Enhancing Toughness of PLA/ZrP Nanocomposite through Reactive Melt-Mixing by Ethylene-Methyl Acrylate-Glycidyl Methacrylate Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
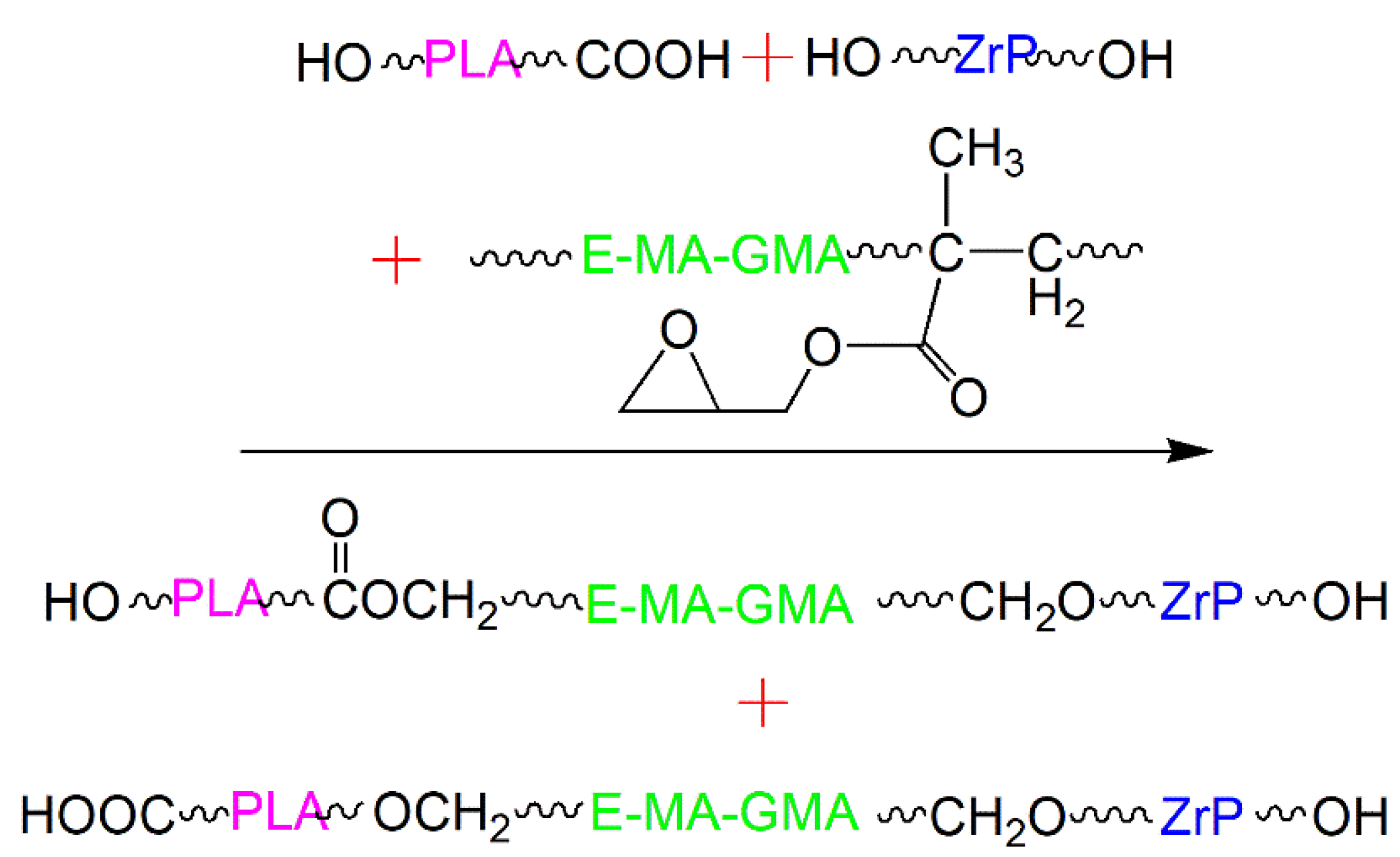
2.3. Reaction Mechanism
2.4. Characterization
2.4.1. Scanning Electron Microscope
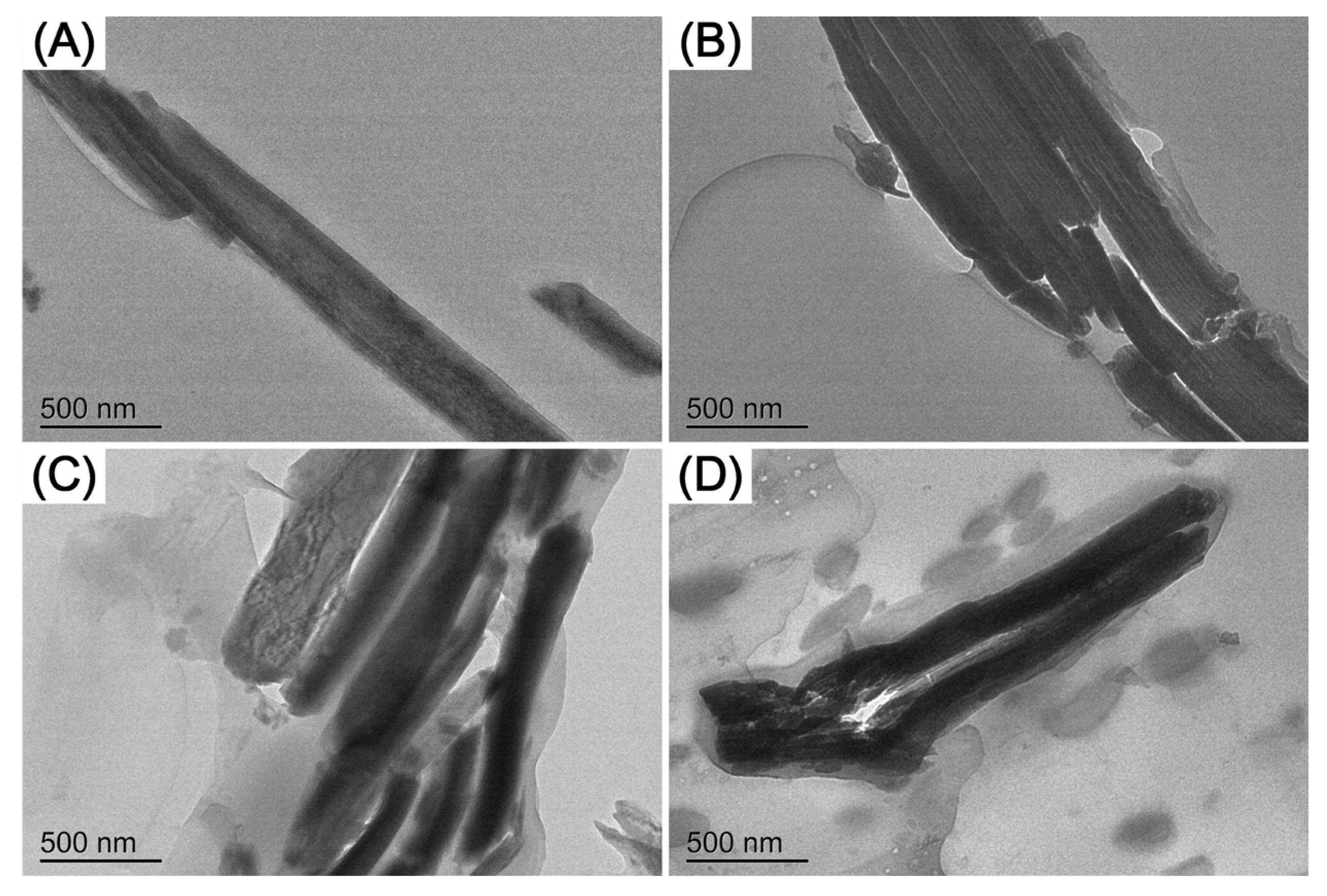
2.4.2. Transmission Electron Microscope
2.4.3. Fourier Transformation Infrared Spectroscopy
2.4.4. Dynamic Mechanical Analysis
2.4.5. Differential Scanning Calorimetry
2.4.6. Thermogravimetric Analysis
2.4.7. Mechanical Properties
3. Results and Discussion
3.1. Micro Morphology Analysis
3.2. Fourier Transformation Infrared Spectroscopy
3.3. Dynamic Mechanical Analysis
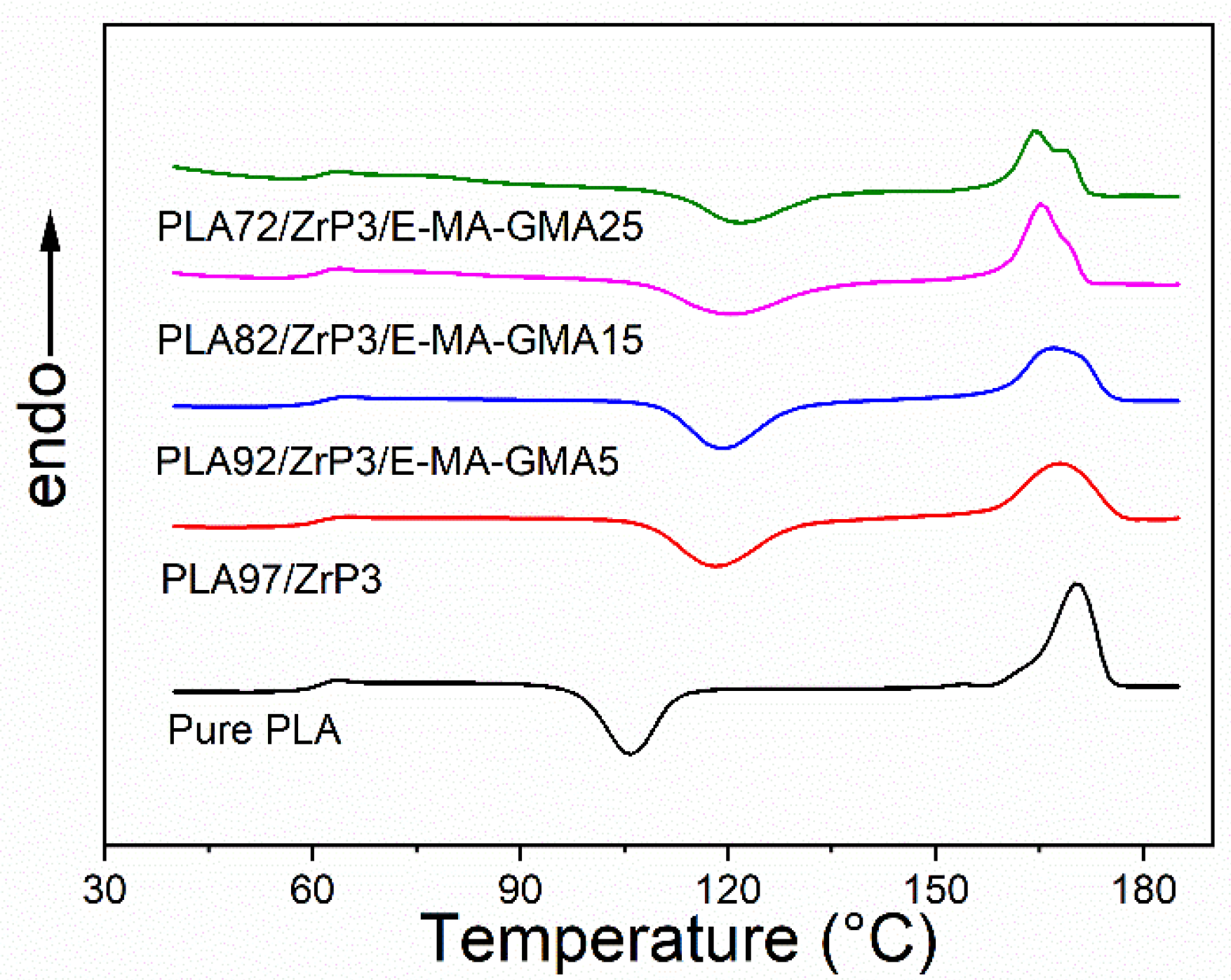
3.4. Melting and Crystalline Behavior
3.5. Thermogravimetric Analysis
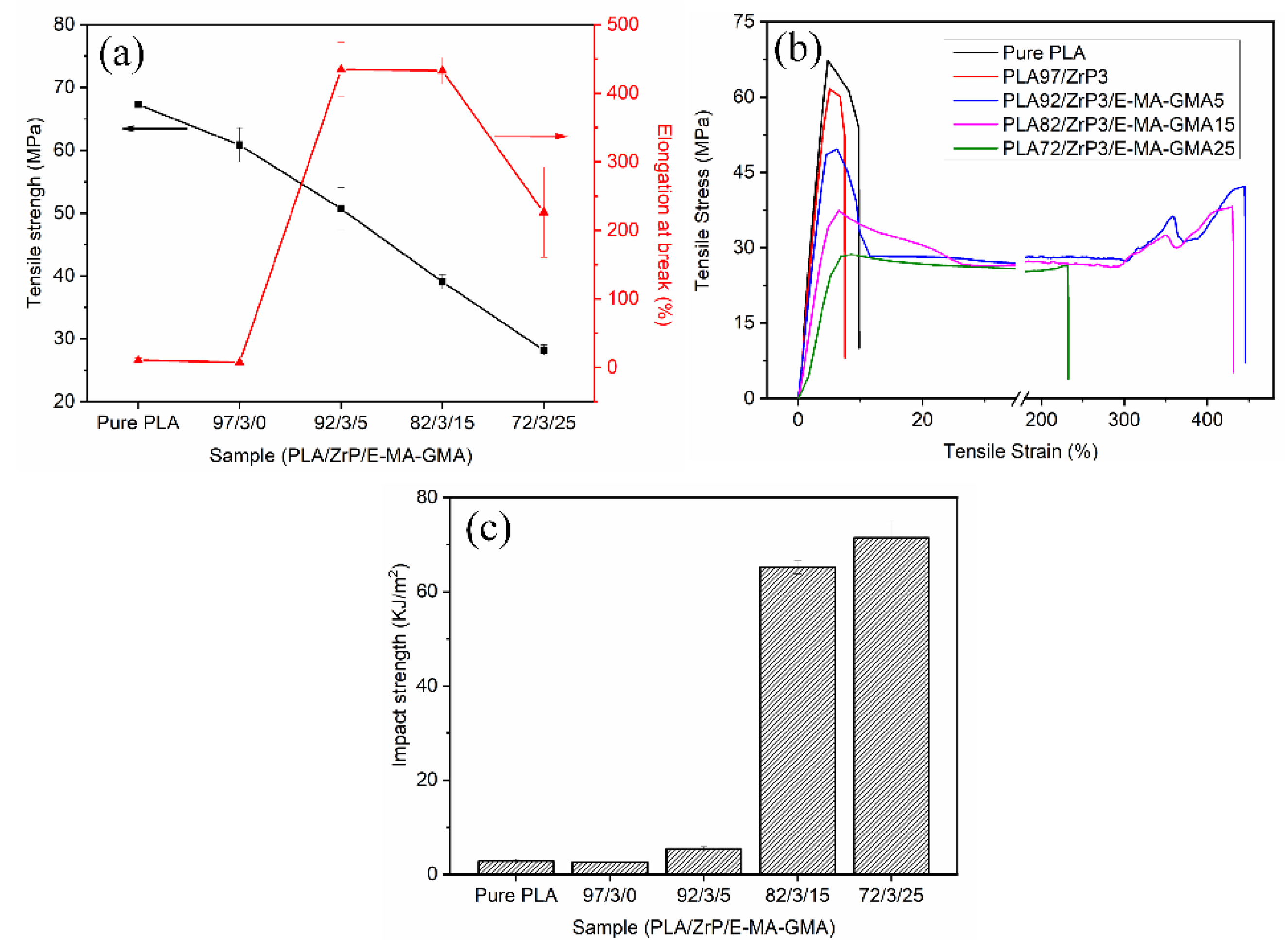
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening Polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Chihaoui, B.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Lignin-containing cellulose fibrils as reinforcement of plasticized PLA biocomposites produced by melt processing using PEG as a carrier. Ind. Crops Prod. 2022, 175, 114287. [Google Scholar] [CrossRef]
