. Polym. Mater. 2021, 3, 4554–4562. [Google Scholar] [CrossRef]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K., 2nd; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 211–221. [Google Scholar] [CrossRef]
- Zgola-Grzeskowiak, A.; Grzeskowiak, T.; Zembrzuska, J.; Lukaszewski, Z. Comparison of biodegradation of poly(ethylene glycol)s and poly(propylene glycol)s. Chemosphere 2006, 64, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Ullio Gamboa, G.V.; Tartara, L.I.; Luna, J.D.; Benoit, J.P.; Palma, S.D. Triamcinolone acetonide-loaded lipid nanocapsules for ophthalmic applications. Int. J. Pharm. 2020, 573, 118795. [Google Scholar] [CrossRef] [PubMed]
- Doty, A.C.; Zhang, Y.; Weinstein, D.G.; Wang, Y.; Choi, S.; Qu, W.; Mittal, S.; Schwendeman, S.P. Mechanistic analysis of triamcinolone acetonide release from PLGA microspheres as a function of varying in vitro release conditions. Eur. J. Pharm. Biopharm. 2017, 113, 24–33. [Google Scholar] [CrossRef]
- Sun, S.; Li, J.; Li, X.; Lan, B.; Zhou, S.; Meng, Y.; Cheng, L. Episcleral drug film for better-targeted ocular drug delivery and controlled release using multilayered poly-ε-caprolactone (PCL). Acta Biomater. 2016, 37, 143–154. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, S.; Li, J.; Nan, K.; Lan, B.; Jin, Y.; Chen, H.; Cheng, L. Sustained release of triamcinolone acetonide from an episcleral plaque of multilayered poly-ε-caprolactone matrix. Acta Biomater. 2014, 10, 126–133. [Google Scholar] [CrossRef]
- Tamboli, V.; Mishra, G.P.; Mitra, A.K. Novel pentablock copolymer (PLA-PCL-PEG-PCL-PLA) based nanoparticles for controlled drug delivery: Effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid Polym. Sci. 2013, 291, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Herbst, F.; Seiffert, S.; Binder, W.H. Dynamic supramolecular poly(isobutylene)s for self-healing materials. Polym. Chem. 2012, 3, 3084–3092. [Google Scholar] [CrossRef]
- Gyor, M.; Wang, H.C.; Faust, R. Living Carbocationic Polymerization of Isobutylene with Blocked Bifunctional Initiators in the Presence of Di-Tert-Butylpyridine as a Proton Trap. J. Macromol. Sci. A 1992, 29, 639–653. [Google Scholar] [CrossRef]
- Orszagh, I.; Nagy, A.; Kennedy, J. Living carbocationic copolymerizations. I. Synthesis and characterization of isobutylene/p‐methylstyrene copolymers. J. Phys. Org. Chem. 1995, 8, 258–272. [Google Scholar] [CrossRef]
- Majoros, I.; Nagy, A.; Kennedy, J. Conventional and living carbocationic polymerizations united. I. A comprehensive model and new diagnostic method to probe the mechanism of homopolymerizations. In Theories and Mechanism of Phase Transitions, Heterophase Polymerizations, Homopolymerization, Addition Polymerization; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1994; Volume 112, pp. 1–114. [Google Scholar]
- Kennedy, J.P. Recent developments in living carbocationic polymerization of alkenes. Macromol. Symp. 1990, 32, 119–129. [Google Scholar] [CrossRef]
- Shen, N.; Bu, J.; Prevot, M.E.; Hegmann, T.; Kennedy, J.P.; Xu, W. Macromolecular Engineering and Additive Manufacturing of Polyisobutylene-Based Thermoplastic Elastomers. II. The Poly(styrene-b-isobutylene-b-styrene)/Poly(phenylene oxide) System. Macromol. Rapid. Commun. 2022, 2200109. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.N.L.; Young, W.-S.; Lewis, R.L.; Bogart, T.D.; Smith, J.R.; Epps, T.H. Systematic Study on the Effect of Solvent Removal Rate on the Morphology of Solvent Vapor Annealed ABA Triblock Copolymer Thin Films. ACS Nano 2012, 6, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Spontak, R.J.; Patel, N.P. Thermoplastic elastomers: Fundamentals and applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]