- Williams, C.K.; Hillmyer, M.A. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, Q.; Ji, X.; Yang, G.; Chen, J.; Fatehi, P. Strong, ductile and biodegradable polylactic acid/lignin-containing cellulose nanofibril composites with improved thermal and barrier properties. Ind. Crops Prod. 2021, 171, 113898. [Google Scholar] [CrossRef]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened renewable PLA reactive multiphase blends system: Phase morphology and performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Schneiderman, D.K.; Francis, L.F.; Bates, F.S. Toughening Glassy Poly(lactide) with Block Copolymer Micelles. ACS Macro Lett. 2016, 5, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, Y.; Bai, H.; Zhang, Q.; Fu, Q. Remarkably Enhanced Impact Toughness and Heat Resistance of poly(l-Lactide)/Thermoplastic Polyurethane Blends by Constructing Stereocomplex Crystallites in the Matrix. ACS Sustain. Chem. Eng. 2016, 4, 111–120. [Google Scholar] [CrossRef]
- Jia, D.; Bai, H.; Liu, Z.; Liang, C.; Qin, Z.; Qiang, F. Stereocomplex crystallites induce simultaneous enhancement in impact toughness and heat resistance of injection-molded polylactide/polyurethane blends. RSC Adv. 2016, 6, 17008–17015. [Google Scholar]
- Jia, S.; Qu, J.; Chen, R.; Wu, C.; Zan, H.; Zhai, S.; Liu, W.; Feng, Y. Effects of thermoplastic polyurethane on the properties of poly(lactic acid)/organo-montmorillonite nanocomposites based on novel vane extruder. Polym. Eng. Sci. 2013, 54, 2292–2300. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, J.; Li, Y.; Ning, Z.; Gang, J.; Qu, J. In-situ thermal reduction and effective reinforcement of graphene nanosheet/poly (ethylene glycol)/poly (lactic acid) nanocomposites. Polym. Adv. Technol. 2015, 25, 1515–1522. [Google Scholar]
- Fang, H.; Jiang, F.; Wu, Q.; Ding, Y.; Wang, Z. Supertough Polylactide Materials Prepared through In Situ Reactive Blending with PEG-Based Diacrylate Monomer. ACS Appl. Mater. Interfaces 2014, 6, 13552–13563. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhu, X.; Na, H.; Zhu, J. How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos. Sci. Technol. 2014, 90, 9–15. [Google Scholar] [CrossRef]
- Becker, J.M.; Pounder, R.J.; Dove, A.P. Synthesis of Poly(lactide)s with Modified Thermal and Mechanical Properties. Macromol. Rapid Commun. 2010, 31, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Fei, L.; Zhang, J.; Na, H.; Jin, Z. Non-planar Ring Contained Polyester Modifying Polylactide to Pursue High Toughness. Compos. Sci. Technol. 2016, 128, 41–48. [Google Scholar]