- Grady, B.P.; Cooper, S.L. 13—Thermoplastic Elastomers. In Science and Technology of Rubber, 3rd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: Burlington, MA, USA, 2005; pp. 555–617. [Google Scholar]
- Motomatsu, M.; Mizutani, W.; Tokumoto, H. Microphase domains of poly(styrene-block-ethylene/butylene-block-styrene) triblock copolymers studied by atomic force microscopy. Polymer 1997, 38, 1779–1785. [Google Scholar] [CrossRef]
- Knoll, A.; Horvat, A.; Lyakhova, K.S.; Krausch, G.; Sevink, G.J.A.; Zvelindovsky, A.V.; Magerle, R. Phase Behavior in Thin Films of Cylinder-Forming Block Copolymers. Phys. Rev. Lett. 2002, 89, 035501. [Google Scholar] [CrossRef]
- Ranade, S.V.; Richard, R.E.; Helmus, M.N. Styrenic block copolymers for biomaterial and drug delivery applications. Acta Biomater. 2005, 1, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Hao, Y.L.; Yao, X.; Zhang, S.; Duan, X.C.; Yin, Y.F.; Xu, M.Q.; Guo, Y.; Li, Z.T.; Zheng, X.C.; et al. Effect of XlogP and Hansen Solubility Parameters on Small Molecule Modified Paclitaxel Anticancer Drug Conjugates Self-Assembled into Nanoparticles. Bioconjugate Chem. 2018, 29, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Small, P.A. Some Factors Affecting the Solubility of Polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Abou-ElNour, M.; Ishak, R.A.H.; Tiboni, M.; Bonacucina, G.; Cespi, M.; Casettari, L.; Soliman, M.E.; Geneidi, A.S. Triamcinolone acetonide-loaded PLA/PEG-PDL microparticles for effective intra-articular delivery: Synthesis, optimization, in vitro and in vivo evaluation. J. Control. Release 2019, 309, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.V.; Miller, K.M.; Richard, R.E.; Chan, A.K.; Allen, M.J.; Helmus, M.N. Physical characterization of controlled release of paclitaxel from the TAXUSTM Express2TM drug-eluting stent. J. Biomed. Mater. Res. A 2004, 71, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.E.; Hoerr, R.A. Drug Release from Novel Rubbery Coatings. Macromol. Symp. 2010, 291–292, 326–329. [Google Scholar] [CrossRef]

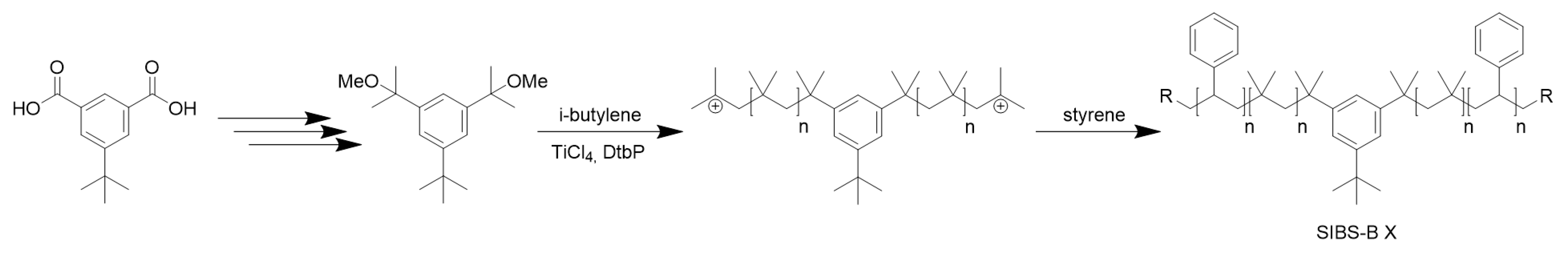

| Sample (SIBS) | Mn (GPC) | PDI | wt % PS |
|---|---|---|---|
| B1 | 10,800 | 1.36 | 6% |
| B2 | 14,000 | 1.8 | 32% |
| B3 | 18,000 | 1.47 | 36% |
| B4 | 22,300 | 1.44 | 38% |
| B5 | 25,200 | 1.65 | 49% |
| Sample | TTank | TExtruder | Pressure (MPa) | Feed (mm·s −1) |
|---|---|---|---|---|
| SIBS-B4 | 210 | 190 | 0.2 | 10 |
| TA1 | 190 | 180 | 0.4 | 10 |
| TA5 | 190 | 180 | 0.4 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilgeroth, P.S.; Thümmler, J.F.; Binder, W.H. 3D Printing of Triamcinolone Acetonide in Triblock Copolymers of Styrene–Isobutylene–Styrene as a Slow-Release System. Polymers 2022, 14, 3742. https://doi.org/10.3390/polym14183742
Hilgeroth PS, Thümmler JF, Binder WH. 3D Printing of Triamcinolone Acetonide in Triblock Copolymers of Styrene–Isobutylene–Styrene as a Slow-Release System. Polymers. 2022; 14(18):3742. https://doi.org/10.3390/polym14183742
Chicago/Turabian StyleHilgeroth, Philipp S., Justus F. Thümmler, and Wolfgang H. Binder. 2022. "3D Printing of Triamcinolone Acetonide in Triblock Copolymers of Styrene–Isobutylene–Styrene as a Slow-Release System" Polymers 14, no. 18: 3742. https://doi.org/10.3390/polym14183742