- Odent, J.; Habibi, Y.; Raquez, J.M.; Dubois, P. Ultra-tough polylactide-based materials synergistically designed in the presence of rubbery ε-caprolactone-based copolyester and silica nanoparticles. Compos. Sci. Technol. 2013, 84, 86–91. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H. Mechanical properties and phase morphology of super-tough PLA/PBAT/EMA-GMA multicomponent blends. Mater. Lett. 2017, 192, 17–20. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Oyama, H.T. Super-tough poly(lactic acid) materials: Reactive blending with ethylene copolymer. Polymer 2009, 50, 747–751. [Google Scholar] [CrossRef]
- Lin, W.; Qu, J.-P. Enhancing Impact Toughness of Renewable Poly(lactic acid)/Thermoplastic Polyurethane Blends via Constructing Cocontinuous-like Phase Morphology Assisted by Ethylene–Methyl Acrylate–Glycidyl Methacrylate Copolymer. Ind. Eng. Chem. Res. 2019, 58, 10894–10907. [Google Scholar] [CrossRef]
- Beryl, J.R.; Xavier, J.R. Mechanical and corrosion protection properties of polymer–clay nanocomposite coatings for mild steel in marine environment. Emergent Mater. 2020, 3, 75–85. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Ismail, A.F.; Matsuura, T. Mixed matrix membrane incorporated with large pore size halloysite nanotubes (HNTs) as filler for gas separation: Morphological diagram. Chem. Eng. J. 2011, 172, 581–590. [Google Scholar] [CrossRef]
- Jerus, P.; Clearfield, A. Kinetics of gas-solid reactions in α-zirconium phosphate. H+/Na+ mixed diffusion coefficients. J. Inorg. Nucl. Chem. 1981, 43, 2117–2120. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Browning, R.L.; Sue, H.J.; Clearfield, A. Effect of Crystallinity on the Intercalation of Monoamine in α-Zirconium Phosphate Layer Structure. Chem. Mater. 2005, 17, 513–514. [Google Scholar] [CrossRef]
- Clearfield, A.; Duax, W.L.; Garces, J.M.; Medina, A.S. On the mechanism of ion exchange in crystalline zirconium phosphates—IV potassium ion exchange of α-zirconium phosphate. J. Inorg. Nucl. Chem. 1972, 34, 329–337. [Google Scholar] [CrossRef]
- Lu, X.; Wei, X.; Huang, J.; Yang, L.; Zhang, G.; He, G.; Wang, M.; Qu, J. Supertoughened Poly(lactic acid)/Polyurethane Blend Material by in Situ Reactive Interfacial Compatibilization via Dynamic Vulcanization. Ind. Eng. Chem. Res. 2014, 53, 17386–17393. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly(3-hydroxybutyrate-co-hydroxyvalerate) and Poly(butylene succinate) with Balanced Properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Wu, H.; Hou, A.; Qu, J.-P. Phase Morphology and Performance of Supertough PLA/EMA–GMA/ZrP Nanocomposites Prepared through Reactive Melt-Blending. ACS Omega 2019, 4, 19046–19053. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Liu, B.; Estep, G.; Zhang, J. Super Toughened Poly(lactic acid) Ternary Blends by Simultaneous Dynamic Vulcanization and Interfacial Compatibilization. Macromolecules 2010, 43, 6058–6066. [Google Scholar] [CrossRef]
- Liu, H.; Song, W.; Feng, C.; Li, G.; Zhang, J. Interaction of Microstructure and Interfacial Adhesion on Impact Performance of Polylactide (PLA) Ternary Blends. Macromolecules 2011, 44, 1513–1522. [Google Scholar] [CrossRef]
- Dong, W.; Jiang, F.; Zhao, L.; You, J.; Cao, X.; Li, Y. PLLA microalloys versus PLLA nanoalloys: Preparation, morphologies, and properties. ACS Appl. Mater. Interfaces 2012, 4, 3667–3675. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, G.; Yin, J.; Wei, J. Reactive compatibilization of high-impact poly(lactic acid)/ethylene copolymer blends catalyzed by N,N-dimethylstearylamine. Polym. Int. 2014, 63, 1263–1269. [Google Scholar] [CrossRef]


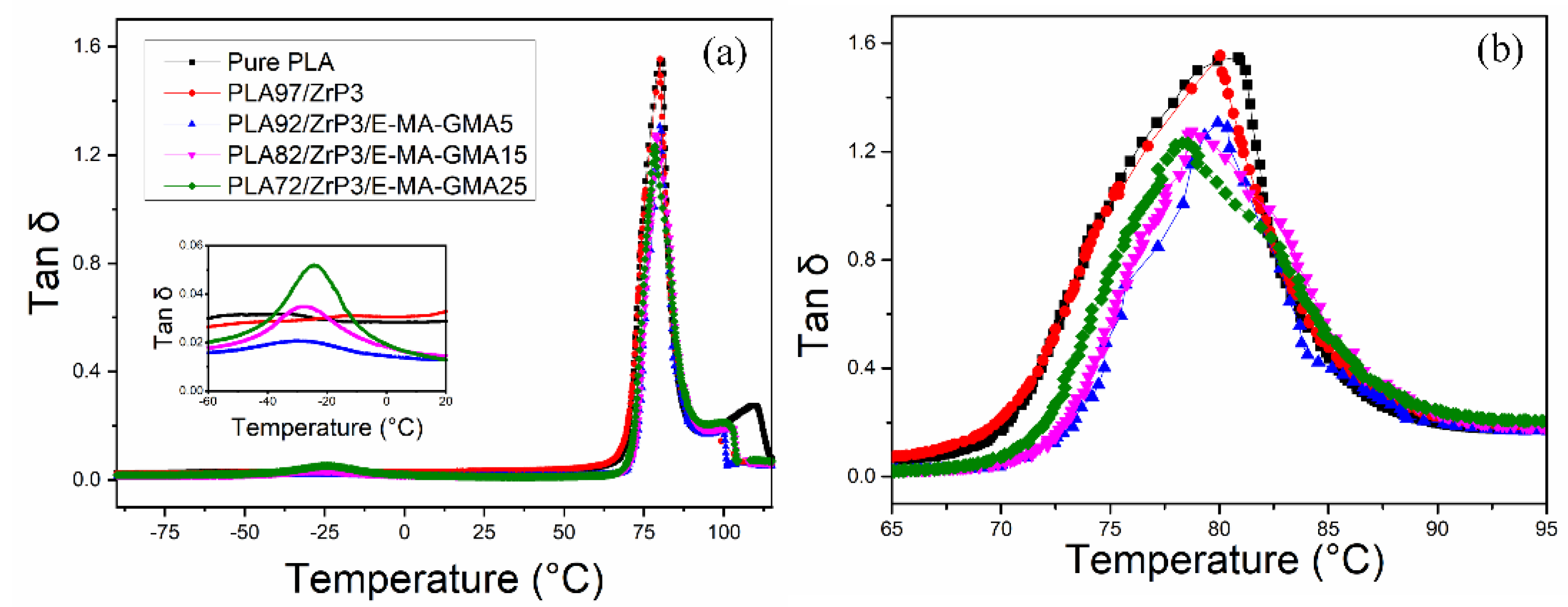

| Compositions | Tg1 (°C) | Tg2 (°C) |
|---|---|---|
| pure PLA | — | 80.4 |
| PLA/ZrP (97/3) | — | 80.0 |
| PLA/ZrP/E-MA-GMA (92/3/5) | −29.9 | 79.9 |
| PLA/ZrP/E-MA-GMA (82/3/15) | −27.5 | 78.7 |
| PLA/ZrP/E-MA-GMA (72/3/25) | −24.2 | 78.3 |
| Compositions | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHcc (J/g) | ΔHm (J/g) | χc (%) |
|---|---|---|---|---|---|---|
| pure PLA | 60.3 | 105.8 | 170.3 | 32.4 | 40.6 | 8.8 |
| PLA/ZrP (97/3) | 60.2 | 118.2 | 168.1 | 30.0 | 34.9 | 5.4 |
| PLA/ZrP/E-MA-GMA (92/3/5) | 60.9 | 119.0 | 167.0 | 26.0 | 30 | 4.6 |
| PLA/ZrP/E-MA-GMA (82/3/15) | 60.9 | 120.5 | 165.3 | 28.1 | 31.1 | 3.9 |
| PLA/ZrP/E-MA-GMA (72/3/25) | 61.0 | 121.6 | 164.5 | 23.3 | 25.2 | 2.8 |
| Compositions | Ti (°C) | Tp (°C) | Tf (°C) | Rmax (%/min) | Char (%) |
|---|---|---|---|---|---|
| pure PLA | 319.0 | 362.4 | 373.6 | 29.63 | 0.82 |
| E-MA-GMA | 398.4 | 457.5 | 491.5 | 20.37 | 0.23 |
| PLA/ZrP (97/3) | 342.9 | 368.2 | 378.4 | 35.98 | 4.04 |
| PLA/ZrP/E-MA-GMA (92/3/5) | 339.8 | 367.2 | 472.1 | 32.15 | 3.07 |
| PLA/ZrP/E-MA-GMA (82/3/15) | 342.9 | 370.5 | 484.9 | 25.43 | 2.96 |
| PLA/ZrP/E-MA-GMA (72/3/25) | 343.8 | 369.9 | 472.3 | 23.31 | 2.77 |
| Compositions | Tensile Strength (MPa) | Tensile Modulus (MPa) | Impact Strength (kJ/m2) | Elongation at Break (%) |
|---|---|---|---|---|
| pure PLA | 67.3 ± 0.1 | 1590 ± 21 | 2.9 ± 0.4 | 10.4 ± 2.8 |
| PLA/ZrP (97/3) | 60.9 ± 2.7 | 1218 ± 45 | 2.6 ± 0.1 | 7.4 ± 0.7 |
| PLA/ZrP/E-MA-GMA (92/3/5) | 50.7 ± 3.3 | 1177 ± 51 | 5.5 ± 0.4 | 434.7 ± 39.8 |
| PLA/ZrP/E-MA-GMA (82/3/15) | 39.1 ± 1.1 | 824 ± 40 | 65.2 ± 1.4 | 432.8 ± 19.1 |
| PLA/ZrP/E-MA-GMA (72/3/25) | 28.2 ± 0.8 | 604 ± 48 | 71.5 ± 3.7 | 226.3 ± 66.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Lu, X.; Li, Y.; Deng, Y.; Lu, J.; Liu, Z.; Wu, H.; Tong, Y.; Qu, J. Enhancing Toughness of PLA/ZrP Nanocomposite through Reactive Melt-Mixing by Ethylene-Methyl Acrylate-Glycidyl Methacrylate Copolymer. Polymers 2022, 14, 3748. https://doi.org/10.3390/polym14183748
Zhu C, Lu X, Li Y, Deng Y, Lu J, Liu Z, Wu H, Tong Y, Qu J. Enhancing Toughness of PLA/ZrP Nanocomposite through Reactive Melt-Mixing by Ethylene-Methyl Acrylate-Glycidyl Methacrylate Copolymer. Polymers. 2022; 14(18):3748. https://doi.org/10.3390/polym14183748
Chicago/Turabian StyleZhu, Chuanbiao, Xiang Lu, Yi Li, Yanhong Deng, Jiuling Lu, Zhigang Liu, Hao Wu, Yi Tong, and Jinping Qu. 2022. "Enhancing Toughness of PLA/ZrP Nanocomposite through Reactive Melt-Mixing by Ethylene-Methyl Acrylate-Glycidyl Methacrylate Copolymer" Polymers 14, no. 18: 3748. https://doi.org/10.3390/polym14183748






